Published online Apr 26, 2022. doi: 10.4330/wjc.v14.i4.190
Peer-review started: March 18, 2021
First decision: September 29, 2021
Revised: November 13, 2022
Accepted: April 3, 2022
Article in press: April 3, 2022
Published online: April 26, 2022
Cardiac magnetic resonance imaging (MRI) is an evolving technology, proving to be a highly accurate tool for quantitative assessment. Most recently, it has been increasingly used in the diagnostic and prognostic evaluation of conditions involving an elevation in troponin or troponinemia. Although an elevation in troponin is a nonspecific marker of myocardial tissue damage, it is a frequently ordered investigation leaving many patients without a specific diagnosis. Fortunately, the advent of newer cardiac MRI protocols can provide additional information. In this review, we discuss several conditions associated with an elevation in troponin such as myocardial infarction, myocarditis, Takotsubo cardiomyopathy, coronavirus disease 2019 related cardiac dysfunction and athlete’s heart syndrome.
Core tip: Cardiac magnetic resonance has excellent spatial resolution to assess ventricular volumes and function. It is also continuing to evolve to provide key diagnostic and prognostic information particularly through the use of gadolinium contrast agent for the conditions presented in this review.
- Citation: Nguyen Nguyen N, Assad JG, Femia G, Schuster A, Otton J, Nguyen TL. Role of cardiac magnetic resonance imaging in troponinemia syndromes. World J Cardiol 2022; 14(4): 190-205
- URL: https://www.wjgnet.com/1949-8462/full/v14/i4/190.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i4.190
Troponinemia describes an elevation in serum troponin levels that can result from a myriad of conditions such as acute myocardial infarction (AMI), takotsubo cardiomyopathy (TTS), myocarditis and athlete’s heart syndrome (AHS). More recently, severe acute respiratory syndrome coronavirus 2 has been linked to cardiac disease and elevated troponin levels. Given that troponinemia is nonspecific, establishing a definitive diagnosis can be difficult. Fortunately, cardiac magnetic resonance imaging (CMRI) has the ability to characterise myocardial tissue and identify unique pathological features of cardiac disease. As a diagnostic tool for these conditions, CMRI imaging may be promising.
This narrative review gives an overview of the diagnostic features and potential role of CMRI in conditions associated with troponinemia such as myocardial infarction (MI), TTS, myocarditis, coronavirus disease 2019 (COVID-19) related cardiovascular disease and AHS (Table 1).
| Condition | Cardiac magnetic resonance imaging features |
| Myocardial infarction | < 1 mo |
| Myocardial oedema present on T2-weighted images, T2 mapping and T1 mapping | |
| Microvascular obstruction revealed as a hypointense core within hyperintense infarct zone in area of LGE | |
| Infarct size can be calculated using pre and post-contrast T1-weighted mapping and ECV assessment | |
| Myocardial necrosis/scar by LGE in a subendocardial or full-thickness pattern within a coronary artery territory | |
| Additionally at < 6 mo | |
| T2-weighted hyperintensity on double inversion recovery turbo spin echo | |
| Takotsubo syndrome | Can help distinguish coexisting CAD or acute myocarditis LGE typically absent |
| Myocardial oedema present on T2-weighted images, T2 mapping and T1 mapping | |
| Accurate assessment of WMAs on cine imaging | |
| Can be useful to identify ventricular thrombus | |
| Myocarditis | Inflammatory hyperaemia demonstrated on T1-weighted images |
| Myocardial oedema on T2-weighted images | |
| Myocardial necrosis/scar by LGE in a subepicardial or mid-wall pattern | |
| Greater T1 and T2 increases with acute inflammation | |
| Pericardial effusion | |
| COVID-19 related cardiac dysfunction | Features similar to that of acute myocarditis |
| Myocardial oedema on T2-weighted images | |
| Myocardial necrosis/scar by LGE in a subepicardial or mid-wall pattern | |
| Myocardial fibrosis using T1-weighted mapping and ECV assessment | |
| Can be useful to identify ventricular thrombus and pericardial effusion | |
| Athlete’s heart | LVH typically < 12 mm |
| Lower ECV with LVH compared to HCM | |
| RV dilatation seen on cine imaging | |
| LGE focal and generally at the RV insertion points |
AMI is one of the most common causes of elevated troponin levels. It is defined by the presence of acute myocardial injury in conjunction with dynamic changes in troponin levels, and evidence of myocardial ischaemia[1]. Electrocardiography (ECG), transthoracic echocardiography (TTE) and invasive coronary angiography are the standard of care in the evaluation of MI. Adjunctive use of CMRI can be useful to confirm the diagnosis, assess chronicity, guide management and aid prognosis. It can be utilised to distinguish the changes seen between an acute and an established, or also called chronic MI. CMRI is also highly accurate at assessing ventricular volumes and function with superior spatial resolution, contrast-to-noise ratio and tissue characterisation compared to TTE.
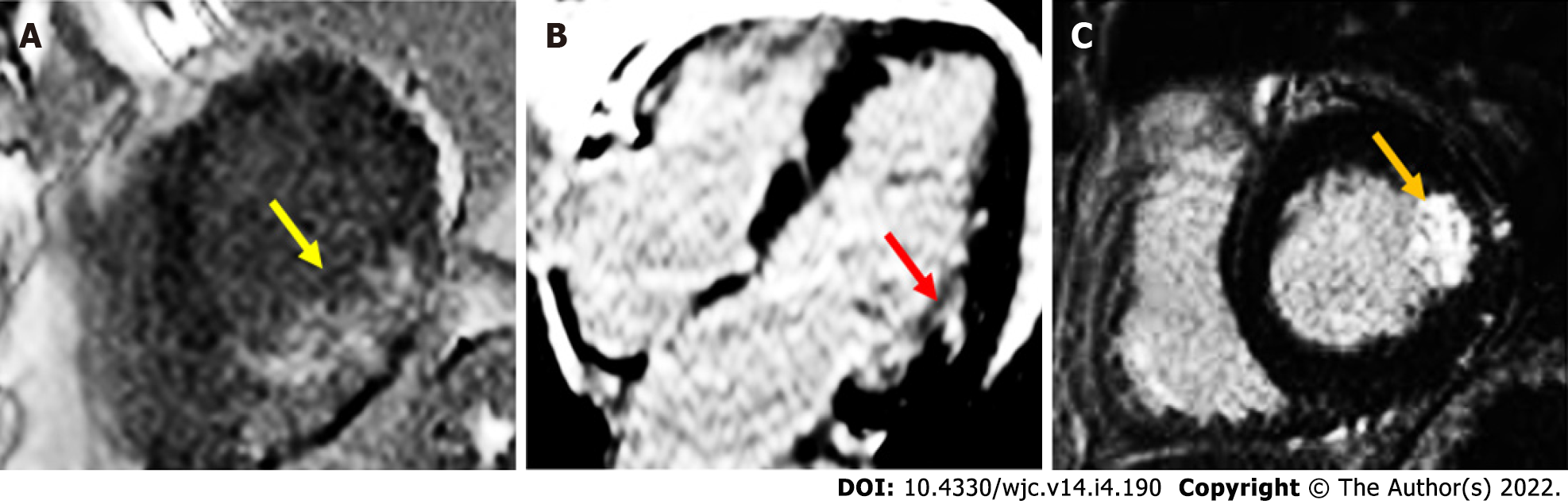
On CMRI, AMI can demonstrate ventricular regional wall motion abnormalities (RWMAs) corresponding with the affected vascular territory on cine images. Intramyocardial haemorrhage may occur, and is represented by a hypointense zone in the infarcted area on T2-imaging or mapping[2]. Following ischaemic insult from coronary artery obstruction, myocardial cellular injury begins in the subendocardial region, and continues to extend towards the subepicardium if there is ongoing oxygen deprivation. This process is known as the “wavefront phenomenon of myocardial death”, named by Reimer et al[3]. Late gadolinium enhancement images characteristically demonstrate a hypodense core surrounded by an area of hyperenhancement (Figure 1), and is found in a subendocardial or transmural distribution depending on the extent of the MI[4-6]. In chronic MI (CMI), cine images will typically show wall thinning, RWMA, and a lack of oedema on T2-weighted images[7]. In a study by Rehwald et al[8], the authors used rabbit models to demonstrate that gadolinium contrast agent uptake was greater in infarcted myocardial tissue. In another study, Kim et al[9] demonstrated that the extent of transmural hyperenhancement reflected the degree of irreversible injury. Native T1-sequences have also been explored by Kali et al[10], who demonstrated CMRI may be useful in diagnosing CMI, and determining likely irreversible injury. It is particularly useful in some situations, such as in renal failure patients, where gadolinium contrast is contraindicated.
Besides determining left ventricular ejection fraction (LVEF) on cine imaging, a detailed assessment of LV deformation measurements can also take place using CMRI[11]. Left ventricular global radial strain, circumferential strain and global longitudinal strain (GLS) have all shown association with increased major adverse cardiac events (MACEs)[12], and GLS has been demonstrated to be an independent predictor of post-MI clinical outcome. Impairment of left atrial strain on CMRI post-MI has also been demonstrated to be an independent predictor for increase in MACEs, as well as improving prognostic value when combined with LVEF[13,14].
It has been demonstrated that in patients with coronary artery disease, those treated with revascularisation have a significantly lower annual mortality rate compared to those treated with medical therapy[15,16]. In these patients, CMRI can be used to assess the viability of a coronary artery territory. The most important parameters are LV end-diastolic wall thickness, quantitative LV systolic or diastolic performance during low-dose dobutamine stress testing, and late gadolinium enhancement (LGE)[17]. For example, in a LV segment with ≤ 50% transmural LGE, a normal dobutamine response is correlated with greater functional recovery after revascularisation[18,19]. In contrast, the presence of ≥ 50% transmural LGE indicates nonviable infarcted tissue[20,21]. This technique is comparable to fluorodeoxyglucose positron emission tomography, which is considered the gold standard in the assessment of myocardial viability[22]. Unfortunately, the role of CMRI can be limited in many healthcare settings, considering factors including machine access, availability of imaging experts, cost and time.
The use of CMRI in assessing prognosis following MI has shown promising results. Assessment for microvascular obstruction (MVO) through the use of first pass perfusion studies during and following gadolinium contrast administration is one of the prognostic features that has been studied. It is implicated in adverse ventricular remodelling, larger infarct size (IS) and poorer clinical outcome[23-25]. van Kranenburg et al[26] have also demonstrated that MVO is an independent predictor for major adverse clinical outcomes at 2 years. Infarct size on CMRI has also been shown to be strongly associated with heart failure hospitalisation and all-cause mortality[27]. More recently, postcontrast T1 mapping has been shown to accurately quantify IS in a small study[28]. CMRI has also demonstrated some correlation between IS and peak troponin I[29], however this has not been a consistent or reliable finding. Intramyocardial haemorrhage has been linked to adverse LV remodelling and increased MACEs, but heterogeneity in imaging techniques mean further study is required[23]. The presence of LGE in patients with symptoms suggestive of MI conferred worse MACEs compared to those without LGE[30]. The prognostic importance of LVEF has been demonstrated in a number of studies[31,32]. Study delay for at least 1 wk following AMI should be considered, to allow for myocardial functional recovery as found by Mather et al[33], and further imaging at up to 6 mo may be required to assess stabilised LVEF[34]. LVEF ≤ 35% and LGE were independently associated with MACEs, with better predictive value than TTE[35]. The extent of LV scarring has also been clearly associated with risk of spontaneous ventricular arrhythmias[36-38]. Assessment of the peri-infarct, or “grey-zone”, surrounding the infarcted core may also play a role in risk stratifying post-MI patients, with increased size posing potential heightened ventricular arrhythmic risk[39]. Yan et al[40] used a semiautomatic software detection system to quantify percentage of abnormal myocardial delayed enhancement of tissue surrounding the infarct core, and noted that it was an independent predictor of post-MI all-cause and cardiovascular mortality.
Incorporation of artificial intelligence-based analyses will likely have a role to play in the future in cardiac outcome and prognosis prediction. It has already been demonstrated that fully automated volumetric and myocardial segmentation assessment are equally effective as manual efforts in predicting MACEs[41].
As it stands, without the availability of randomised controlled data or larger studies, the actions to be taken if high risk CMRI features are seen are not completely clear[42,43].
TTS, which is also known as Takotsubo cardiomyopathy, transient apical ballooning syndrome, broken heart syndrome and stress-induced cardiomyopathy, is a condition of transient LV dysfunction that is typically triggered by physical or emotional stress[44]. TTS mimics MI with often indistinguishable clinical presentation, ECG changes and cardiac enzyme elevation, but without angiographic evidence of acute obstructive coronary artery disease or plaque rupture[44,45]. Given the transient nature of TTS, traditionally it was thought of as a benign condition however more recent data suggests this is misguided, with complications comparable to those seen in patients with the acute coronary syndrome[46,47]. CMRI is increasingly used to diagnose and evaluate complications of TTS in both the acute and subacute setting, particularly in those with atypical features, or bystander coronary artery disease[48].
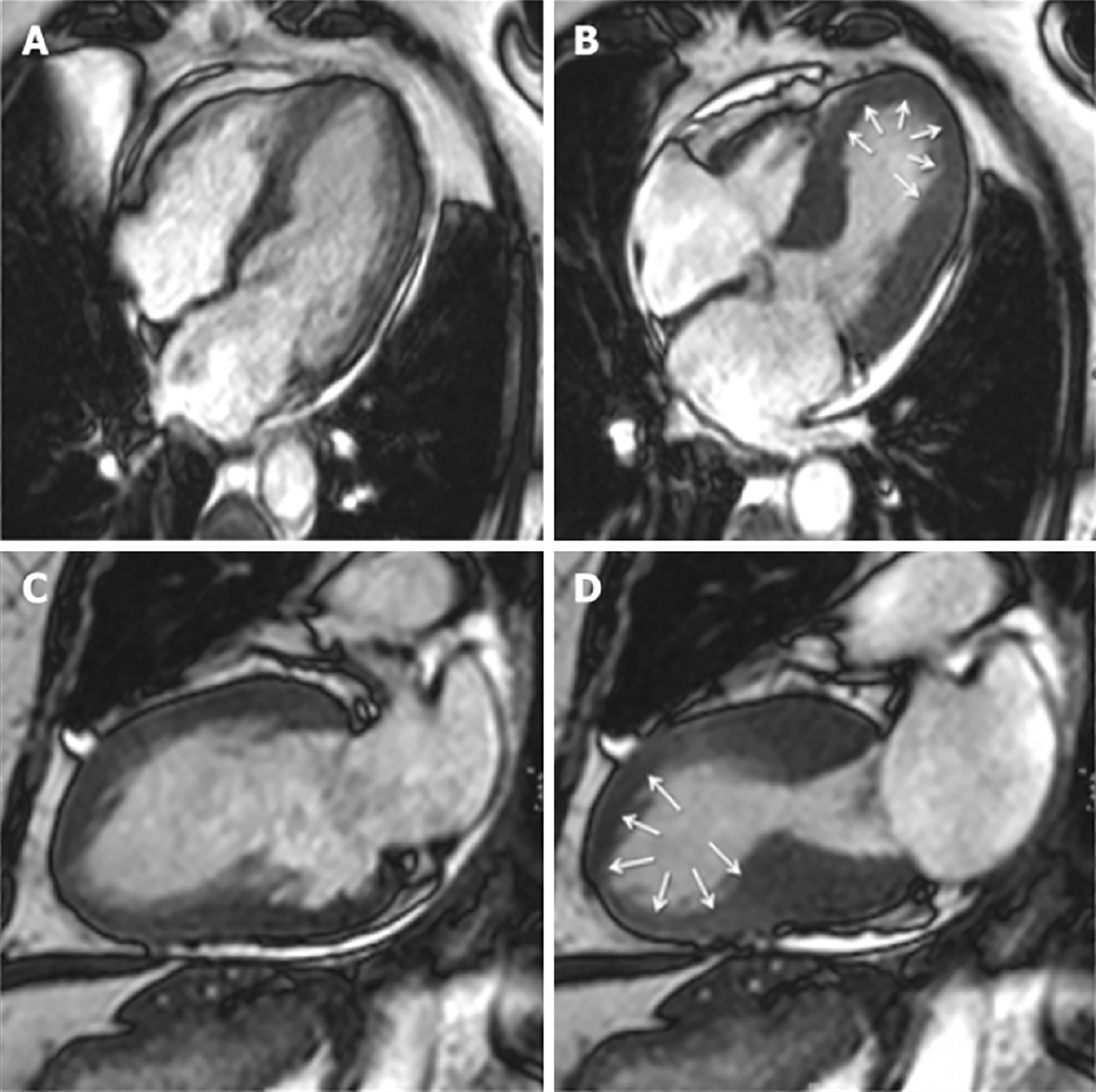
In the acute setting, CMRI can define TTS by excluding other aetiologies such as MI and myocarditis and identifying RWMAs that extend beyond a single coronary artery distribution[49] (Figure 2). One of the hallmarks is reversible myocardial inflammation corresponding to RWMA[31,44]. CMRI can assess myocardial inflammation and oedema with T2-weighted images[44,50-52].
In the subacute phase, its strength in identifying subtle RWMA makes it the ideal modality to accurately assess for resolution of regional dysfunction, with full recovery being a criteria confirmation of diagnosis[44].
Late gadolinium-enhanced imaging is a valuable adjunct in confirming a diagnosis of TTS when there is coexisting coronary artery disease or suspicion for myocarditis. It is widely believed that in TTS, there is an absence of LGE on CMRI[31], however, there are studies challenging this notion, having demonstrated LGE in patients with TTS in the acute phase[52-54]. It should be noted that LGE in this setting is transient, resolving on serial imaging to confirm the diagnosis of TTS, and has been associated with increased incidence cardiogenic shock and a longer timeframe for resolution of wall motion abnormalities[55,56]. In contrast, patients with myocardial infarction will have focal subendocardial or transmural LGE evident, while those with myocarditis typically will have a mid-wall distribution of LGE[57].
In addition to confirming the diagnosis of TTS, CMRI is useful in identifying complications such as mitral regurgitation and LV outflow tract obstruction seen on blood flow imaging, pericardial effusion seen on black blood T1-weighted sequences, and ventricular (including apical) thrombi not visualised on TTE, during early gadolinium (EGE) sequences. Thrombi will appear as a low signal intensity without gadolinium uptake, in comparison to the high intensity signal from the blood pool[50,52].
It has been hypothesised that the elevated catecholamines observed in TTS have a role in the microvascular dysfunction noted in patients with TTS, correlating with improvement in myocardial function[58]. While not established in TTS, there is emerging evidence in the utility of quantitative perfusion CMRI to more objectively assess the role that microvascular dysfunction plays in this syndrome, and is subject to further research[59].
Acute myocarditis is an inflammatory cardiomyopathy secondary to infectious and noninfectious conditions, sometimes associated with symptoms of heart failure developing over ≤ 3 mo. The clinical presentation can be nonspecific and may include chest pain, heart failure, cardiogenic shock, arrhythmias and/or sudden cardiac death. Early investigations may demonstrate elevated troponin levels, elevated acute phase reactants such as C-reactive protein, erythrocyte sedimentation rate and eosinophil count. An ECG may be normal, show nonspecific abnormalities or be similar to the pattern of acute pericarditis and AMI. Most importantly, it is important to exclude alternative causes such as MI. Due to the variable clinical presentation, the gold standard for diagnosis remains an endomyocardial biopsy (EMB), which is an invasive procedure that carries risk of life-threatening complications. CMRI may provide a noninvasive alternative for the assessment of myocarditis[60].
CMRI has become an important tool in the assessment of myocardial inflammation in patients with suspected myocarditis. Assessment of gross abnormalities includes changes in ventricular size and geometry, regional and global wall motion abnormalities and identification of pericardial effusion. In addition, there are techniques to assess microscopic markers of myocardial inflammation such as T1-weighted sequences for detection of myocardial hyperaemia, LGE for myocardial necrosis, fibrosis or scars and T2-weighted imaging to identify oedema[61-63]. Following early CMRI data, consensus diagnostic criteria were released and incorporated into the Lake Louise criteria[60] (LLC) (Table 2). The use of newer mapping techniques such as for native T1 and T2, and quantification of extracellular volume (ECV), in comparison to the LLC, appear superior in the diagnosis of acute myocarditis, with positive predictive value of 90% versus 71%[64].
| Two out of three criteria must be met to be consistent with myocardial inflammation: |
| Regional or global myocardial signal intensity increase in T2-weighted images |
| Increased global myocardial early gadolinium enhancement ratio between myocardium and skeletal muscle in gadolinium-enhanced T1-weighted images |
| At least one focal lesion with nonischaemic regional distribution in inversion recovery-prepared gadolinium enhanced T1-weighted images (late gadolinium enhancement) |
LGE has been shown to be highly accurate in the diagnosis of myocarditis with a high correlation with EMB[65]. Acute myocarditis is associated with subepicardial or mid-wall late gadolinium enhancement most commonly in the lateral, inferolateral or inferior wall[66-68] (Figure 3). In particular, small studies have suggested specific patterns for certain viruses: parvovirus B19 is associated with the lateral wall while human herpes virus 6 is linked to the septal wall[69]. The presence of LGE on follow-up studies denotes areas of irreversible myocardial injury[62,65].
CMRI diagnostic accuracy in the workup for chronic myocarditis (> 14 d) is not as well established compared to acute myocarditis, with T2-mapping providing the only discernible additional diagnostic benefit together with the LLC[66,70].
Although CMRI has demonstrated prognostic guidance in acute myocarditis, cardiac enzyme markers do not reflect the degree of myocardial injury or permanent scarring as demonstrated on CMRI LGE[70]. There are insufficient data available to relate CMRI features to independent risk of ventricular arrhythmias, although this is clearly raised in the context of impaired LV function[67,71]. In a meta-analysis, the presence of LGE, particularly anteroseptal location, has been found to be an independent risk factors for adverse cardiac outcomes, including all-cause mortality, cardiac mortality including sudden cardiac death, and MACEs[72].
COVID-19 infection has variable presentations, most commonly involving respiratory symptoms. However, since the declaration of the pandemic in March 2020, there have been increasing reports of cardiovascular disease. The incidence has been reported to be ≥ 40%, depending on the definition or population sampled[73-76]. Postmortem studies of confirmed COVID-19 cases have demonstrated the presence of the virus in the myocardium, but not necessarily with consistent expression of cardiac sequelae[77]. There are numerous mechanisms involved in myocardial injury, which include direct viral invasion and host innate immunity response, hypoxia, micro- and macrovascular thrombosis, inflammatory injury and stress-induced cardiomyopathy[78].
Considering the multifaceted components of COVID-19-induced myocardial injury, it should not be expected that the imaging findings of this infection would duplicate that of a viral myocarditis syndrome alone. Cardiac involvement in COVID-19 infection may not be present with clinically severe cardiac symptoms[75,79], and even though echocardiography is a sensitive tool to identify gross cardiac dysfunction, LVEF may be normal[77]. There have been reports of primary cardiac involvement in COVID-19[80], where CMRI can be useful in identifying acute viral myocarditis features, as well as evidence of thrombosis such as LV apical thrombus.
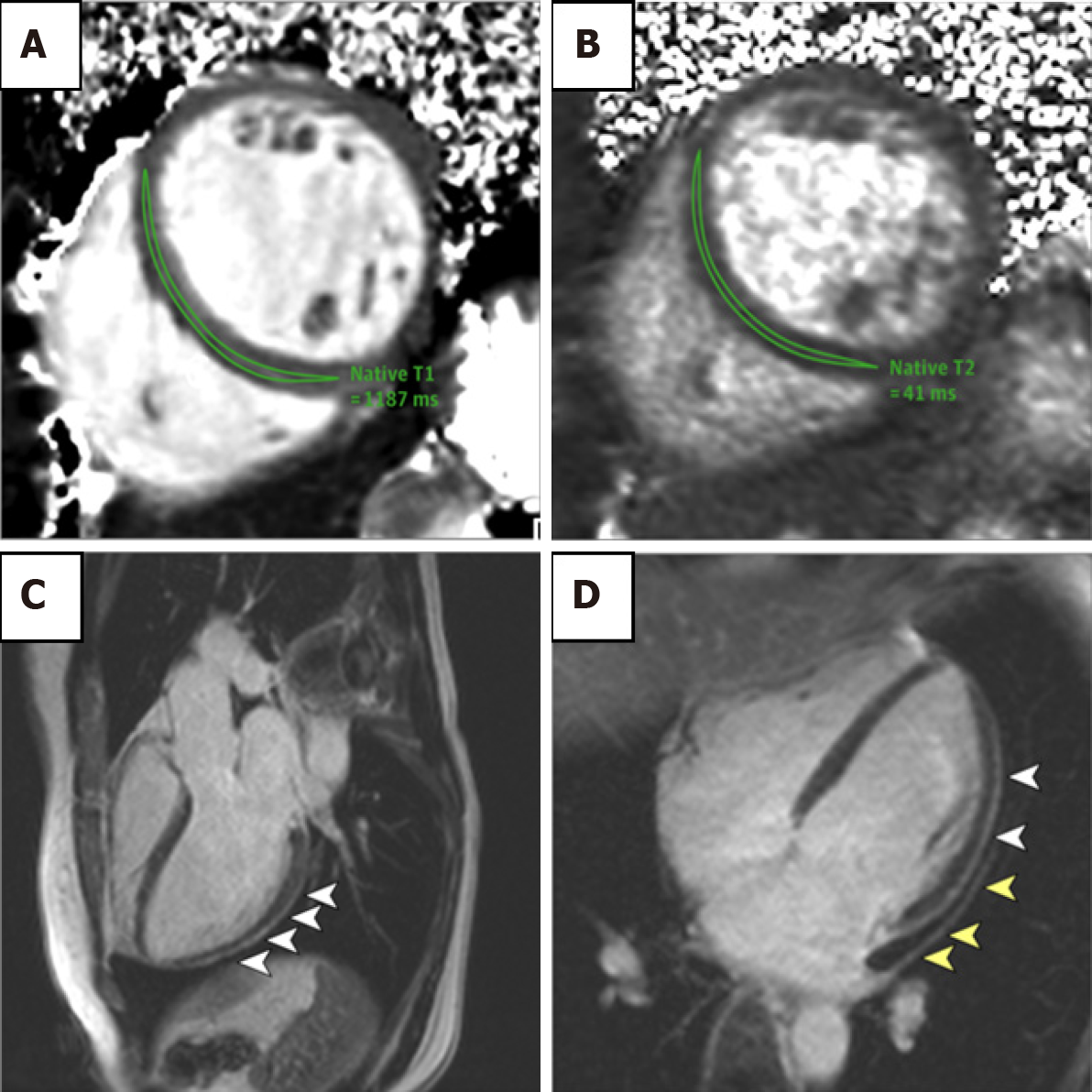
Studies using CMRI have demonstrated the severity of cardiovascular involvement following acute infection. In a report by Huang et al[81], the authors noted that 57% of patients had myocardial oedema or LGE on CMRI performed > 1 mo after development of infection (Figure 4). This suggests an ongoing pathological process affecting the myocardium. Historically, the LGE distribution in acute viral myocarditis involved the lateral and inferior walls. However with COVID-19, LGE patterns have been reported as subepicardial, mid-wall or subendocardial mimicking AMI[75]. Of note, there are no studies available where participants have baseline cardiac MRI data prior to COVID-19 infection. In addition, T2-signal hyperintensity tended to favour the interventricular septum, anterior and anterolateral walls, as well as basal inferior and mid-chamber. T1-mapping and ECV also demonstrated increased values, suggestive of myocardial fibrosis[75,81].
A developing use for CMRI is in the diagnosis suspected COVID-19-vaccine-associated myocarditis. These events tend to occur more frequently in young male patients after the second dose of mRNA vaccine[82,83]. Patients typically present with chest pain, troponin elevations, and abnormal CMRI findings[84]. CMRI abnormalities include myocardial oedema, hyperaemia and LGE, which are expected findings in acute myocarditis[85-89]. To date, there are no specific features for COVID-19-vaccine-associated myocarditis.
Evidence for the outcome of COVID-19-induced myocardial injury continues to evolve. Studies have found that elevation in troponin T levels confer significantly increased risk of mortality[73,90,91]. However, whether this and other markers of myocardial injury are byproducts of disease severity, or directly contribute to morbidity and mortality, remains to be elucidated. Currently, there is no long-term data on COVID-19 effects on the cardiovascular system, but considering its global impact, the identification, monitoring and study of outcomes is critical, with CMRI likely to play an essential role[92,93].
Competitive sports level training can lead to a condition known as AHS, defined by complex cardiac chamber remodelling, ventricular systolic impairment and abnormalities involving the electrical conduction system. Electrocardiogram changes can include first-degree atrioventricular block, incomplete right bundle branch block, early repolarisation and isolated increased QRS voltages that may meet criteria for LV hypertrophy (LVH)[94]. Transient troponin elevation occurs with moderate-to-high intensity exercise[95,96]. The role of CMRI is also continuing to evolve in helping distinguish AHS from conditions such as hypertrophic cardiomyopathy (HCM) and arrhythmogenic cardiomyopathy (ACM), which can have similar ECG and TTE features.
Although ventricular hypertrophy and dilatation can occur in both ventricles, impairment of systolic function following prolonged exercise tends to observed more frequently in the right ventricle (Figure 5A). In early forms of the disease, diastolic dysfunction is often observed, first defined by a reduction in mitral E:A ratio on echocardiographic Doppler imaging[97-100]. A hallmark of AHS is increased LV mass[101]. Unfortunately, this is not a discriminating feature and can overlap with other conditions such as HCM and ACM[102]. In particular, differentiating AHS from mild HCM, with LV wall thickness range 13–15 mm, is critical in preventing adverse outcomes for athletes. Despite early reports suggesting that different cardiac conditions can lead to particular patterns of LVH, this has not been demonstrated in subsequent studies[103,104]. Cessation of training usually leads to LVH regression and improvement in clinical outcomes.
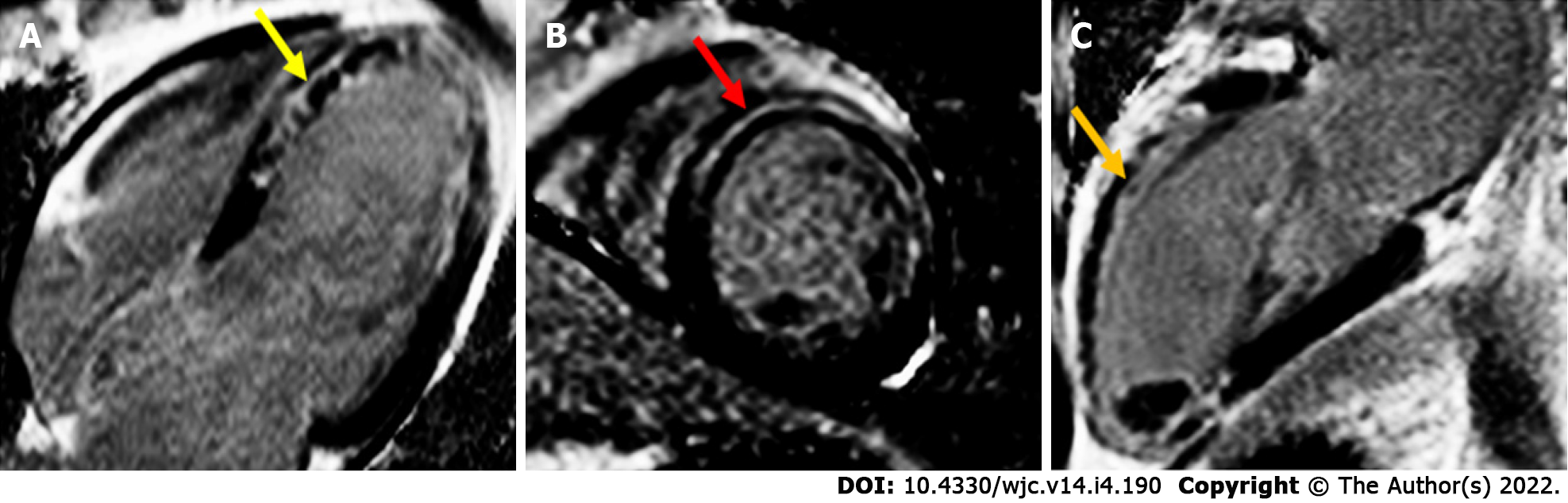
The advancement of CMRI technology may help shed light on the potential long-term effects of competitive level exercise and help differentiate different cardiac conditions. LV cavity size (LV end-diastolic and end-systolic diameter) in AHS is usually larger than HCM, particularly if the end-diastolic diameter exceeds 54 mm[100]. CMRI can also provide accurate morphology assessment for excessive trabeculation and noncompaction cardiomyopathy, if it cannot be clearly delineated on echocardiography[105]. The use of more advanced CMRI tissue characterisation techniques such as T1 mapping and ECV assessment is also helpful. Athletes have been demonstrated to have lower ECV, likely as a result of myocyte enlargement, compared to nonathletes. Conversely, in HCM, there is increased ECV[106,107]. The role of LGE to distinguish AHS compared to HCM is not yet certain. Domenech-Ximenos et al[106] found that focal LGE was more prevalent in intensive endurance athletes compared to healthy subjects (37.6% vs 2.8%), with a typical pattern at the right ventricular (RV) insertion points. This may overlap with the LGE distribution in HCM[107].
Ventricular dilatation has been noted in athletes compared to healthy nonathletic individuals[108,109]. LV dilatation is less severe as compared to the RV dilatation. Stress echocardiography observation of improvement in LVEF by > 11%, and presence of mid-wall LGE may help to discriminate between AHS and pathological dilated cardiomyopathy[95,96,110,111] but this has not yet been investigated in large studies. In a meta-analysis performed by D’Ascenzi et al[102], in high-performance athletes, RV end-diastolic volume (EDV) and end-systolic volume (ESV) exhibited the greatest relative increase in ventricular remodelling, compared to baseline parameters. This may be in response to increased venous return and other hemodynamic changes. The increase in RV size in athletes has led to situations where the dimensions meet part of the ACM criteria[112]. CMRI can be useful to improve spatial resolution in cases with poor echocardiographic windows. It can quantify function, identify RWMAs, and determine the presence of myocardial fibrosis and fibrofatty infiltration, to evaluate ACM versus AHS. In one study, Zaidi et al[113] found that the presence of RV ejection fraction < 45%, the ratio of RV EDV to LV EDV > 1.1/1, RV RWMA and LGE found together in athletes was highly indicative of ACM.
Exercise CMRI may provide addition diagnostic information by comparing the difference between adaptive responses and ventricular pathology in AHS, as well as potential prognostic information. The development of in-scanner CMRI exercise protocols with excellent reproducibility has been important in facilitating studies on the difference in physiological and pathological responses of athletes[114,115]. During exercise, elite athletes with evidence of ventricular arrhythmias had an increase in RV EDV, decrease in RV ESV, and, as a result, had reduced RV ejection fraction compared to athletes with no evidence of ventricular arrhythmia and healthy controls[116]. Of note, stress TTE yielded similar sensitivity in identifying exercise induced RV dysfunction.
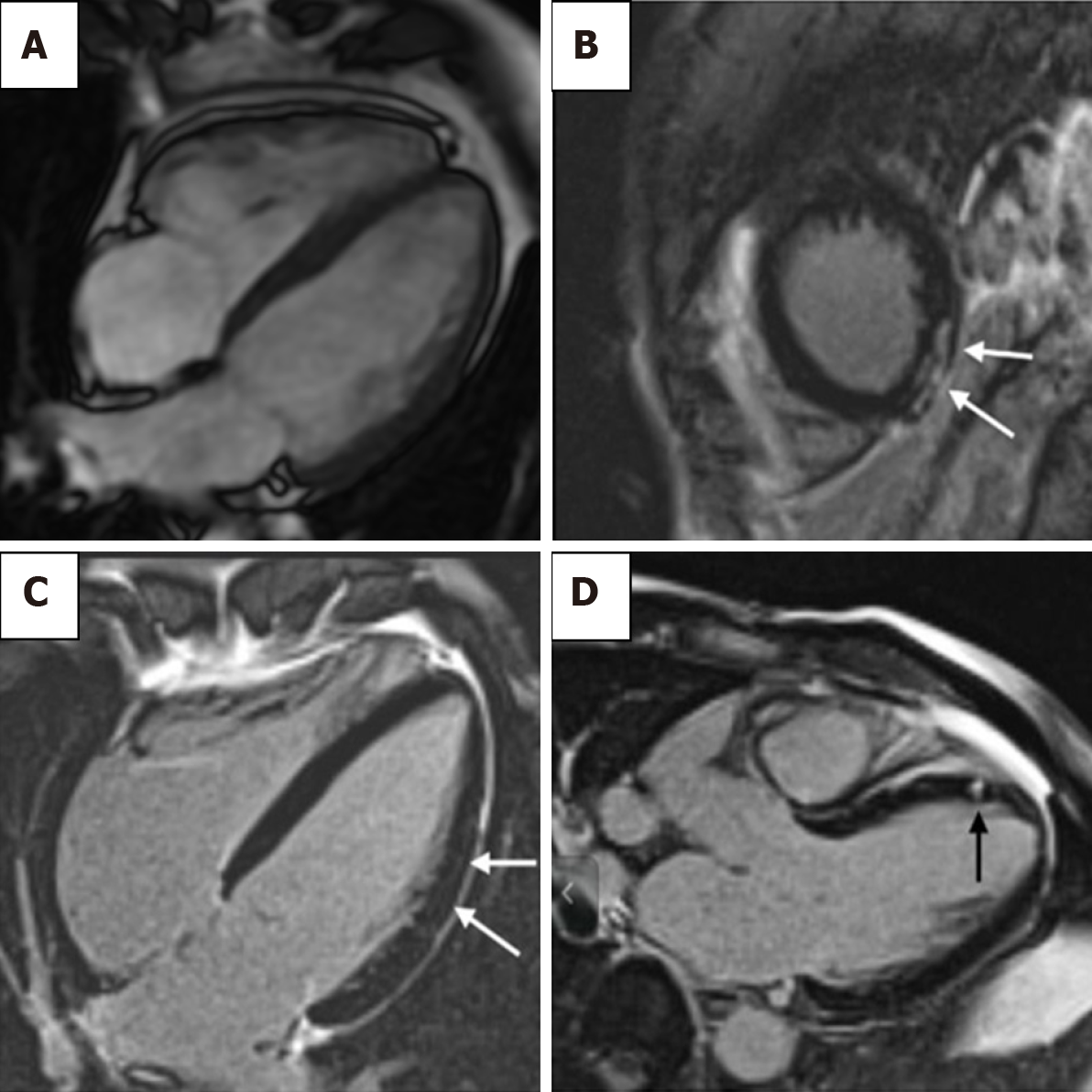
The use of CMRI has been explored in AHS adverse outcome prognostication. In the context of excellent spatial and temporal resolution of CMRI, there appears to be no difference in resting in cardiac volumes of elite athletes with and without evidence of ventricular arrhythmias[116]. LGE at the junction of the right ventricle and interventricular septum has previously been noted in athletes, but is not related to any clinical sequelae. A pattern of myocardial wall fibrosis in AHS has otherwise not consistently been demonstrated, or has been affected by confounding factors such as veteran athletes with coronary artery atherosclerotic plaques[112,117-119] (Figure 5B–5D). Zorzi et al[120] did note that in athletes who presented with a history of ventricular arrhythmias and subsequently found to have LV LGE, a striae subepicardial – midmyocardial lateral LV wall distribution pattern was more prevalent. There are no long-term data on outcome of incidental finding of myocardial LGE[114,121-123]. The definitive role of CMRI for prognostication in AHS remains to be defined outside of its roles in excluding other important diagnoses such as HCM and ARC.
Traditionally, gross morphological cine scanning and determination of LGE location in the myocardial wall has formed key diagnostic features in the use of CMRI for many conditions. The advent of more advanced techniques such as T1 and T2 mapping has allowed a deeper understanding of the pathophysiological process involved.
The limitations for the use of CMRI include: (1) Access to CMR facilities with trained staff to perform the scan and process the images; (2) Standardised protocols; (3) Duration of procedure; (4) High cost; and (5) Lack of superiority to cheaper, faster and more accessible imaging modalities.
Moving forward, improving access to CMRI, increasing the number of skilled personnel and developing clear scanning guidelines are needed. Further research including large randomised trials are necessary to further define the role of CMRI in the assessment of MI, TTS, myocarditis, AHS and COVID-19-related cardiac conditions.
Troponinemia or an elevation in serum troponin levels can result from several different conditions making the diagnosis difficult. CMRI provides a powerful insight into the pathological mechanisms of disease, diagnostic features, as well as potential prognosis. With advancement in technology and research, this will only continue to improve.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kwok W, United States; Roncalli J, France S-Editor: Ma YJ L-Editor: Kerr C P-Editor: Ma YJ
| 1. | Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation. 2018;138:e618-e651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1019] [Cited by in F6Publishing: 1604] [Article Influence: 320.8] [Reference Citation Analysis (0)] |
| 2. | Bulluck H, Dharmakumar R, Arai AE, Berry C, Hausenloy DJ. Cardiovascular Magnetic Resonance in Acute ST-Segment-Elevation Myocardial Infarction: Recent Advances, Controversies, and Future Directions. Circulation. 2018;137:1949-1964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 111] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 3. | Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786-794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1447] [Cited by in F6Publishing: 1332] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 4. | Ichikawa Y, Sakuma H, Suzawa N, Kitagawa K, Makino K, Hirano T, Takeda K. Late gadolinium-enhanced magnetic resonance imaging in acute and chronic myocardial infarction. Improved prediction of regional myocardial contraction in the chronic state by measuring thickness of nonenhanced myocardium. J Am Coll Cardiol. 2005;45:901-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Thiele H, Kappl MJ, Conradi S, Niebauer J, Hambrecht R, Schuler G. Reproducibility of chronic and acute infarct size measurement by delayed enhancement-magnetic resonance imaging. J Am Coll Cardiol. 2006;47:1641-1645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Mahrholdt H, Wagner A, Holly TA, Elliott MD, Bonow RO, Kim RJ, Judd RM. Reproducibility of chronic infarct size measurement by contrast-enhanced magnetic resonance imaging. Circulation. 2002;106:2322-2327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 310] [Cited by in F6Publishing: 290] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 7. | Rajiah P, Desai MY, Kwon D, Flamm SD. MR imaging of myocardial infarction. Radiographics. 2013;33:1383-1412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Rehwald WG, Fieno DS, Chen EL, Kim RJ, Judd RM. Myocardial magnetic resonance imaging contrast agent concentrations after reversible and irreversible ischemic injury. Circulation. 2002;105:224-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 273] [Cited by in F6Publishing: 243] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 9. | Kim HW, Farzaneh-Far A, Kim RJ. Cardiovascular magnetic resonance in patients with myocardial infarction: current and emerging applications. J Am Coll Cardiol. 2009;55:1-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 10. | Kali A, Choi EY, Sharif B, Kim YJ, Bi X, Spottiswoode B, Cokic I, Yang HJ, Tighiouart M, Conte AH, Li D, Berman DS, Choi BW, Chang HJ, Dharmakumar R. Native T1 Mapping by 3-T CMR Imaging for Characterization of Chronic Myocardial Infarctions. JACC Cardiovasc Imaging. 2015;8:1019-1030. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 11. | Ersbøll M, Valeur N, Mogensen UM, Andersen MJ, Møller JE, Velazquez EJ, Hassager C, Søgaard P, Køber L. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2013;61:2365-2373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 12. | Eitel I, Stiermaier T, Lange T, Rommel KP, Koschalka A, Kowallick JT, Lotz J, Kutty S, Gutberlet M, Hasenfuß G, Thiele H, Schuster A. Cardiac Magnetic Resonance Myocardial Feature Tracking for Optimized Prediction of Cardiovascular Events Following Myocardial Infarction. JACC Cardiovasc Imaging. 2018;11:1433-1444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 13. | Schuster A, Backhaus SJ, Stiermaier T, Navarra JL, Uhlig J, Rommel KP, Koschalka A, Kowallick JT, Lotz J, Gutberlet M, Bigalke B, Kutty S, Hasenfuss G, Thiele H, Eitel I. Left Atrial Function with MRI Enables Prediction of Cardiovascular Events after Myocardial Infarction: Insights from the AIDA STEMI and TATORT NSTEMI Trials. Radiology. 2019;293:292-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Nayyar D, Nguyen T, Pathan F, Vo G, Richards D, Thomas L, Dimitri H, Otton J. Cardiac magnetic resonance derived left atrial strain after ST-elevation myocardial infarction: an independent prognostic indicator. Cardiovasc Diagn Ther. 2021;11:383-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Allman KC, Shaw LJ, Hachamovitch R, Udelson JE. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: a meta-analysis. J Am Coll Cardiol. 2002;39:1151-1158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 981] [Cited by in F6Publishing: 904] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 16. | Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900-2907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1163] [Cited by in F6Publishing: 1079] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 17. | Charoenpanichkit C, Hundley WG. The 20 year evolution of dobutamine stress cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Bove CM, DiMaria JM, Voros S, Conaway MR, Kramer CM. Dobutamine response and myocardial infarct transmurality: functional improvement after coronary artery bypass grafting--initial experience. Radiology. 2006;240:835-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Motoyasu M, Sakuma H, Ichikawa Y, Ishida N, Uemura S, Okinaka T, Isaka N, Takeda K, Nakano T. Prediction of regional functional recovery after acute myocardial infarction with low dose dobutamine stress cine MR imaging and contrast enhanced MR imaging. J Cardiovasc Magn Reson. 2003;5:563-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Hillenbrand HB, Kim RJ, Parker MA, Fieno DS, Judd RM. Early assessment of myocardial salvage by contrast-enhanced magnetic resonance imaging. Circulation. 2000;102:1678-1683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 133] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Perazzolo Marra M, Lima JA, Iliceto S. MRI in acute myocardial infarction. Eur Heart J. 2011;32:284-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Bax JJ, Wijns W, Cornel JH, Visser FC, Boersma E, Fioretti PM. Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: comparison of pooled data. J Am Coll Cardiol. 1997;30:1451-1460. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 360] [Cited by in F6Publishing: 303] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 23. | Hamirani YS, Wong A, Kramer CM, Salerno M. Effect of microvascular obstruction and intramyocardial hemorrhage by CMR on LV remodeling and outcomes after myocardial infarction: a systematic review and meta-analysis. JACC Cardiovasc Imaging. 2014;7:940-952. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 167] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 24. | Eitel I, de Waha S, Wöhrle J, Fuernau G, Lurz P, Pauschinger M, Desch S, Schuler G, Thiele H. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1217-1226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 288] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 25. | Symons R, Pontone G, Schwitter J, Francone M, Iglesias JF, Barison A, Zalewski J, de Luca L, Degrauwe S, Claus P, Guglielmo M, Nessler J, Carbone I, Ferro G, Durak M, Magistrelli P, Lo Presti A, Aquaro GD, Eeckhout E, Roguelov C, Andreini D, Vogt P, Guaricci AI, Mushtaq S, Lorenzoni V, Muller O, Desmet W, Agati L, Janssens S, Bogaert J, Masci PG. Long-Term Incremental Prognostic Value of Cardiovascular Magnetic Resonance After ST-Segment Elevation Myocardial Infarction: A Study of the Collaborative Registry on CMR in STEMI. JACC Cardiovasc Imaging. 2018;11:813-825. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | van Kranenburg M, Magro M, Thiele H, de Waha S, Eitel I, Cochet A, Cottin Y, Atar D, Buser P, Wu E, Lee D, Bodi V, Klug G, Metzler B, Delewi R, Bernhardt P, Rottbauer W, Boersma E, Zijlstra F, van Geuns RJ. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging. 2014;7:930-939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 235] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 27. | Stone GW, Selker HP, Thiele H, Patel MR, Udelson JE, Ohman EM, Maehara A, Eitel I, Granger CB, Jenkins PL, Nichols M, Ben-Yehuda O. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J Am Coll Cardiol. 2016;67:1674-1683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 327] [Cited by in F6Publishing: 417] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 28. | Bulluck H, Hammond-Haley M, Fontana M, Knight DS, Sirker A, Herrey AS, Manisty C, Kellman P, Moon JC, Hausenloy DJ. Quantification of both the area-at-risk and acute myocardial infarct size in ST-segment elevation myocardial infarction using T1-mapping. J Cardiovasc Magn Reson. 2017;19:57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Ingkanisorn WP, Rhoads KL, Aletras AH, Kellman P, Arai AE. Gadolinium delayed enhancement cardiovascular magnetic resonance correlates with clinical measures of myocardial infarction. J Am Coll Cardiol. 2004;43:2253-2259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 211] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 30. | Kwong RY, Chan AK, Brown KA, Chan CW, Reynolds HG, Tsang S, Davis RB. Impact of unrecognized myocardial scar detected by cardiac magnetic resonance imaging on event-free survival in patients presenting with signs or symptoms of coronary artery disease. Circulation. 2006;113:2733-2743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 558] [Cited by in F6Publishing: 523] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 31. | Eitel I, Kubusch K, Strohm O, Desch S, Mikami Y, de Waha S, Gutberlet M, Schuler G, Friedrich MG, Thiele H. Prognostic value and determinants of a hypointense infarct core in T2-weighted cardiac magnetic resonance in acute reperfused ST-elevation-myocardial infarction. Circ Cardiovasc Imaging. 2011;4:354-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 32. | El Aidi H, Adams A, Moons KG, Den Ruijter HM, Mali WP, Doevendans PA, Nagel E, Schalla S, Bots ML, Leiner T. Cardiac magnetic resonance imaging findings and the risk of cardiovascular events in patients with recent myocardial infarction or suspected or known coronary artery disease: a systematic review of prognostic studies. J Am Coll Cardiol. 2014;63:1031-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 33. | Mather AN, Fairbairn TA, Artis NJ, Greenwood JP, Plein S. Timing of cardiovascular MR imaging after acute myocardial infarction: effect on estimates of infarct characteristics and prediction of late ventricular remodeling. Radiology. 2011;261:116-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Ripa RS, Nilsson JC, Wang Y, Søndergaard L, Jørgensen E, Kastrup J. Short- and long-term changes in myocardial function, morphology, edema, and infarct mass after ST-segment elevation myocardial infarction evaluated by serial magnetic resonance imaging. Am Heart J. 2007;154:929-936. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Pontone G, Guaricci AI, Andreini D, Solbiati A, Guglielmo M, Mushtaq S, Baggiano A, Beltrama V, Fusini L, Rota C, Segurini C, Conte E, Gripari P, Dello Russo A, Moltrasio M, Tundo F, Lombardi F, Muscogiuri G, Lorenzoni V, Tondo C, Agostoni P, Bartorelli AL, Pepi M. Prognostic Benefit of Cardiac Magnetic Resonance Over Transthoracic Echocardiography for the Assessment of Ischemic and Nonischemic Dilated Cardiomyopathy Patients Referred for the Evaluation of Primary Prevention Implantable Cardioverter-Defibrillator Therapy. Circ Cardiovasc Imaging. 2016;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Scott PA, Morgan JM, Carroll N, Murday DC, Roberts PR, Peebles CR, Harden SP, Curzen NP. The extent of left ventricular scar quantified by late gadolinium enhancement MRI is associated with spontaneous ventricular arrhythmias in patients with coronary artery disease and implantable cardioverter-defibrillators. Circ Arrhythm Electrophysiol. 2011;4:324-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Scott PA, Rosengarten JA, Curzen NP, Morgan JM. Late gadolinium enhancement cardiac magnetic resonance imaging for the prediction of ventricular tachyarrhythmic events: a meta-analysis. Eur J Heart Fail. 2013;15:1019-1027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Acosta J, Fernández-Armenta J, Borràs R, Anguera I, Bisbal F, Martí-Almor J, Tolosana JM, Penela D, Andreu D, Soto-Iglesias D, Evertz R, Matiello M, Alonso C, Villuendas R, de Caralt TM, Perea RJ, Ortiz JT, Bosch X, Serra L, Planes X, Greiser A, Ekinci O, Lasalvia L, Mont L, Berruezo A. Scar Characterization to Predict Life-Threatening Arrhythmic Events and Sudden Cardiac Death in Patients With Cardiac Resynchronization Therapy: The GAUDI-CRT Study. JACC Cardiovasc Imaging. 2018;11:561-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 39. | Roes SD, Borleffs CJ, van der Geest RJ, Westenberg JJ, Marsan NA, Kaandorp TA, Reiber JH, Zeppenfeld K, Lamb HJ, de Roos A, Schalij MJ, Bax JJ. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging. 2009;2:183-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 341] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 40. | Yan AT, Shayne AJ, Brown KA, Gupta SN, Chan CW, Luu TM, Di Carli MF, Reynolds HG, Stevenson WG, Kwong RY. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 576] [Cited by in F6Publishing: 545] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 41. | Schuster A, Lange T, Backhaus SJ, Strohmeyer C, Boom PC, Matz J, Kowallick JT, Lotz J, Steinmetz M, Kutty S, Bigalke B, Gutberlet M, de Waha-Thiele S, Desch S, Hasenfuß G, Thiele H, Stiermaier T, Eitel I. Fully Automated Cardiac Assessment for Diagnostic and Prognostic Stratification Following Myocardial Infarction. J Am Heart Assoc. 2020;9:e016612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2018;138:e272-e391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 249] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 43. | Elayi CS, Charnigo RJ, Heron PM, Lee BK, Olgin JE. Primary Prevention of Sudden Cardiac Death Early Post-Myocardial Infarction: Root Cause Analysis for Implantable Cardioverter-Defibrillator Failure and Currently Available Options. Circ Arrhythm Electrophysiol. 2017;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 44. | Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur Heart J. 2018;39:2032-2046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 641] [Cited by in F6Publishing: 832] [Article Influence: 166.4] [Reference Citation Analysis (0)] |
| 45. | Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. [Myocardial stunning due to simultaneous multivessel coronary spasms: a review of 5 cases]. J Cardiol. 1991;21:203-214. [PubMed] [Cited in This Article: ] |
| 46. | Barrera-Ramirez CF, Jimenez-Mazuecos JM, Alfonso F. Apical thrombus associated with left ventricular apical ballooning. Heart. 2003;89:927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 47. | Scally C, Rudd A, Mezincescu A, Wilson H, Srivanasan J, Horgan G, Broadhurst P, Newby DE, Henning A, Dawson DK. Persistent Long-Term Structural, Functional, and Metabolic Changes After Stress-Induced (Takotsubo) Cardiomyopathy. Circulation. 2018;137:1039-1048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 48. | Lyon AR, Akashi YJ. Use of cardiac MRI to diagnose Takotsubo syndrome. Nat Rev Cardiol. 2015;12:669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 49. | Eitel I, Behrendt F, Schindler K, Kivelitz D, Gutberlet M, Schuler G, Thiele H. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. Eur Heart J. 2008;29:2651-2659. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 50. | Bratis K. Cardiac Magnetic Resonance in Takotsubo Syndrome. Eur Cardiol. 2017;12:58-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 51. | Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, Schuler G, Schulz-Menger J, Thiele H, Friedrich MG. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 393] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 52. | Gunasekara MY, Mezincescu AM, Dawson DK. An Update on Cardiac Magnetic Resonance Imaging in Takotsubo Cardiomyopathy. Curr Cardiovasc Imaging Rep. 2020;13:1-8. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Avegliano G, Huguet M, Costabel JP, Ronderos R, Bijnens B, Kuschnir P, Thierer J, Tobón-Gomez C, Martinez GO, Frangi A. Morphologic pattern of late gadolinium enhancement in Takotsubo cardiomyopathy detected by early cardiovascular magnetic resonance. Clin Cardiol. 2011;34:178-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 54. | Rolf A, Nef HM, Möllmann H, Troidl C, Voss S, Conradi G, Rixe J, Steiger H, Beiring K, Hamm CW, Dill T. Immunohistological basis of the late gadolinium enhancement phenomenon in tako-tsubo cardiomyopathy. Eur Heart J. 2009;30:1635-1642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 55. | Nakamori S, Matsuoka K, Onishi K, Kurita T, Ichikawa Y, Nakajima H, Ishida M, Kitagawa K, Tanigawa T, Nakamura T, Ito M, Sakuma H. Prevalence and signal characteristics of late gadolinium enhancement on contrast-enhanced magnetic resonance imaging in patients with takotsubo cardiomyopathy. Circ J. 2012;76:914-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 56. | Naruse Y, Sato A, Kasahara K, Makino K, Sano M, Takeuchi Y, Nagasaka S, Wakabayashi Y, Katoh H, Satoh H, Hayashi H, Aonuma K. The clinical impact of late gadolinium enhancement in Takotsubo cardiomyopathy: serial analysis of cardiovascular magnetic resonance images. J Cardiovasc Magn Reson. 2011;13:67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 57. | Satoh H, Sano M, Suwa K, Saitoh T, Nobuhara M, Saotome M, Urushida T, Katoh H, Hayashi H. Distribution of late gadolinium enhancement in various types of cardiomyopathies: Significance in differential diagnosis, clinical features and prognosis. World J Cardiol. 2014;6:585-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 75] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 58. | Lyon AR, Citro R, Schneider B, Morel O, Ghadri JR, Templin C, Omerovic E. Pathophysiology of Takotsubo Syndrome: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77:902-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 104] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 59. | Ojha V, Khurana R, Ganga KP, Kumar S. Advanced cardiac magnetic resonance imaging in takotsubo cardiomyopathy. Br J Radiol. 2020;93:20200514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 60. | Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S, Aletras A, Laissy JP, Paterson I, Filipchuk NG, Kumar A, Pauschinger M, Liu P; International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475-1487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1600] [Cited by in F6Publishing: 1627] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 61. | Friedrich MG, Strohm O, Schulz-Menger J, Marciniak H, Luft FC, Dietz R. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis. Circulation. 1998;97:1802-1809. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 346] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 62. | Mahrholdt H, Goedecke C, Wagner A, Meinhardt G, Athanasiadis A, Vogelsberg H, Fritz P, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance assessment of human myocarditis: a comparison to histology and molecular pathology. Circulation. 2004;109:1250-1258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 775] [Cited by in F6Publishing: 688] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 63. | Abdel-Aty H, Boyé P, Zagrosek A, Wassmuth R, Kumar A, Messroghli D, Bock P, Dietz R, Friedrich MG, Schulz-Menger J. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. J Am Coll Cardiol. 2005;45:1815-1822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 574] [Cited by in F6Publishing: 531] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 64. | Lurz P, Luecke C, Eitel I, Föhrenbach F, Frank C, Grothoff M, de Waha S, Rommel KP, Lurz JA, Klingel K, Kandolf R, Schuler G, Thiele H, Gutberlet M. Comprehensive Cardiac Magnetic Resonance Imaging in Patients With Suspected Myocarditis: The MyoRacer-Trial. J Am Coll Cardiol. 2016;67:1800-1811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 273] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 65. | Citro R, Lyon AR, Meimoun P, Omerovic E, Redfors B, Buck T, Lerakis S, Parodi G, Silverio A, Eitel I, Schneider B, Prasad A, Bossone E. Standard and advanced echocardiography in takotsubo (stress) cardiomyopathy: clinical and prognostic implications. J Am Soc Echocardiogr. 2015;28:57-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 66. | Hunold P, Schlosser T, Vogt FM, Eggebrecht H, Schmermund A, Bruder O, Schüler WO, Barkhausen J. Myocardial late enhancement in contrast-enhanced cardiac MRI: distinction between infarction scar and non-infarction-related disease. AJR Am J Roentgenol. 2005;184:1420-1426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 67. | Grün S, Schumm J, Greulich S, Wagner A, Schneider S, Bruder O, Kispert EM, Hill S, Ong P, Klingel K, Kandolf R, Sechtem U, Mahrholdt H. Long-term follow-up of biopsy-proven viral myocarditis: predictors of mortality and incomplete recovery. J Am Coll Cardiol. 2012;59:1604-1615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 348] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 68. | Aquaro GD, Perfetti M, Camastra G, Monti L, Dellegrottaglie S, Moro C, Pepe A, Todiere G, Lanzillo C, Scatteia A, Di Roma M, Pontone G, Perazzolo Marra M, Barison A, Di Bella G; Cardiac Magnetic Resonance Working Group of the Italian Society of Cardiology. Cardiac MR With Late Gadolinium Enhancement in Acute Myocarditis With Preserved Systolic Function: ITAMY Study. J Am Coll Cardiol. 2017;70:1977-1987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 289] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 69. | Mahrholdt H, Wagner A, Deluigi CC, Kispert E, Hager S, Meinhardt G, Vogelsberg H, Fritz P, Dippon J, Bock CT, Klingel K, Kandolf R, Sechtem U. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114:1581-1590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 567] [Cited by in F6Publishing: 570] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 70. | Berg J, Kottwitz J, Baltensperger N, Kissel CK, Lovrinovic M, Mehra T, Scherff F, Schmied C, Templin C, Lüscher TF, Heidecker B, Manka R. Cardiac Magnetic Resonance Imaging in Myocarditis Reveals Persistent Disease Activity Despite Normalization of Cardiac Enzymes and Inflammatory Parameters at 3-Month Follow-Up. Circ Heart Fail. 2017;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 71. | Mavrogeni S, Petrou E, Kolovou G, Theodorakis G, Iliodromitis E. Prediction of ventricular arrhythmias using cardiovascular magnetic resonance. Eur Heart J Cardiovasc Imaging. 2013;14:518-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Georgiopoulos G, Figliozzi S, Sanguineti F, Aquaro GD, di Bella G, Stamatelopoulos K, Chiribiri A, Garot J, Masci PG, Ismail TF. Prognostic Impact of Late Gadolinium Enhancement by Cardiovascular Magnetic Resonance in Myocarditis: A Systematic Review and Meta-Analysis. Circ Cardiovasc Imaging. 2021;14:e011492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 73. | Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811-818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2516] [Cited by in F6Publishing: 2703] [Article Influence: 675.8] [Reference Citation Analysis (0)] |
| 74. | Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, Chaudhry F, De Freitas JK, Fayad ZA, Pinney SP, Levin M, Charney A, Bagiella E, Narula J, Glicksberg BS, Nadkarni G, Mancini DM, Fuster V; Mount Sinai COVID Informatics Center. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J Am Coll Cardiol. 2020;76:533-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 515] [Cited by in F6Publishing: 509] [Article Influence: 127.3] [Reference Citation Analysis (0)] |
| 75. | Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa-Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered From Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020;5:1265-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1240] [Cited by in F6Publishing: 1350] [Article Influence: 337.5] [Reference Citation Analysis (0)] |
| 76. | Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2428] [Cited by in F6Publishing: 2872] [Article Influence: 718.0] [Reference Citation Analysis (0)] |
| 77. | Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss HP, Blankenberg S, Püschel K, Westermann D. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020;5:1281-1285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 551] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 78. | Giustino G, Pinney SP, Lala A, Reddy VY, Johnston-Cox HA, Mechanick JI, Halperin JL, Fuster V. Coronavirus and Cardiovascular Disease, Myocardial Injury, and Arrhythmia: JACC Focus Seminar. J Am Coll Cardiol. 2020;76:2011-2023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 139] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 79. | Li X, Wang H, Zhao R, Wang T, Zhu Y, Qian Y, Liu B, Yu Y, Han Y. Elevated Extracellular Volume Fraction and Reduced Global Longitudinal Strains in Participants Recovered from COVID-19 without Clinical Cardiac Findings. Radiology. 2021;299:E230-E240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 80. | Gravinay P, Issa N, Girard D, Camou F, Cochet H. CMR and serology to diagnose COVID-19 infection with primary cardiac involvement. Eur Heart J Cardiovasc Imaging. 2021;22:133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 81. | Huang L, Zhao P, Tang D, Zhu T, Han R, Zhan C, Liu W, Zeng H, Tao Q, Xia L. Cardiac Involvement in Patients Recovered From COVID-2019 Identified Using Magnetic Resonance Imaging. JACC Cardiovasc Imaging. 2020;13:2330-2339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 389] [Cited by in F6Publishing: 362] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 82. | Jain SS, Steele JM, Fonseca B, Huang S, Shah S, Maskatia SA, Buddhe S, Misra N, Ramachandran P, Gaur L, Eshtehardi P, Anwar S, Kaushik N, Han F, Chaudhuri NR, Grosse-Wortmann L. COVID-19 Vaccination-Associated Myocarditis in Adolescents. Pediatrics. 2021;148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 83. | Tano E, San Martin S, Girgis S, Martinez-Fernandez Y, Sanchez Vegas C. Perimyocarditis in Adolescents After Pfizer-BioNTech COVID-19 Vaccine. J Pediatric Infect Dis Soc. 2021;10:962-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 43] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 84. | Bozkurt B, Kamat I, Hotez PJ. Myocarditis With COVID-19 mRNA Vaccines. Circulation. 2021;144:471-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 556] [Cited by in F6Publishing: 538] [Article Influence: 179.3] [Reference Citation Analysis (0)] |
| 85. | Kim HW, Jenista ER, Wendell DC, Azevedo CF, Campbell MJ, Darty SN, Parker MA, Kim RJ. Patients With Acute Myocarditis Following mRNA COVID-19 Vaccination. JAMA Cardiol. 2021;6:1196-1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 217] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 86. | Larson KF, Ammirati E, Adler ED, Cooper LT Jr, Hong KN, Saponara G, Couri D, Cereda A, Procopio A, Cavalotti C, Oliva F, Sanna T, Ciconte VA, Onyango G, Holmes DR, Borgeson DD. Myocarditis After BNT162b2 and mRNA-1273 Vaccination. Circulation. 2021;144:506-508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 111] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 87. | Montgomery J, Ryan M, Engler R, Hoffman D, McClenathan B, Collins L, Loran D, Hrncir D, Herring K, Platzer M, Adams N, Sanou A, Cooper LT Jr. Myocarditis Following Immunization With mRNA COVID-19 Vaccines in Members of the US Military. JAMA Cardiol. 2021;6:1202-1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 359] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 88. | Marshall M, Ferguson ID, Lewis P, Jaggi P, Gagliardo C, Collins JS, Shaughnessy R, Caron R, Fuss C, Corbin KJE, Emuren L, Faherty E, Hall EK, Di Pentima C, Oster ME, Paintsil E, Siddiqui S, Timchak DM, Guzman-Cottrill JA. Symptomatic Acute Myocarditis in 7 Adolescents After Pfizer-BioNTech COVID-19 Vaccination. Pediatrics. 2021;148. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 245] [Article Influence: 81.7] [Reference Citation Analysis (2)] |
| 89. | Vollmann D, Eiffert H, Schuster A. Acute Perimyocarditis Following First Dose of mRNA Vaccine Against COVID-19. Dtsch Arztebl Int. 2021;118:546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070-2079. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 349] [Cited by in F6Publishing: 319] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 91. | Metkus TS, Sokoll LJ, Barth AS, Czarny MJ, Hays AG, Lowenstein CJ, Michos ED, Nolley EP, Post WS, Resar JR, Thiemann DR, Trost JC, Hasan RK. Myocardial Injury in Severe COVID-19 Compared With Non-COVID-19 Acute Respiratory Distress Syndrome. Circulation. 2021;143:553-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 92. | Sanghvi SK, Schwarzman LS, Nazir NT. Cardiac MRI and Myocardial Injury in COVID-19: Diagnosis, Risk Stratification and Prognosis. Diagnostics (Basel). 2021;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 93. | Mitrani RD, Dabas N, Goldberger JJ. COVID-19 cardiac injury: Implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020;17:1984-1990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 161] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 94. | Corrado D, Pelliccia A, Heidbuchel H, Sharma S, Link M, Basso C, Biffi A, Buja G, Delise P, Gussac I, Anastasakis A, Borjesson M, Bjørnstad HH, Carrè F, Deligiannis A, Dugmore D, Fagard R, Hoogsteen J, Mellwig KP, Panhuyzen-Goedkoop N, Solberg E, Vanhees L, Drezner J, Estes NA 3rd, Iliceto S, Maron BJ, Peidro R, Schwartz PJ, Stein R, Thiene G, Zeppilli P, McKenna WJ; Section of Sports Cardiology, European Association of Cardiovascular Prevention and Rehabilitation. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur Heart J. 2010;31:243-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 564] [Cited by in F6Publishing: 513] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 95. | Marshall L, Lee KK, Stewart SD, Wild A, Fujisawa T, Ferry AV, Stables CL, Lithgow H, Chapman AR, Anand A, Shah ASV, Dhaun N, Strachan FE, Mills NL, Ross MD. Effect of Exercise Intensity and Duration on Cardiac Troponin Release. Circulation. 2020;141:83-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 96. | Shave R, Oxborough D. Exercise-induced cardiac injury: evidence from novel imaging techniques and highly sensitive cardiac troponin assays. Prog Cardiovasc Dis. 2012;54:407-415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 97. | Middleton N, Shave R, George K, Whyte G, Hart E, Atkinson G. Left ventricular function immediately following prolonged exercise: A meta-analysis. Med Sci Sports Exerc. 2006;38:681-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 98. | Carrió I, Serra-Grima R, Berná L, Estorch M, Martinez-Duncker C, Ordoñez J. Transient alterations in cardiac performance after a six-hour race. Am J Cardiol. 1990;65:1471-1474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 99. | La Gerche A, Connelly KA, Mooney DJ, MacIsaac AI, Prior DL. Biochemical and functional abnormalities of left and right ventricular function after ultra-endurance exercise. Heart. 2008;94:860-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 172] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 100. | Caselli S, Maron MS, Urbano-Moral JA, Pandian NG, Maron BJ, Pelliccia A. Differentiating left ventricular hypertrophy in athletes from that in patients with hypertrophic cardiomyopathy. Am J Cardiol. 2014;114:1383-1389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 101. | Morganroth J, Maron BJ, Henry WL, Epstein SE. Comparative left ventricular dimensions in trained athletes. Ann Intern Med. 1975;82:521-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 532] [Cited by in F6Publishing: 472] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 102. | D'Ascenzi F, Anselmi F, Piu P, Fiorentini C, Carbone SF, Volterrani L, Focardi M, Bonifazi M, Mondillo S. Cardiac Magnetic Resonance Normal Reference Values of Biventricular Size and Function in Male Athlete's Heart. JACC Cardiovasc Imaging. 2019;12:1755-1765. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 103. | Pluim BM, Zwinderman AH, van der Laarse A, van der Wall EE. The athlete's heart. A meta-analysis of cardiac structure and function. Circulation. 2000;101:336-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 613] [Cited by in F6Publishing: 618] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 104. | Galanti G, Stefani L, Mascherini G, Di Tante V, Toncelli L. Left ventricular remodeling and the athlete's heart, irrespective of quality load training. Cardiovasc Ultrasound. 2016;14:46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 105. | Swoboda PP, McDiarmid AK, Erhayiem B, Broadbent DA, Dobson LE, Garg P, Ferguson C, Page SP, Greenwood JP, Plein S. Assessing Myocardial Extracellular Volume by T1 Mapping to Distinguish Hypertrophic Cardiomyopathy From Athlete's Heart. J Am Coll Cardiol. 2016;67:2189-2190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 106. | Domenech-Ximenos B, Sanz-de la Garza M, Prat-González S, Sepúlveda-Martínez A, Crispi F, Duran-Fernandez K, Perea RJ, Bijnens B, Sitges M. Prevalence and pattern of cardiovascular magnetic resonance late gadolinium enhancement in highly trained endurance athletes. J Cardiovasc Magn Reson. 2020;22:62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 107. | Rubinshtein R, Glockner JF, Ommen SR, Araoz PA, Ackerman MJ, Sorajja P, Bos JM, Tajik AJ, Valeti US, Nishimura RA, Gersh BJ. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3:51-58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 308] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 108. | Prakken NH, Velthuis BK, Teske AJ, Mosterd A, Mali WP, Cramer MJ. Cardiac MRI reference values for athletes and nonathletes corrected for body surface area, training hours/week and sex. Eur J Cardiovasc Prev Rehabil. 2010;17:198-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 109. | Pelliccia A, Culasso F, Di Paolo FM, Maron BJ. Physiologic left ventricular cavity dilatation in elite athletes. Ann Intern Med. 1999;130:23-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 376] [Cited by in F6Publishing: 352] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 110. | Gati S, Sharma S. Determinants of the athlete's heart: a cardiovascular magnetic resonance imaging study. Eur J Prev Cardiol. 2020;27:536-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 111. | Gati S, Sharma S, Pennell D. The Role of Cardiovascular Magnetic Resonance Imaging in the Assessment of Highly Trained Athletes. JACC Cardiovasc Imaging. 2018;11:247-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 112. | Heidbüchel H, Hoogsteen J, Fagard R, Vanhees L, Ector H, Willems R, Van Lierde J. High prevalence of right ventricular involvement in endurance athletes with ventricular arrhythmias. Role of an electrophysiologic study in risk stratification. Eur Heart J. 2003;24:1473-1480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 275] [Cited by in F6Publishing: 280] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 113. | Zaidi A, Sheikh N, Jongman JK, Gati S, Panoulas VF, Carr-White G, Papadakis M, Sharma R, Behr ER, Sharma S. Clinical Differentiation Between Physiological Remodeling and Arrhythmogenic Right Ventricular Cardiomyopathy in Athletes With Marked Electrocardiographic Repolarization Anomalies. J Am Coll Cardiol. 2015;65:2702-2711. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 114. | La Gerche A, Claessen G, Van de Bruaene A, Pattyn N, Van Cleemput J, Gewillig M, Bogaert J, Dymarkowski S, Claus P, Heidbuchel H. Cardiac MRI: a new gold standard for ventricular volume quantification during high-intensity exercise. Circ Cardiovasc Imaging. 2013;6:329-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 175] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 115. | Le TT, Bryant JA, Ting AE, Ho PY, Su B, Teo RC, Gan JS, Chung YC, O'Regan DP, Cook SA, Chin CW. Assessing exercise cardiac reserve using real-time cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2017;19:7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 116. | La Gerche A, Claessen G, Dymarkowski S, Voigt JU, De Buck F, Vanhees L, Droogne W, Van Cleemput J, Claus P, Heidbuchel H. Exercise-induced right ventricular dysfunction is associated with ventricular arrhythmias in endurance athletes. Eur Heart J. 2015;36:1998-2010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 131] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 117. | Maestrini V, Merghani A, Rosmini S, Cox A, Bulluck H, Culotta V, Cheang M, Fontana M, Treibel TA, Abdel-Gadir A, Sharma S, Moon J. CMR findings in high endurance veteran athletes - a 247 subject study. J Cardiovasc Magn Reson. 2016;18:O38. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 118. | La Gerche A, Burns AT, Mooney DJ, Inder WJ, Taylor AJ, Bogaert J, Macisaac AI, Heidbüchel H, Prior DL. Exercise-induced right ventricular dysfunction and structural remodelling in endurance athletes. Eur Heart J. 2012;33:998-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 509] [Cited by in F6Publishing: 508] [Article Influence: 39.1] [Reference Citation Analysis (1)] |
| 119. | Breuckmann F, Möhlenkamp S, Nassenstein K, Lehmann N, Ladd S, Schmermund A, Sievers B, Schlosser T, Jöckel KH, Heusch G, Erbel R, Barkhausen J. Myocardial late gadolinium enhancement: prevalence, pattern, and prognostic relevance in marathon runners. Radiology. 2009;251:50-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 120. | Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, Pilichou K, Migliore F, Rizzo S, Giorgi B, De Conti G, Sarto P, Serratosa L, Patrizi G, De Maria E, Pelliccia A, Basso C, Schiavon M, Bauce B, Iliceto S, Thiene G, Corrado D. Nonischemic Left Ventricular Scar as a Substrate of Life-Threatening Ventricular Arrhythmias and Sudden Cardiac Death in Competitive Athletes. Circ Arrhythm Electrophysiol. 2016;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 192] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 121. | Androulakis E, Swoboda PP. The Role of Cardiovascular Magnetic Resonance in Sports Cardiology; Current Utility and Future Perspectives. Curr Treat Options Cardiovasc Med. 2018;20:86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 122. | Pujadas S, Doñate M, Li CH, Merchan S, Cabanillas A, Alomar X, Pons-Llado G, Serra-Grima R, Carreras F. Myocardial remodelling and tissue characterisation by cardiovascular magnetic resonance (CMR) in endurance athletes. BMJ Open Sport Exerc Med. 2018;4:e000422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Plácido R, Cunha Lopes B, Almeida AG, Rochitte CE. The role of cardiovascular magnetic resonance in takotsubo syndrome. J Cardiovasc Magn Reson. 2016;18:68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |