INTRODUCTION
Sustained ventricular tachycardia (VT) is common in coronary heart disease, usually occurring in the setting of acute myocardial ischemia/infarction or later as scar-related arrhythmia.
Catecholamines have been described in the treatment of VT in the past in both animal and human models[1-5]. In clinical practice, however, their use is restricted to a few indications. Epinephrine is indicated in the setting of cardiopulmonary resuscitation for shock-resistant ventricular fibrillation (VF), pulseless electrical activity or asystole[6,7]. Currently, isoproterenol is recommended as an antiarrhythmic treatment for arrhythmic storm in Brugada syndrome[8].
Acute therapy for sustained VT depends on hemodynamic tolerance. In the case of VT with hemodynamic instability, electrical cardioversion is the standard of care. In cases of recurrence, intravenous (IV) amiodarone is the drug of choice. However, resistance to this drug, even in association with a beta-blocker, has been described[9-11].
We report three cases of coronary heart disease presenting with hemodynamically unstable sustained monomorphic VT, resistant to beta-blockers and amiodarone, in which arrhythmias were rapidly terminated by single-bolus low doses of IV epinephrine.
CASE REPORT
Patient 1
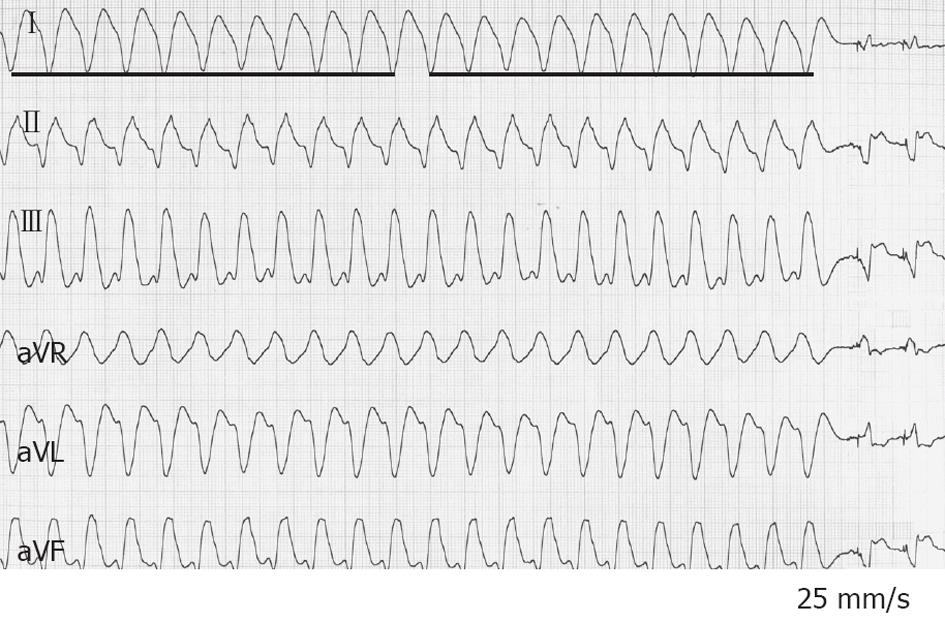
A 47-year-old man, a smoker without past medical history, was admitted to the Cardiac Care Unit (CCU) due to severe fatigue and palpitations. Physical examination was normal with the exception of a heart rate (HR) of 170 beats/min. Blood pressure (BP) was 125/86 mmHg. A VT was documented on a standard 12-lead electrocardiogram (ECG) (Figure 1). An IV dose of 300 mg amiodarone administered over 5 min failed to stop VT, and the patient then developed hemodynamic instability with a drop in BP to 89/46 mmHg, and profuse sweating without loss of consciousness. Because of rapid hemodynamic alteration and no available anesthesiologist to perform sedation for electrical cardioversion according to the protocol of this remote medical hospital, a single slow infusion of epinephrine (1/10 000, 1 mg within 1 min) was administered. VT stopped within 30 s. Termination was preceded by an increase in BP up to 130/84 mmHg and a slight increase in VT rate from 170 to 180 beats/min (Figure 2). The side effects of epinephrine were chest discomfort, nausea, and anxiousness. ECG in sinus rhythm (SR) after cessation of VT showed a pathologic Q wave in inferior leads (Figure 3). Echocardiography performed in SR showed a dilated left ventricle (62 mm), and left ventricular ejection fraction (LVEF) of 40% with inferior wall akinesia. Troponin I was slightly elevated at 0.82 ng/mL. No electrolyte disturbances were observed. Coronarography showed occlusion of the middle segment of the right coronary artery and a bare-metal stent was implanted. Continuous ECG monitoring did not record any ectopic ventricular beat in the following days. The patient was discharged seven days later.
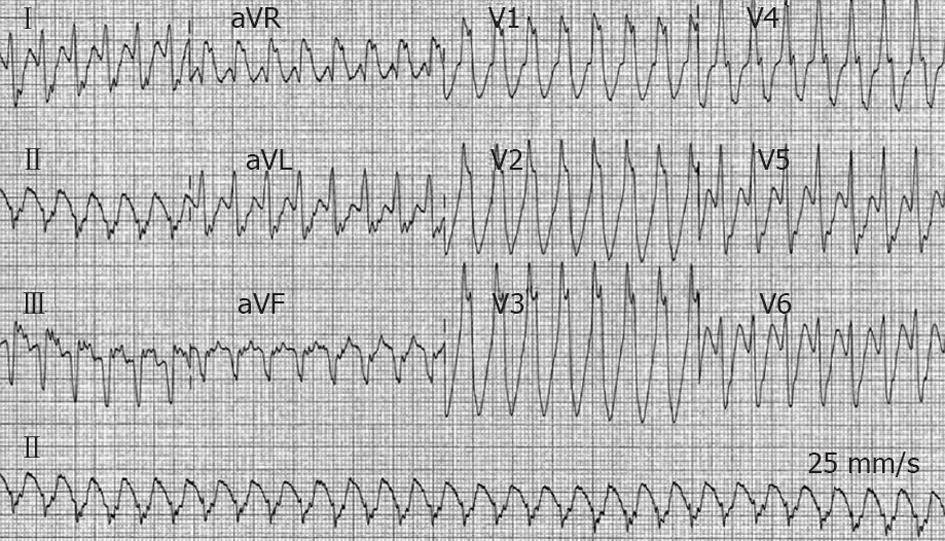
Figure 1 Twelve-lead electrocardiogram from a 47-year-old man (patient 1) with acute inferior myocardial infarction.
Ventricular tachycardia at a heart rate of 170 beats/min with right bundle branch block morphology and left axis deviation is present.
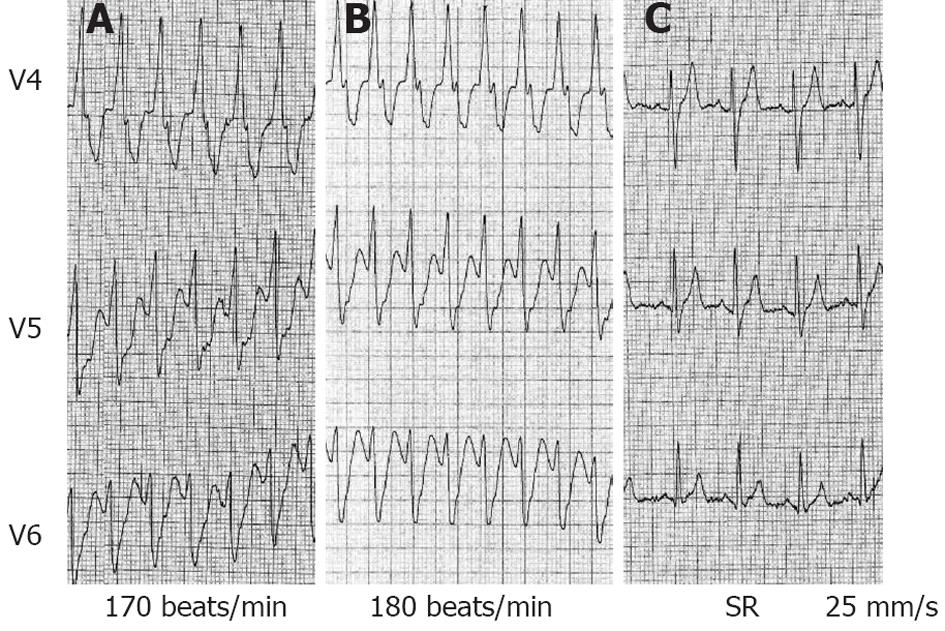
Figure 2 V4 to V6 leads also from patient 1.
A: Ventricular tachycardia (VT) at admission with a heart rate (HR) of 170 beats/min, blood pressure (BP) 125/86 mmHg. Amiodarone (300 mg intravenous) did not interrupt VT, no substantial change was observed in VT cycle length, BP dropped to 89/46 mmHg; B: Immediately after a bolus of epinephrine (1 mg over 30 s), an increase in HR up to 180 beats/min was observed; C: Electrocardiogram 30 s after epinephrine bolus: sinus tachycardia (HR 110 beats/min) after VT termination.
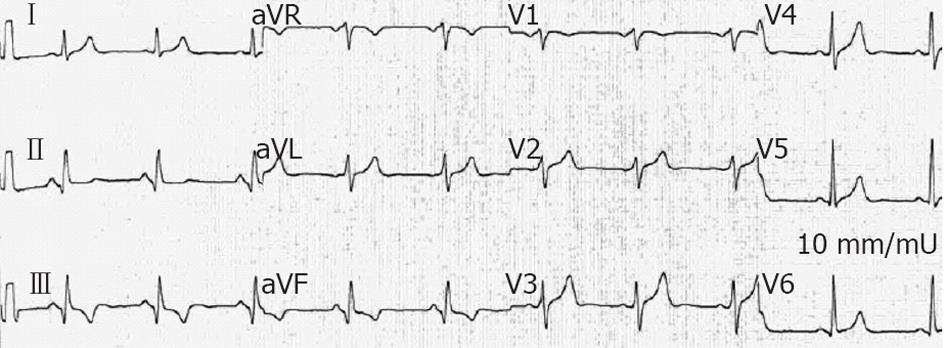
Figure 3 In patient 1, electrocardiogram performed 2 d after the reduction of ventricular tachycardia showed pathologic Q wave displaying a sequelae of a transmural myocardial infarction in inferior leads.
Patient 2
A 64-year-old male was admitted to the CCU due to sustained VT with a HR of 140 beats/min (Figure 4A). Past medical history showed an inferior myocardial infarction (MI) with low ejection fraction and an implantable cardioverter-defibrillator (ICD) for secondary prevention. He was on bisoprolol 10 mg daily associated with amiodarone 200 mg daily added three months prior for recurrent non-sustained VT. On arrival, he was conscious and had a palpable pulse although BP was 85/50 mmHg; ICD interrogation was not attempted due to unavailability of this expertise at the time of patient management. One slow-rate infusion of IV epinephrine (1/10 000; 0.5 mg in 30 s) was then administered. VT termination occurred approximately 30 s after the end of the epinephrine infusion. Cessation was preceded by a slight increase in the VT rate (from 140 to 148 beats/min). During epinephrine administration the patient experienced brief chest discomfort and headache. Twelve-lead ECG in SR displayed pathologic Q waves in inferior and lateral leads (Figure 4B). Troponin I was slightly elevated (0.57 ng/mL). Serum electrolytes were within normal limits. Two-D echocardiography indicated a LVEF of 35%. Coronary angiography revealed a restenosis of the right coronary artery and a sub-occlusion of the ostium of a marginal artery. A drug-eluting stent was implanted in both lesions. An ICD interrogation before discharge did not register any arrhythmic event as the programming VT zone (150 beats/min) was higher than the patient’s VT (140 beats/min). No further episodes of VT were observed during hospitalization, and the patient was discharged seven days later.
Figure 4 Electrocardiogram tracings from a 64-year-old man with ischemic dilated cardiomyopathy with an implanted cardioverter-defibrillator, on chronic treatment with bisoprolol and amiodarone (patient 2).
A: Ten-lead electrocardiogram (ECG) at admission with sustained ventricular tachycardia (VT) at 140 beats/min with a left bundle branch block morphology and right axis deviation, blood pressure was 85/50 mmHg; B: Twelve-lead ECG 30 s after intravenous epinephrine bolus (0.5 mg over 30 s) shows sinus rhythm with pathologic Q waves in inferior leads. VT termination was preceded by a slight increase in VT rate from 140 to 148 beats/min (not shown).
Patient 3
A 67-year-old man was admitted to the CCU due to an arrhythmic storm. The patient had a previous history of two MI (inferior and anterior) with a large akinetic area in the anterior wall and a low ejection fraction (LVEF < 20%). He was on chronic treatment with carvedilol (12.5 mg twice daily) and amiodarone (200 mg daily). An ICD had been implanted for primary prevention. As the patient developed permanent atrial fibrillation and remained symptomatic despite an optimal pharmacological regimen, the ICD was upgraded to CRT-D because of the need for chronic ventricular pacing. Four months previously, the patient had been admitted for syncope. At that time, ICD interrogation confirmed an arrhythmic storm. Coronary angiography showed an occlusion of the first marginal artery, but no intervention was performed due to the absence of viability. He underwent successful radiofrequency catheter ablation of two inducible monomorphic VTs. Four months later, the patient was re-admitted to the CCU due to another arrhythmic storm.
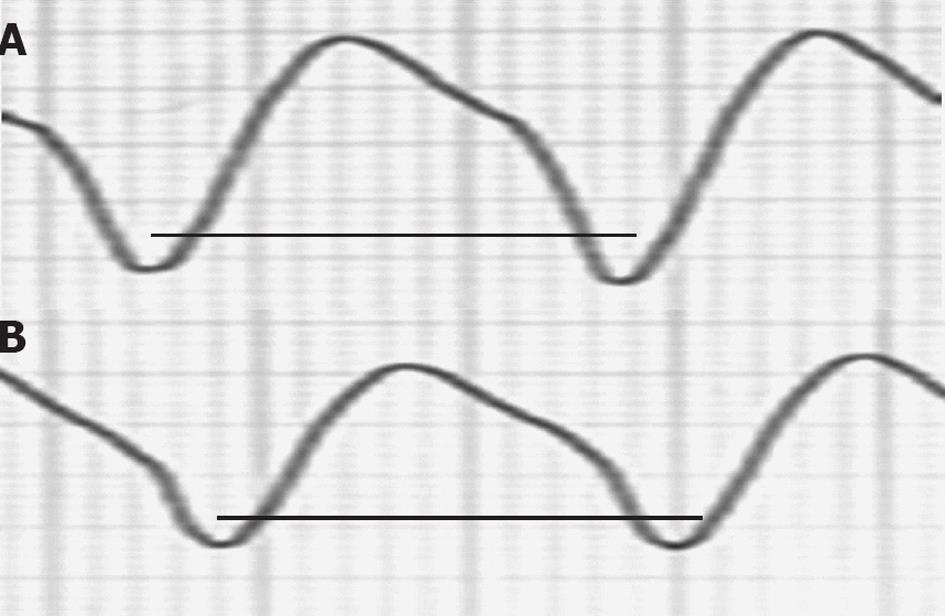
On admission to the CCU, a tachycardia with a VT pattern was recorded (Figure 5). BP was 90/45 mmHg. No electrolyte disturbance was observed. Troponin I was within normal limits. Once in the CCU, he experienced six more appropriate ICD shocks within 5 min. The underlying rhythm was VT with a HR of 140-150 beats/min. The ICD was programmed with one VT zone from 130 to 200 and the VF zone > 200 beats/min. Appropriate ICD shocks were delivered, but were followed by recurrence of the VT. Amiodarone (150 mg IV over 15 min) failed to terminate VT and decreased BP to 70/40 mmHg. Different protocols of direct manual overdrive pacing also failed to interrupt VT. One “aggressive” attempt transformed VT to VF managed by the ICD. Less than 60 s later, the VT re-appeared. BP steadily continued to decrease down to 65/30 mmHg. A bolus IV injection of epinephrine (1/10 000, 0.5 mg in 30 s) was administered while his BP was continuously monitored. Following a BP increase up to 125/85 mmHg, VT terminated within 90 s, preceded by a small shortening of VT cycle length (Figure 6). Treatment was supplemented by amiodarone IV (300 mg over 20 min) and atenolol IV (25 mg over 15 min). The next day, he underwent coronary angioplasty of the first marginal artery. The patient remained free of arrhythmias.
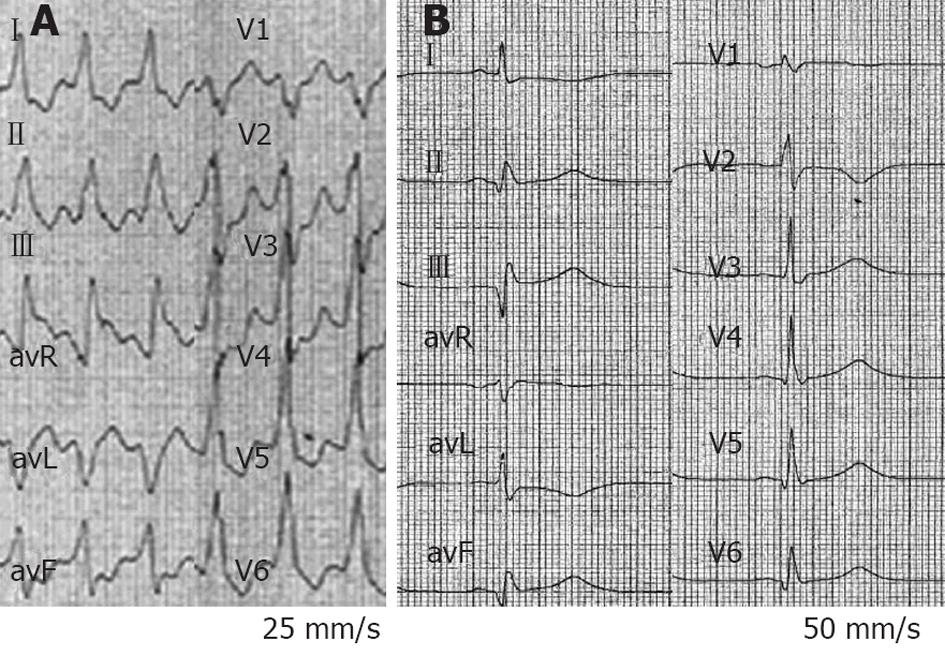
Figure 5 Six-lead electrocardiogram from a 47-year-old man in arrhythmic storm with ischemic dilated cardiomyopathy and an implanted cardioverter-defibrillator (patient 3).
A sustained ventricular tachycardia (VT) with a heart rate of 140 beats/min is observed, blood pressure (BP) was 90/45 mmHg. intravenous amiodarone failed to terminate VT and decreased BP to 70/40 mmHg. Epinephrine (0.5 mg over 30 s) increased BP up to 125/85 mmHg, and VT terminated within 90 s, preceded by a small shortening of VT cycle length. At the end of the tracing, VT stopped. The bold line on the left side of the picture includes ten VT cycles; the bold line on the right side includes the last ten VT cycles before interruption.
Figure 6 A ventricular tachycardia of 140 beats/min from a 47-year-old man in arrhythmic storm with ischemic dilated cardiomyopathy and an implanted cardioverter-defibrillator (patient 3) was interrupted by Epinephrine (0.
5 mg over 30 s) after a small cycle length shortening. A: First ventricular tachycardia (VT) cycle of the patient in Figure 5; B: Last VT cycle before VT interruption. The two bold lines have the same length. A small VT cycle shortening before interruption is noted.
DISCUSSION
We illustrated three cases of drug-resistant sustained monomorphic VT with hemodynamic instability, in which the arrhythmia was terminated with IV epinephrine. All patients had coronary heart disease, but whether acute coronary syndrome was concerned is difficult to state. Indeed, a moderate troponin increase is also seen after tachyarrhythmias and post-VT ECGs did not show either ST-segment elevation or depression. Amiodarone was ineffective in all patients. Two of the patients had an ICD. One ICD carrier experienced an arrhythmic storm. Termination of long-duration (> 2 h) VT was observed within 30-90 s after the administration of epinephrine, an alpha- and beta-sympathomimetic agent. An arrhythmic storm was stopped after epinephrine infusion. No short-term VT recurrence was observed, or in the patient with an arrhythmic storm.
VT is a common arrhythmia in coronary heart disease[12,13]. The standard of care for sustained VT with a pulse is to use either anti-arrhythmic drugs or external electrical shock. Advanced Cardiac Life Support (ACLS) protocol recommends the use of amiodarone, procainamide, mexiletine, sotalol or lidocaine as anti-arrhythmic drugs[7,8]. In clinical practice, amiodarone is the most commonly used anti-arrhythmic before electrical cardioversion[9,10].
When to use epinephrine in sustained VT
In cases of drug-resistant poorly tolerated VT, immediate external electrical cardioversion must be attempted. However, there are cases in which VT recurs immediately after the shock, and cardioversion involves the need for anesthesia when the patient is still conscious. Although shocks delivered by ICD often effectively terminate VT, in some patients VT can restart quickly, as the ICD is unable to prevent arrhythmia. In this context of arrhythmic storm, sedation and anti-arrhythmic drugs are of great interest[14]. However, in the setting of coronary disease, either acute phase or scar-related VT, effective drug therapy is limited to lidocaine, mexiletine, procainamide, amiodarone and beta-blockers[15,16]. However, the first three cited drugs are not commonly available or used in remote medical centers or in low-income countries. Moreover, in some cases, ICD-carriers live or are travelling in a part of the world where ACLS is not provided at all, and only amiodarone is available. In the past, some case reports described interruption of sustained VT using intravenous sympathomimetic amines[2-4]. Based on the cases reported herein, low doses of IV epinephrine may be able to terminate sustained monomorphic VT, when the arrhythmia is refractory to amiodarone used alone or in combination with beta-blockers and electrical cardioversion.
Possible mechanisms of VT reduction by epinephrine
Two possible mechanisms may underlie the interruption of a monomorphic sustained VT by low doses of intravenous epinephrine.
Effects on BP, baroreceptor stimulation and coronary perfusion: Epinephrine has an alpha-sympathomimetic effect by which systemic BP is known to increase. This increase in BP could have two different effects. The rise in BP could result in better coronary artery perfusion, thus inhibiting ischemia-induced VT (patient 1). Alternatively, a baroreceptor-mediated increase in parasympathetic tone via the carotid body could lead to VT termination via vagal stimulation[17]. The latter hypothesis is difficult to demonstrate because vagal myocardial innervation is not completely defined in humans[18]. Finally, a direct effect of epinephrine on coronary perfusion, through coronary vasodilatation, is a possible mechanism in ischemia-related VT[19].
Modification of conduction properties: Conduction velocity and refractoriness of the myocardium are modified in opposite ways by epinephrine and beta-blockers and/or amiodarone. Adrenaline-dependent increased conduction velocity with shortening of VT cycle length could be responsible for extinction of the circuit by a simple mechanism of head-tail conjunction. Considering a circus movement reentry as the mechanism of the VT, the acceleration of the VT by epinephrine will translate into progressive shortening of the excitable gap until it will be so short that the “head” meets the “tail”, and then the circuit becomes extinct. This being the case, termination of the VT will be preceded by a faster epinephrine-induced, small transient VT acceleration in all 3 patients. A change in myocardial fiber stretch by epinephrine could also modify the circuit components favoring VT interruption[20].
On the other hand, amiodarone could limit the refractoriness shortening of epinephrine which, combined with an increased conduction velocity determined by epinephrine itself, might be responsible for tachycardia interruption because of a residual refractoriness prolongation of part of the circuit[21-23]. Tonet et al[24,25] have shown that beta-receptor blockade by beta-blocker agents and chronic amiodarone therapy converts VT to SR via prolongation of the refractory period and cycle length. Thus, pre-treatment with amiodarone might enhance the effectiveness of IV epinephrine which, reducing repolarization dispersion, interrupts reentry and could be useful in cases of electrical storm.
Detailed analysis of the cases reported herein can support both hypotheses. In all patients, elevation of BP preceded VT termination, suggesting a mechanism of better coronary artery perfusion or parasympathetic stimulation[26,27]. Otherwise, in all patients, the cycle length of the VT shortened just before the return to SR, suggesting a modification in myocardial conduction properties with an increase in conduction velocity and a subsequent shortening of the VT cycle length able to induce reentry interruption by a head-tail conjunction mechanism. All patients were previously treated with amiodarone, whose antiarrhythmic properties mainly concern refractoriness prolongation, especially when taken on a chronic basis[28]. A possible intricate electrophysiological mechanism could also be conjectured, that is, a residual prolonged refractoriness by beta-blocker and amiodarone pre-treatment combined with increased velocity when epinephrine is administered[24].
Precautions on the use of epinephrine for VT
The most frequent and clinically significant side effects of IV epinephrine were not observed in our patients, which may be explained by the lower doses (≤ 1 mg) used[29]. The potential complications of epinephrine while performing cardiopulmonary resuscitation were described for doses greater than those that are the standard[8,9]. VF induction in subjects treated with bolus or continuous infusion of epinephrine for severe hypotension or cardiogenic shock have been reported, and the conversion of VT to VF is a matter of real concern[6,7]. It is of interest to note that in experimental models of chronically infarcted canine hearts, selective beta1-adrenergic stimulation alone does not cause dispersion of myocardial refractoriness and does not cause significant proarrhythmia[30]. Also, in dogs with experimentally induced myocardial infarction, infusion of isoproterenol increases the incidence of inducible VT, but does not facilitate the induction of VF[31]. In any case, given the potentially harmful intervention in the setting of coronary heart disease, further research to explore the mechanism of action, its effectiveness and its safety is mandatory. In our patients, evidence of acute ischemia was modest with small increases in troponin level; this factor is to be considered when deciding whether to use epinephrine.
Clinical implications
It is well known that spontaneous episodes of VT can accelerate, slow, or even terminate spontaneously. However, in all cases, the termination of a long-duration (> 2 h) VT was rapidly observed after 90 s or less following epinephrine administration, preceded by a shortening of the VT cycle length before SR restoration, strongly suggesting an epinephrine-related effect. Although the use of sympathomimetic amines to treat sustained monomorphic ischemic VT could be questionable, mainly because its rationale is against the current paradigm of the pathophysiology of ischemic VT as well as the wide availability of appropriate drugs and external cardioversion, the effect observed in our cases merits further investigation. Remote medical centers and under-resourced countries with difficulties providing ACLS management may be concerned. We might also speculate on a possible protective effect of amiodarone and beta-blocking treatment for the prevention of the degenerative arrhythmic effects of epinephrine itself. In fact, VT cycle length was slightly shortened in all patients, without any change in VT morphology or VF degeneration.
Study limitations
Some limitations merit consideration. First, as these are only three observational cases, this remains a non-conclusive assessment of the efficacy and safety of this unconventional “heroic” intervention. Second, although all subjects had beta-blocker and/or amiodarone resistant VT, another antiarrhythmic drugs such as lidocaine or mexiletine were not attempted before the epinephrine regimen. Third, the treatment of patients 1 and 2 is not a conventional one and should not, therefore, be advocated at the first intention. Fourth, one of the questions raised by our observations is whether VT was actually self-limiting or truly stopped by the epinephrine injection; however, the rapid onset of action in a longstanding sustained monomorphic VT serves to support considering it a direct effect of epinephrine.
Conclusion
Low dose IV epinephrine may terminate sustained monomorphic VT refractory to amiodarone used alone or in combination with beta-blockers and electrical cardioversion. Although potentially harmful, epinephrine may be an alternative effective therapy for refractory VT, particularly in remote medical centers and low income countries where advanced cardiac life support is under-provided. This treatment requires a clinical study.