Copyright
©2010 Baishideng Publishing Group Co.
World J Cardiol. Jun 26, 2010; 2(6): 150-159
Published online Jun 26, 2010. doi: 10.4330/wjc.v2.i6.150
Published online Jun 26, 2010. doi: 10.4330/wjc.v2.i6.150
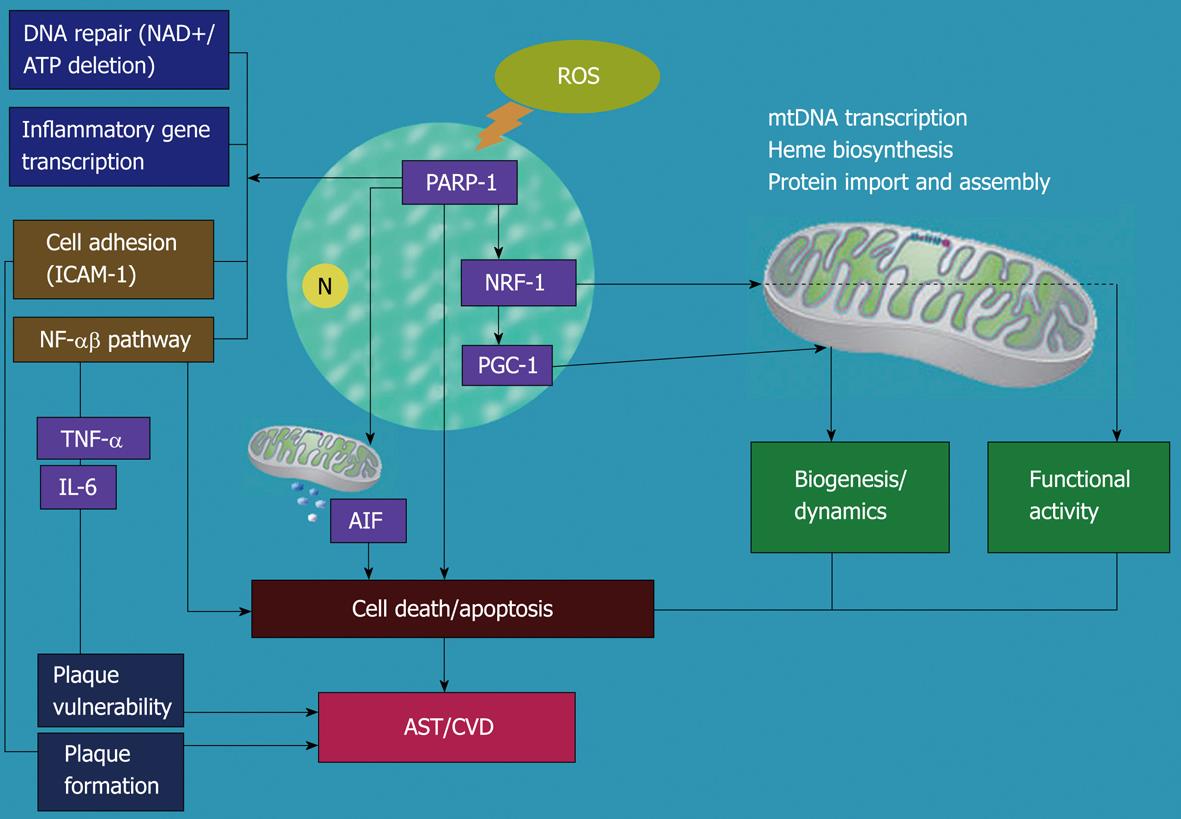
Figure 1 The molecular regulation of nuclear and mitochondrial cross-talk signaling on ROS-triggered AST/VCD development.
ROS activate PARP-1 and cause AST primarily by increasing endothelial and smooth muscle cell death (mitochondria-dependent or not) and triggering inflammatory reactions. In addition, proinflammatory cytokines and chemokines induce plaque formation and plaque vulnerability that leads to deterioration in the later stages of AST. However, PARP-1 can also participate in NRF-1 regulation or indirectly activate PGC-1 to improve mitochondrial biofunctions such as biogenesis and fusion. This mechanism defends against ROS-induced progression of AST by inhibiting mitochondrial-mediated apoptosis and against the occurrence of AST at an early stage. ROS: Reactive oxygen species; PARP-1: Poly(ADP-ribose) polymerase-1; NRF-1: Nuclear respiratory factor 1; PGC-1: Peroxisome proliferator-activated receptor γ coactivator-1; mtDNA: Mitochondrial DNA; AIF: Apoptosis-inducing-factor; NF-κB: Nuclear factor κ-light-chain-enhancer of activated B cells; TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6; AST: Atherosclerosis; CVD: Cardiovascular diseases; N: Nucleus.
- Citation: Chang JC, Kou SJ, Lin WT, Liu CS. Regulatory role of mitochondria in oxidative stress and atherosclerosis. World J Cardiol 2010; 2(6): 150-159
- URL: https://www.wjgnet.com/1949-8462/full/v2/i6/150.htm
- DOI: https://dx.doi.org/10.4330/wjc.v2.i6.150