Published online Nov 26, 2022. doi: 10.4330/wjc.v14.i11.576
Peer-review started: March 13, 2022
First decision: June 8, 2022
Revised: July 4, 2022
Accepted: October 27, 2022
Article in press: October 27, 2022
Published online: November 26, 2022
Since 2010, the European Society of Cardiology has extended prescription criteria for oral antithrombotic therapy (OAT) in atrial fibrillation (AF). Direct oral anticoagulants (DOACs) were upgraded from an IIAa recommendation in 2012 to an IA in 2016. In real-world scenarios, however, OAC prescription is still suboptimal, mainly for DOACs.
To evaluate OAT temporal prescription patterns in a cohort of patients hospitalized with AF in a Cardiology Department.
A retrospective observational study was conducted on a cohort of hospitalized patients in a secondary setting (Trapani, Italy) from 2010 to 2021 with AF as the main or secondary diagnosis. For 4089 consecutive patients, the variables extracted from the Cardiology department database were: Sex, age, time of hospitalization, antithrombotic therapy (warfarin, acenocoumarol, apixaban, dabigatran, edoxaban, rivaroxaban, aspirin, clopidogrel, other antiplatelet agents, low molecular weight heparin, and fondaparinux), diagnosis at discharge and used resources. Basal features are presented as percentage values for categorized variables and as mean +/- SD for categorized once.
From January 1st, 2010 to October 6th, 2021, 25132 patients were hospitalized in our department; 4089 (16.27%, mean age 75.59+/-10.82) were discharged with AF diagnosis; of them, 2245 were males (54.81%, mean age 73.56+/-11.45) and 1851 females (45.19%, mean age 78.06+/-9.47). Average length of stay was 5.76+/-4.88 days; 154 patients died and 88 were moved to other Departments/Structures. AF was the main diagnosis in 899 patients (21.94%). The most frequent main diagnosis in patients with AF was acute myocardial infarction (1973 discharges, 48.19%). The most frequent secondary cardiac diagnosis was chronic coronary syndrome (1864 discharges, 45.51%), and the most frequent secondary associated condition was arterial hypertension (1010 discharges, 24.66%). For the analysis of antithrombotic treatments, the final sample included 3067 patients, after excluding in-hospital deaths, transferred out or self-discharged patients, as well as discharges lacking indications for prescribed treatments. OAC treatment increased significantly (35.63% in 2010-2012 vs 61.18% in 2019-2021, +25.55%, P < 0.0001), in spite of any antiplatelet agent use. This rise was due to increasing use of DOACs, with or without antiplatelet agents, from 3.04% in 2013-2015 to 50.06% in 2019-2021 (+47.02%, P < 0.0001) and was greater for factor Xa inhibitors, especially apixaban. In addition, treatment with a vitamin K antagonist, in spite of any antiplatelet agent use, decreased from 35.63% in 2010-2012 to 11.12% in 2019-2021 (-24.48%, P < 0.0001), as well as any antiplatelet therapy, alone or in double combination, (49.18% in 2010-2012 vs 34.18% in 2019-2021, -15.00%, P < 0.0001); and patients not receiving antithrombotic therapy declined with time (14.58% in 2010-2012 vs 1.97% in 2021, P < 0.0001).
Real-world patients with AF are elderly and affected by cardiovascular and non-cardiovascular diseases. The percentage of patients on OAT and DOACs increased. These data suggest a slow, gradual guidelines implementation process.
Core Tip: In this study, the proportion of patients on oral antithrombotic therapy, with or without an antiplatelet agent, increased significantly from 2010 to 2021. This rise was due to increasing use of direct oral anticoagulants, with or without antiplatelet agents. At the same time, there was a gradual decline in the use of vitamin K antagonists, with or without antiplatelet drugs, and of antiplatelet therapy, alone or in double combination, while the proportion of patients not receiving antithrombotic therapy decreased. These data suggest a slow and gradual guidelines implementation process.
- Citation: Abrignani MG, Lombardo A, Braschi A, Renda N, Abrignani V, Lombardo RM. Time trends in antithrombotic therapy prescription patterns: Real-world monocentric study in hospitalized patients with atrial fibrillation. World J Cardiol 2022; 14(11): 576-598
- URL: https://www.wjgnet.com/1949-8462/full/v14/i11/576.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i11.576
Atrial fibrillation (AF) represents the most common type of sustained cardiac arrhythmia and an emerging epidemic throughout the world, affecting 1%–2% of the adult population[1]. Its prevalence rises steeply from 0.1% in patients < 60 years to approximately 20% in those ≥ 85 years[2,3]. With the progressive aging population and improved survival from other forms of cardiovascular disease[3], both AF prevalence and incidence have been progressively increasing[2,4-6], becoming a significant public health burden.
AF is often associated with increased rates of death, hospitalization, cardiovascular and non-cardiovascular complications, and degraded quality of life, and is a known independent cardiac risk factor (fourfold to fivefold) for ischemic stroke, due to high thromboembolic risk[7-9]. This risk is greater in the elderly (in patients 80-89 years old it reaches 23.5%)[7]. Up to 15%-20% of all strokes are due to AF. AF is often associated with other cardiovascular risk factors or conditions, such as diabetes mellitus, arterial hypertension, chronic coronary syndromes, or heart failure, linked to a further increase in thromboembolic risk[10].
Contemporary registry-based observational studies from various geographical regions have consistently shown that patients with thromboembolic complications, particularly ischemic stroke and systemic thromboembolism (acute mesenteric ischemia, and acute limb ischemia) and AF, have a worse prognosis, more disability, longer hospital stays, more medical and neurologic complications, and greater case fatality rates than those without AF[11]. This increases health-care related costs and reduces quality of life[12]. Stroke prevention is therefore central to the management of AF and is a major public health priority.
Fortunately, among patients with AF, stroke, thromboembolic events and death risk may be up to two-thirds mitigated via the usage of oral anticoagulants (OACs), that it is superior to no treatment or antiplatelet agents such as acetylsalicylic acid (ASA), until recently a treatment choice, in patients with different stroke risk profiles[13-17]. The net clinical benefit is almost universal, except for patients with a very low stroke risk.
The European Society of Cardiology (ESC)[18-21] as well as other societies[22-25], in their evidence-based guidelines dedicated to AF, have widened since 2010 the indications for antithrombotic therapy, and now claim OACs as the appropriate treatment for stroke prevention in most patients (namely with additional stroke risk factors, introducing use of the CHA2DS2-VASc and HAS-BLED scores for stroke and bleeding risk stratification, respectively. All patients with non-valvular AF (NVAF), except those who are at low risk or with contraindications, require antithrombotic prophylaxis in order to prevent thromboembolism[18-21].
OACs include vitamin K antagonists (VKAs) and, in recent years, direct oral anticoagulants (DOACs). VKAs (in particular warfarin, historically the first-line stroke prevention option and the only available OAC for decades) are effective for preventing stroke by up to two-thirds, regardless of renal function, and with minor costs[26]. The good anticoagulation control with VKAs is assessed by high time in the therapeutic range (TTR). However, previous randomized controlled trials and real-life settings have controversies regarding TTR values[27]. In low TTR values, VKAs were found to be associated with severe complications, and a minimum TTR of 58% should be achieved to expect a net benefit from being on OAC therapy[28]. VKAs have, however, important limitations such as a narrow therapeutic window, requirement for close monitoring and frequent follow-ups, drug–drug and drug–food interactions, unpredictable dose-response effects, and a slow onset and ebbing of action. As a result, in the past years many AF patients received ASA, other antiplatelet agents, or both, or no antithrombotic treatment[6].
Management of AF patients has dramatically improved following the introduction of DOACs, comprising factor Xa inhibitors, such as apixaban, edoxaban, and rivaroxaban, and direct thrombin inhibitors (dabigatran etexilate), first approved in 2010, that showed numerous advantages over warfarin. DOACs rapidly became preferred by both clinicians and patients due to their easier usage: easy dosing schedule, rapid onset of action, more predictable efficacy which allows a fixed-dose regimen, no need for frequent international normalized ratio (INR) controls and fewer interactions with co-medication or with food[29-31]. In terms of stroke and systemic thromboembolism prevention, all DOACs were demonstrated at least to be non-inferior and in some respects superior (e.g. fewer intracranial hemorrhages) compared with warfarin in randomized controlled trials[32-35], even in older populations[36]. Recently, there has been a significant price drop in DOACs and meta-analyses of randomized controlled trials[37-39], as well as observational data[40-42] confirm their efficacy and real-life effectiveness. However, DOACs have higher costs and need adjustment based on renal function.
Currently, all four DOACs are approved in Italy. The European Medicine Agency (EMA) authorized dabigatran and rivaroxaban use in 2008 (they became available for use in clinical practice on the Italian market in 2013). The EMA approved apixaban in 2011 (available in Italy since January 2014), and edoxaban (available since June 2015). Since 2013, the Agenzia Italiana del Farmaco authorized (AIFA) them for cardiovascular risk reduction in NVAF[43].
After the release of DOAC, several European and North American scientific societies updated their guidelines, now recommending DOACs as first choice treatment in most patients with NVAF[19-21,24,25]. The changes in guidelines, coupled with the emergence of DOACs, whose use has been steadily increasing over a decade[17], have the potential to transform clinical practice patterns.
It is important, however, that AF guidelines are adhered to, as non-adherence to OACs is associated with increased ischemic stroke and mortality in high-risk patients[44].
Notwithstanding the increasing percentage of patients treated with DOACs[45,46], observational studies and administrative databases widely reported the suboptimal use of OACs for stroke prevention[47-51].
In the past, patients with NVAF remained untreated for several reasons, including overestimation of patient bleeding risk and underestimation of stroke risk by physicians[52], and the presence of comorbidities, mainly in elderly patients[53]. Sociodemographic and economic factors can influence prescription patterns[54-56]. On the other hand, DOAC prescription is subject to prior authorization in the Italian as well as in other National Health Systems[57,58]. Until recently, regulatory criteria placed DOAC as a second line therapy, limiting their use to patients in which VKA are contraindicated, or with objective difficulties in accessing INR control facilities, or with high intracranial hemorrhage risk[57].
Nevertheless, real-world studies in this population, are still scarce, in particular there is limited evidence on temporal trends of contemporary AF management since the introduction of DOACs[46,59-61], and treatment patterns at single country level are less known. In Italy, since their introduction, the rate of DOAC utilization is one of the lowest in Europe[43,58,61-63]. Thus, an updated analysis of DOAC treatment in Italy for NVAF patients could be useful.
Knowledge obtained from real-world scenarios may suggest strategies to improve the entire AF care process[63]. The questions are: do prescribers follow current guidelines for OACs prescription in AF patients, and has adherence to guidelines changed over time?
In this paper, the authors discuss the actual real-world status and the change, in its temporal trend, in the prescription of antithrombotic treatments in patient with AF consecutively discharged from a Cardiology Unit during an almost twelve-year period. We hypothesized that adherence to OACs prescription according to guidelines recommendations for patients with AF would improve over time.
This was a retrospective, single-center, observational study conducted in the Cardiology Unit of S. Antonio Abate Hospital of Trapani (Western Sicily, Italy). This unit takes care of all cardiovascular diseases and is also equipped with a cardiac catheterization and electrophysiology laboratory.
We reviewed the database of medical records of all patients aged ≥ 18 years who were consecutively discharged from a reference cardiology center from January 2010 to 2021. The following inclusion criteria were applied: any diagnosis of AF (both main and secondary) at discharge from hospital and hospitalization not resulting in death. Patients without indication of prescribed drugs were excluded.
We collected data on demographic and clinical characteristics, including age and sex, main and secondary diagnosis at discharge, diagnostic and therapeutic procedures, and prescribed antithrombotic treatments from the discharge medication list.
The presence of AF was ascertained during the hospital stay by medical history taking, in-hospital diagnosis by 12-lead electrocardiography, or 24-h Holter monitoring.
The discharge diagnosis codes assessed for each patient and the diagnostic and therapeutic procedures were classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM).
We considered: AF, ICD-9-CM code 42731; cardiovascular diseases (angina pectoris, ICD-9-CM codes 4111, 4131 and 4139; acute myocardial infarction, ICD-9-CM code 410; chronic coronary syndromes, ICD-9-CM codes 412, 414, 429, V4581, V4582; cardiomyopathies, ICD-9-CM codes 402 and 425; valvular diseases, ICD-9-CM codes: 394, 396, 397, 424, 394, V433; peripheral vascular disease, ICD-9-CM codes: 433.1, 440.2, 443.9; acute and chronic heart failure, ICD-9-CM codes: 428, 5184; cardiac arrhythmias, ICD-9-CM codes 426, 427, 727.89; endocarditis, ICD-9-CM codes 421, 424; pulmonary embolism, ICD-9-CM code 415; aortic aneurysm, ICD-9-CM code 493, chest pain, ICD-9-CM codes 786, V717), and other concomitant diseases, such as diabetes mellitus, ICD-9-CM code: 250; arterial hypertension, ICD-9-CM codes: 401–404; dyslipidemias, ICD-9-CM code 272; pulmonary diseases (chronic bronchitis, ICD-9-CM code: 491; asthma, ICD-9-CM code: 493; other, 518, 519, 492, 466, 491, 485, 486, 515, 518, V126; sleep apnea, ICD-9-CM codes: 780.51, 780.53, 780.57, 780.54); disorders of the thyroid gland, ICD-9-CM codes: 240–246; cerebrovascular diseases (stroke/transient ischemic attack (TIA)/hemorrhagic stroke, ICD-9-CM codes: 430–436, 438, 442, 4370); dementia, ICD-9-CM code: 290; other cerebral degenerations, ICD-9-CM code: 331; anemia, ICD-9-CM codes 280, 282, 283, 285; obesity, ICD-9-CM code 278; renal diseases, ICD-9-CM codes 584, 585, V560; neoplastic diseases, ICD-9-CM codes: 1419, 1420, 1479, 1512, 1519, 1534, 1537, 1539, 1540, 1541, 1561, 1590, 1599, 1619, 1629, 1749, 179,185, 1882, 1889, 1890, 1970, 1976, 1980, 1985.
In order to evaluate resource usage related to AF management we considered: length of hospital stay; diagnostic test prescription (such as echocardiogram, ICD-9-CM code: 8872; other ultrasound scan tests, ICD-9-CM code: 887; stress tests, ICD-9-CM code: 894; coronary angiography, ICD-9-CM codes: 885, 3721; peripheral angiography, ICD-9-CM code: 884; Holter ECG monitoring, ICD-9-CM code: 895; computed tomography, ICD-9-CM codes: 8703, 8704, 8741, 8742, 8801; and magnetic resonance imaging, ICD-9-CM code: 889); and interventional procedures (such as electrical cardioversion, ICD-9-CM code: 996; cardiac pacemaker implantation, ICD-9-CM codes: 377, 378; automatic cardiac defibrillator implantation, ICD-9-CM code: 379; percutaneous transluminal coronary angioplasty, ICD-9-CM codes: 885, 3721; peripheral percutaneous transluminal angioplasty, ICD-9-CM codes: 004, 0066, 3950; coronary stenting, ICD-9-CM codes: 004, 360, 377; and peripheral stenting, ICD-9-CM code: 3990).
We searched antithrombotic drug prescription at discharge based on the Anatomical Therapeutic Chemical (ATC) Classification System codes.
The OACs in this study included VKA (warfarin and acenocoumarol) and DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban); antiplatelet drugs (aspirin, clopidogrel, ticlopidine, ticagrelor, and prasugrel), low molecular weight heparin (LMWH) and fondaparinux were also considered. We defined a subject as receiving a VKA prescription if he/she redeemed a discharge prescription with a drug having ATC code B01AA03 (warfarin) or B01AA07 (acenocoumarol). On the other hand, we defined DOAC users as those subjects redeeming prescriptions of dabigatran (ATC code: B01AE07), rivaroxaban (ATC code: B01AF01), apixaban (ATC code: B01AF02), and edoxaban (ATC code: B01AF03).
The patients were then further stratified into the following main categories: (1) Monotherapy with VKAs; (2) monotherapy with DOACs; (3) OAC therapy (VKAs or DOACs); (4) single antiplatelet therapy (SAPT); (5) double antiplatelet therapy (DAPT); (6) double antithrombotic therapy (DAT) (one OAC and one antiplatelet drug); (7) triple antithrombotic therapy (TAT) (DAPT plus OAC); and (8) without therapy.
Drug choice was based on the knowledge and expertise of each prescriber, thus ensuring the collection of real-life data.
We retrieved anonymized medical records stored in the Cardiology Unit databases, collected in electronic case report forms (Microsoft Office Access 2013, Redmond, Washington, United States). Data quality was monitored electronically as well as through periodic medical and data quality reviews, on-site monitoring, and audits.
This study was conducted in accordance with the World Medical Association Declaration of Helsinki. In compliance with privacy laws, the patients’ identification codes were encrypted using a unique and anonymous personal identification code. According to the Italian law for confidentiality data, informed consent was not required for using anonymized retrospective information. Each patient, however, signed a written informed consent form at hospital admission, agreeing to the use of his/her data in anonymous form for any aim of medical research. No additional follow-up visits or testing was performed beyond those carried out as part of routine clinical care.
Data cleaning was performed by verifying minimum and maximum values and by analyzing missing data. Data from patients with missing values were not removed from the analyses of general AF patterns but removed from the analysis of treatment patterns.
The analysis provides descriptive statistics to summarize data patterns. Standard descriptive statistical methods were used to analyze the patient’s demographics and clinical status, and to evaluate the proportion of treated patients in each drug category. The considered variables were year of discharge, age, sex, length of stay, discharge diagnosis, undertaken diagnostic and therapeutic procedures and prescribed drugs at discharge. Once the database was cleaned, a descriptive analysis was undertaken. Continuous variables were reported as mean and standard deviation (± SD), whereas categorical variables were expressed as absolute and relative frequencies with percentages, as appropriate. Continuous variables were compared using the Student’s t. Categorical data were compared using the χ2 test or Fisher’s exact test, as appropriate. A two-tailed P value < 0.05 was considered statistically significant. The statistical analysis was performed using Microsoft Office Excel 2013 (Redmond, Washington, United States) and MedCalc (https://wwwmedcalc.org), and graphs were created using Microsoft Office Excel 2013 (Redmond, Washington, United States). The statistical methods of this study were reviewed by the authors themselves.
For the at-discharge analysis, we included data from 25132 discharges from January 1, 2010 to October 6, 2021.
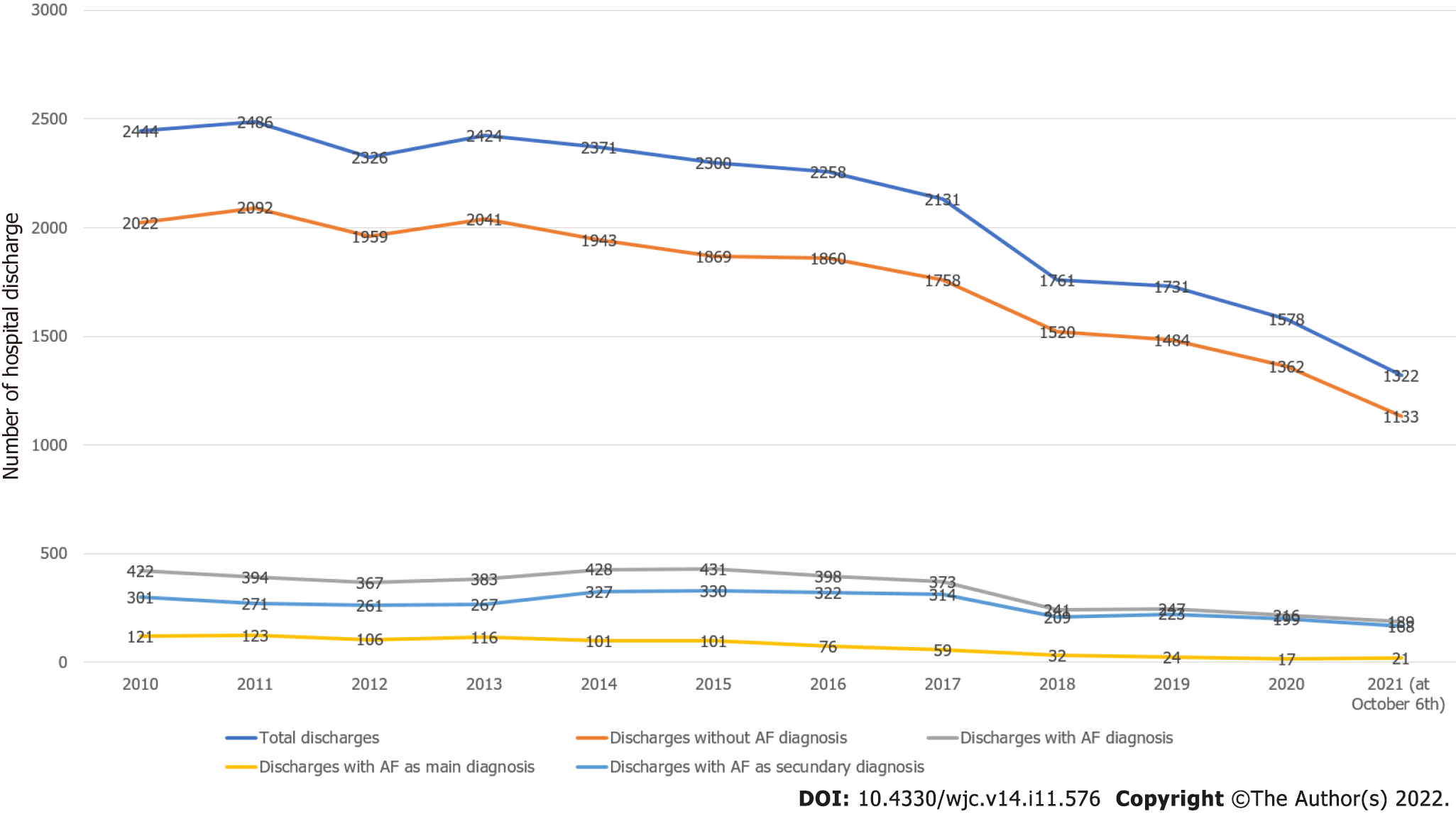
A diagnosis of AF was present in 4089 discharges (16.27%). Figure 1 shows the behavior of hospital discharges in the considered period. Total discharges decreased from 2444 in 2010 to 1532 in 2020 (2021 data were not considered because they were partial) (-37.32%). Discharges without AF diagnosis decreased from 2022 in 2010 to 1362 in 2020 (-30.66%). Discharges with AF diagnosis decreased from 422 in 2010 to 216 in 2020 (-48.81%). The decrease in discharges with AF diagnosis was significantly superior to the decrease in discharges without AF diagnosis (-18.15, P < 0.0001). Discharges with AF as the main diagnosis decreased from 121 in 2010 to 17 in 2020 (-85.95%). Discharges with AF as a secondary diagnosis decreased from 301 in 2010 to 199 in 2020 (-33.88%). The decrease in discharges with AF as the main diagnosis was superior to the decrease in discharges with AF as a secondary diagnosis (-52.07, P < 0.0001).
Among AF patients, 1851 were females (45.19%) and 2245 males (54.81%), with a male/female ratio of 1.21. The mean age of AF patients was 75.59+/-10.82 years. Mean age was lower in males (73.56+/-11.45) than in females (78.06+/-9.47) (P < 0.0001).
AF was the main diagnosis in 899 discharges (21.94%) and the secondary diagnosis in 3190 discharges (88.06%). AF as the secondary diagnosis was observed more frequently than as the main diagnosis (+66.12%, P < 0.0001).
Other main diagnoses are shown in Table 1. The prevalence in this table is related to the total sample. The most frequent main diagnosis in patients with AF was acute myocardial infarction. Secondary diagnoses are shown in Table 2. The prevalence in this table is related to the total sample. Of course, the sum of these percentages exceeds 100%, as a patient could have more comorbidities at the same time. The most frequent secondary cardiac diagnosis was chronic coronary syndrome, and the most frequent secondary associated condition was arterial hypertension.
| Diagnosis | n | % |
| AMI | 1973 | 48.19 |
| Cardiac arrhythmias | 210 | 5.37 |
| Chest pain | 201 | 4.91 |
| Heart failure | 143 | 3.49 |
| Chronic coronary syndrome | 80 | 1.95 |
| Cardiomyopathies | 77 | 1.88 |
| Peripheral artery disease | 74 | 1.81 |
| Stable angina | 55 | 1.34 |
| Unstable angina | 45 | 1.10 |
| Pericarditis | 29 | 0.71 |
| Valvular heart diseases | 27 | 0.66 |
| Shock | 21 | 0.51 |
| Pulmonary embolism | 22 | 0.49 |
| Other | 233 | 15.65 |
| Secondary diagnosis | n | % |
| Cardiac | ||
| Chronic coronary syndromes | 1864 | 45.51 |
| Cardiomyopathies | 1221 | 29.81 |
| Valvular heart diseases | 595 | 14.53 |
| Heart failure | 406 | 9.91 |
| Arrhythmias | 119 | 2.91 |
| Angina pectoris | 91 | 2.22 |
| Extra-cardiac | ||
| Arterial hypertension | 1010 | 24.66 |
| Renal diseases | 985 | 24.05 |
| Diabetes mellitus | 932 | 22.75 |
| Lung diseases | 617 | 15.06 |
| Dyslipidemias | 345 | 8.42 |
| Cerebrovascular & psychiatric diseases | 313 | 7.64 |
| Thyroid diseases | 152 | 3.71 |
| Anemia | 166 | 4.05 |
| Peripheral artery diseases | 146 | 3.56 |
| Obesity | 137 | 3.12 |
| Neoplastic diseases | 83 | 2.03 |
With regard to resource utilization, mean length of stay was 5.76+/-4.88 days in the total sample. Mean length of stay was 3.37+/-2.92 days in discharges with AF as the main diagnosis, and 6.48+/-5.11 days in discharges with AF as a secondary diagnosis. Length of stay was lower in discharges with AF as the main diagnosis (P < 0.0001).
Diagnostic and therapeutic procedures are shown in Table 3. The prevalence in this table is related to the total sample. Of course, the sum of these percentages exceeds 100%, as a patient could have received more diagnostic and therapeutic procedures at the same time. The most frequently used procedure was echocardiogram, whereas the most frequently performed intervention was percutaneous transluminal coronary angioplasty (PTCA)/stenting. Healthcare utilization was noticeable in this AF group.
| Procedures | n | % |
| Diagnostic | ||
| Echocardiogram | 3588 | 87.60 |
| Coronary angiography | 804 | 19.63 |
| Dynamic ECG monitoring | 458 | 11.18 |
| CT scan | 299 | 7.30 |
| Other echography | 212 | 5.18 |
| Stress test | 139 | 3.39 |
| Therapeutic | ||
| Coronary PTCA/stenting | 941 | 22.97 |
| PM implantation | 258 | 6.30 |
| ICD implantation | 90 | 2.20 |
| Peripheral vessels angiography | 90 | 2.20 |
| Peripheral vessels PTCA/stenting | 60 | 1.46 |
| Electric cardioversion | 56 | 3.71 |
For the analysis of antithrombotic treatments, we excluded in-hospital deaths, transferred out or self-discharged patients, as well as discharges lacking indications for prescribed treatments. The final sample was made of 3067 patients with AF diagnosis and known therapy. Figure 2 shows the flowchart of the study.
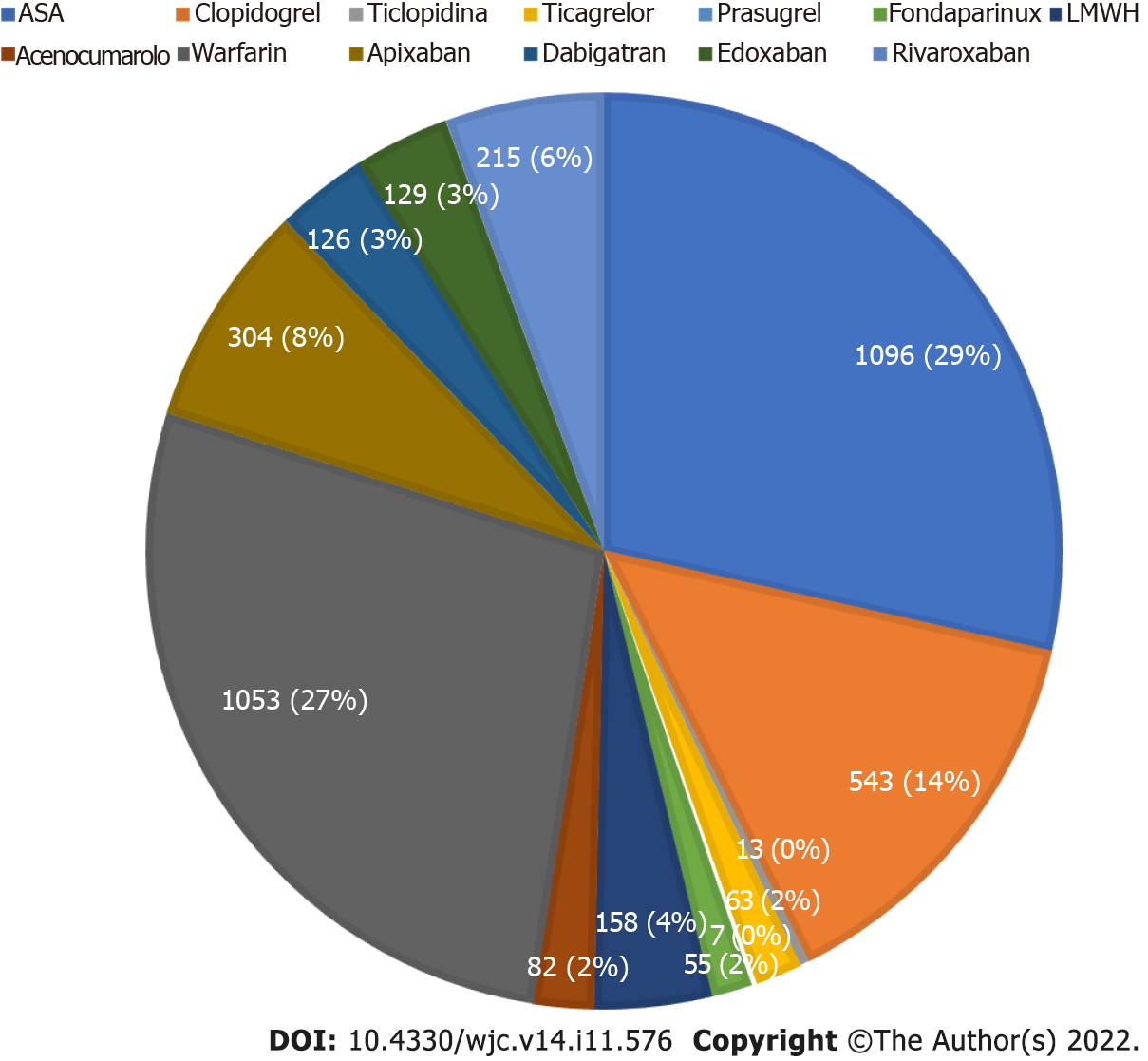
Antithrombotic drugs prescribed at discharge are shown in Figure 3. ASA was the most utilized drug (29% of prescribed drugs), followed by warfarin (27%) and clopidogrel (14%). VKAs were prescribed in 29% of total antithrombotic drugs. Among them, warfarin was undoubtedly the most prescribed drug (92.77% vs 7.23% of acenocoumarol). DOACs were prescribed in 20% of total drugs. Among them, apixaban was the most prescribed (39% of all DOACs). Antiplatelet agents were prescribed in 45% of drugs. Among them ASA was the most prescribed (63.65%), followed by clopidogrel (31.53%).
Changes over time in the prescribed antithrombotic drugs are shown in Table 4 (absolute numbers). After an initial increase, VKAs prescription progressively decreased. Antiplatelet drugs prescription decreased progressively over time. In contrast, DOAC prescription increased sharply from 0.5% in 2013 to 57% in 2021. The percentage of prescribed OACs increased from 27% in 2010 to 64% in 2021, whereas the percentage of patients without any antithrombotic therapy decreased from 13% in 2010 to 4% in 2021.
| Yr | Warfarin | Acenocumarole | Dabigatran | Rivaroxaban | Apixaban | Edoxaban | Aspirin | Clopidogrel | Ticlopidine | Ticagrelor | Prasugrel | LMWH | Fondaparinux | No therapy |
| 2010 | 32 | 3 | 41 | 35 | 1 | 2 | 17 | |||||||
| 2011 | 93 | 3 | 82 | 30 | 2 | 24 | ||||||||
| 2012 | 100 | 11 | 99 | 47 | 1 | 58 | ||||||||
| 2013 | 177 | 12 | 2 | 191 | 73 | 7 | 7 | 1 | 17 | 4 | 65 | |||
| 2014 | 114 | 7 | 10 | 108 | 28 | 12 | 7 | 4 | 41 | |||||
| 2015 | 149 | 12 | 18 | 11 | 138 | 40 | 5 | 13 | 1 | 20 | 1 | 55 | ||
| 2016 | 127 | 6 | 29 | 25 | 3 | 138 | 48 | 13 | 5 | 26 | 34 | |||
| 2017 | 116 | 17 | 18 | 24 | 46 | 16 | 95 | 63 | 7 | 9 | 6 | 30 | ||
| 2018 | 56 | 4 | 20 | 45 | 54 | 21 | 52 | 43 | 4 | 3 | 1 | 7 | ||
| 2019 | 43 | 4 | 40 | 40 | 67 | 21 | 68 | 55 | 2 | 3 | 2 | 2 | ||
| 2020 | 31 | 1 | 19 | 30 | 55 | 27 | 49 | 46 | 5 | 7 | 5 | 7 | ||
| 2021 | 15 | 2 | 29 | 17 | 46 | 41 | 35 | 35 | 4 | 2 | 8 |
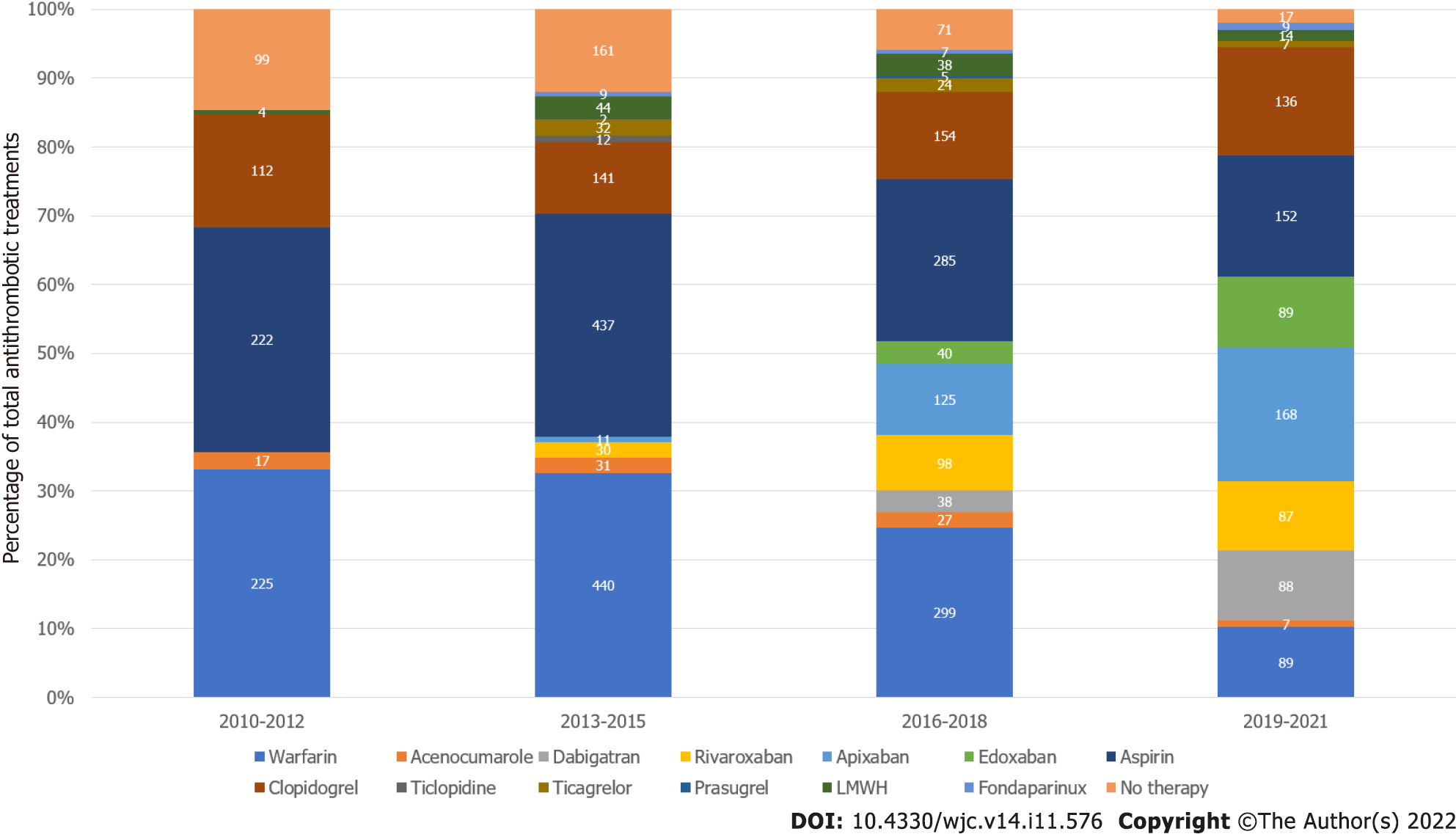
In order to avoid an accentuation of year-to-year variability, data on treatment were grouped in 3-year periods, as shown in Figure 4. VKAs prescription decreased from 35.63% in 2010-2012 to 11.12% in 2019-2021 (-24.48%, P < 0.0001). Antiplatelet drugs prescription decreased from 49.18% in 2010-2012 to 34.18% in 2019-2021 (-15.00%, P < 0.0001). On the contrary, DOACs prescription increased from 3.04% in 2013-2015 to 50.06% in 2019-2021 (+47.02%, P < 0.0001). OAC prescription increased from 35.63% in 2010-2012 to 61.18% in 2019-2021 (+25.55%, P < 0.0001), whereas the percentage of patients without any antithrombotic therapy decreased from 14.58% in 2010-2012 to 1.97% in 2021 (P < 0.0001).
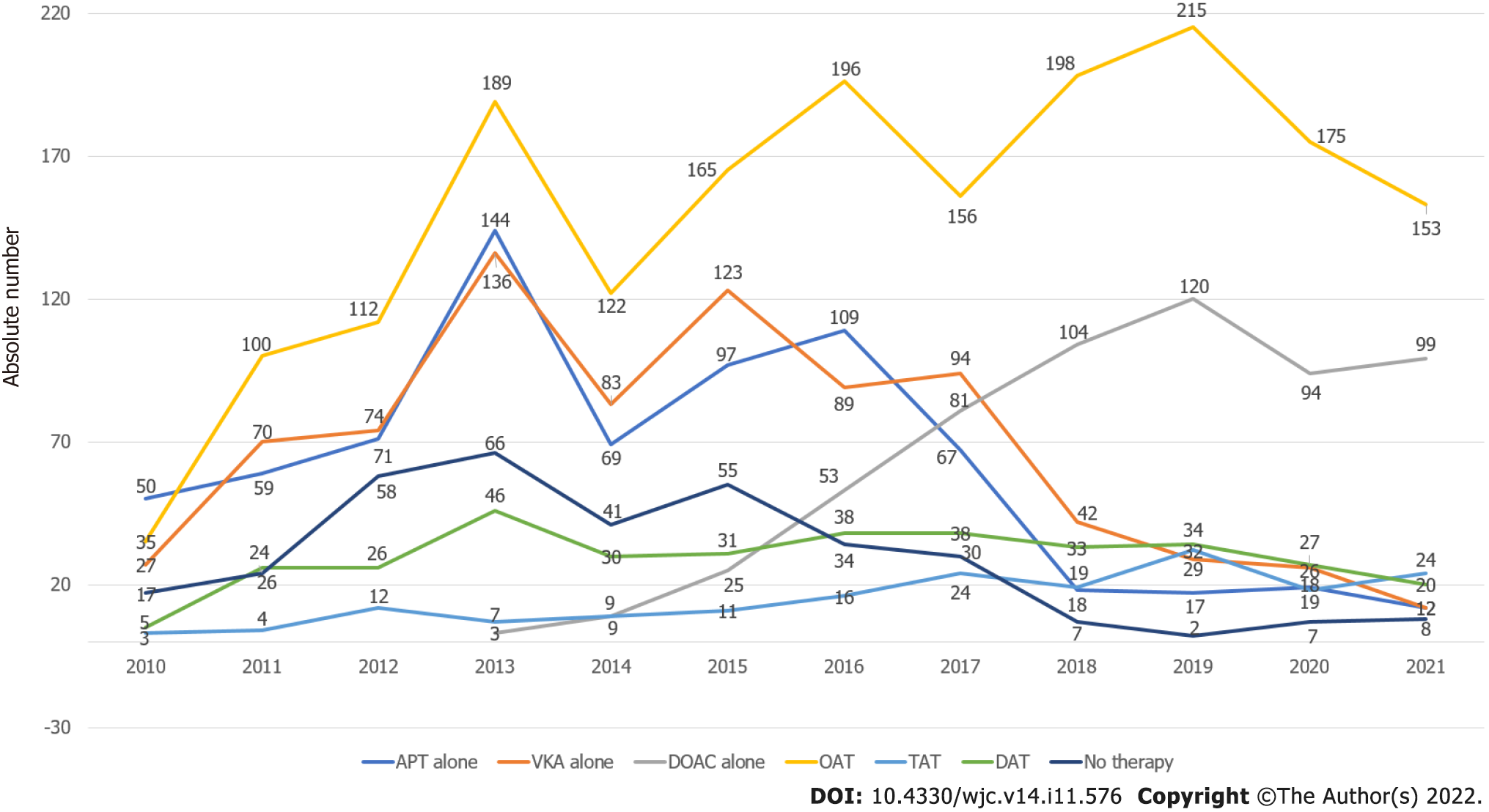
It should be considered that antithrombotic treatments can be combined variously among patients, particularly as our population sample consisted of a large percentage of acute and chronic coronary heart disease patients. Thus, in Figure 5 we show the behavior over time of various antithrombotic combinations. SAPT, DAPT, VKAs, and no therapy decreased over time, whereas DOACs, DAT, TAT and global OACs increased over time.
Finally, these data have been corrected according to the total number of discharges, as their number is not stable over time, as shown in this study. Figure 6 shows the trend in prescribed antithrombotic therapy during the study period. The more relevant data are the sharp increase in patients treated with DOAC (from 0.78% in 2013 to 52.38% in 2021, P < 0.0001) and with OACs (from 34.31 in 2010 to 80.95 in 2021, P < 0.0001); conversely, the number of patients not receiving any antithrombotic therapy decreased from 16.67 in 2010 to 4.23 in 2021 (P < 0.0003).
In this retrospective, single center, contemporary real-world study we examined clinical characteristics, resource utilization, and temporal trends over a twelve-year interval in antithrombotic therapy prescription pattern in a cohort of patients discharged from a cardiology unit with a diagnosis of AF.
Discharges with AF diagnosis decreased over time, and the decrease in discharges with AF as the main diagnosis was significantly superior to the decrease in discharges with AF as the secondary diagnosis. We observed that AF patients were elderly, and predominantly male, with a high prevalence of concomitant cardiac and extra-cardiac diseases. Healthcare utilization in this group of patients was noticeable in terms of both diagnostic and therapeutic procedures.
In terms of antithrombotic treatments, from 2010 to 2021 patients on OAC therapy increased significantly, regardless of antiplatelet drugs use. The increasing use of DOACs, namely factor Xa (FXa) inhibitors (especially apixaban), can explain this phenomenon. Contextually, VKA use, regardless of antiplatelet treatments, declined, like antiplatelet therapy, alone or in double combination, while the proportion of patients not receiving antithrombotic therapy decreased.
In this study, a diagnosis of AF was present in 4089 on 25132 discharges from 2010 to 2021. Total discharges decreased (-37.32%) from 2010 to 2020. This phenomenon may be explained by the shift in medical treatments from hospital to territory. Also discharges with and without AF diagnosis decreased from 2010 to 2020 (respectively -48.81% and -30.66%), but the decrease in discharges with AF diagnosis was greater than the decrease in discharges without AF diagnosis (-18.15, P < 0.0001). Although the incidence and prevalence of AF are expected to increase due to progressive growth in the number of elderly people in the general population, our sample reflects only patients hospitalized in a cardiology unit. Thus, the decrease in discharges with AF diagnosis may be explained, in general, by the decrease in total hospital admissions and, in particular, by the reduction in admission of patients with a paroxysmal AF, that is now considered inappropriate; in fact, the decrease in discharges with AF as the main diagnosis was significantly superior to the decrease in discharges with AF as the secondary diagnosis (-52.07, P < 0.0001). However, AF as a secondary diagnosis was observed more often than as the main diagnosis (+66.12, P < 0.0001). This was due to the real-world nature of this observational study, focused on a global sample of patients admitted to a cardiology unit.
We observed that AF patients were elderly (mean age was 75.59+/-10.82 years), as shown in other studies[29,46,52,64,65]. Males accounted for the majority of patients in the whole study group: the male/female ratio was 1.21. This confirms the data from other studies[29,59,65,66]. An opposite trend, with a greater prevalence in women, was observed by Ermini[63]. The mean age was lower in males (73.56+/-11.45) than in females (78.06+/-9.47) (P < 0.0001).
The most frequent main diagnosis in patients with AF was acute myocardial infarction. The most frequent secondary cardiac diagnosis was chronic coronary syndrome, and the most frequent secondary associated condition was arterial hypertension. A high prevalence of concomitant cardiac and extra-cardiac diseases was shown, in particular arterial hypertension, diabetes mellitus, heart failure, and coronary artery disease. This profile of comorbidities at baseline was in agreement with previous analyses[46,53,63,67,68]. In the Akershus Cardiac Examination 1950 study, 87.6% of men with AF and 86.4% of women with AF had comorbidities, compared with 74.4% and 66.3%, respectively, without AF[3]. Thus, our subjects reflected real-world clinical practice, including a large proportion of patients with advanced age and many comorbidities.
With regard to resource utilization, mean length of stay was 5.76+/-4.88 days in the total sample. Mean length of stay was 3.37+/-2.92 days in discharges with AF as the main diagnosis, significantly lower than in discharges with AF as a secondary diagnosis (6.48+/-5.11, P < 0.0001). The procedure most frequently used was echocardiogram, whereas the most frequently performed intervention was PTCA/stenting. Thus, healthcare utilization in this group of patients is noticeable in terms of both diagnostic and therapeutic procedures.
Our study was able to show the trends in antithrombotic treatments in AF patients. During the study periods, several guidelines on antithrombotic AF management have been published. In practice, the ESC guidelines progressively extended the indication for OAC, excluded antiplatelet treatment, and gave greater importance to DOACs[18-21].
It is known, however, that real-world guideline implementation is not a simple process. The increasing prevalence of AF and AF-related comorbidities proves the need for comprehensive prevention and management strategies. The challenge is the optimization of therapy for each patient. However, there are still gaps in optimal stroke prevention[17].
Thus, the main purpose of this study was to investigate whether guideline recommendations in terms of antithrombotic treatment were actually applied in clinical practice, by evaluating antithrombotic treatment patterns in Italian patients with a discharge diagnosis of AF. In this setting, several disease-specific, prospective observational studies and registry programs were created to better understand AF populations, their demography, treatments, and clinical outcomes at world[45,46,69-72] and European level[9,10,44,73-76]. In addition, observational data are available from America[6,51,71,77,78], Europe[52,55,58,59,62,79-83], and Asia[53,84-93].
A progressive improvement in the guideline-recommended antithrombotic prophylaxis of stroke in AF patients, mainly in newly diagnosed cases, has been shown by these studies[59]; however, most of them belong to the pre-DOAC era, and there is still much to learn about how DOACs are being used in clinical practice. The present study adds to the few studies that have investigated the prescription pattern of antithrombotic agents in AF patients in recent years: Proietti[76] evaluated patients from Belgium, Denmark, Greece, Italy, Norway, Poland, Portugal, Romania, and the Netherlands; Huisman[45,46] evaluated patients from Asia, Africa/Middle East, Europe, Latin America and North America; and Apenteng[59] studied United Kingdom patients.
In our study, antiplatelet agents were prescribed in 45% of total drugs. Among them ASA was the most prescribed (63.65%), followed by clopidogrel (31.53%). ASA was also, in total, the most utilized drug, followed by warfarin and clopidogrel. SAPT, however, decreased significantly over time from 49.18% in 2010-2012 to 34.18% in 2019-2021 (-15.00%, P < 0.0001). Antiplatelet therapy was also commonly prescribed in other studies, regardless of whether there was coexistent myocardial infarction or coronary artery disease[74,75]. Other studies showed, for example, that antiplatelet agents were used in 30% of all patients with AF[63] and in 36% in the pre-DOAC era[58]. Antiplatelet agents are particularly used in the elderly; in 18.3%, 18.9%, 18.9%, and 18.7% of patients aged 75–< 80, 80–< 85, 85–< 90, and ≥ 90 years, respectively[87]. In other studies, treatment with ASA was also the most common (41.7% of patients in GARFIELD[45,46]), whereas in others was very low (3.3%)[68]. The proportion of patients treated with antiplatelet agents other than ASA was low (3.4%)[45,46], in contrast to the high clopidogrel use in our data. These differences may be explained by the fact that we studied unselected cardiology patients with high prevalence of acute and chronic coronary syndromes, and in whom antiplatelet therapy was still prescribed routinely with or without oral anticoagulation. Minor use of antiplatelet agents over time as sole therapy for stroke prevention in AF is a common finding and other studies showed a downward trend from 36% to 17% (in GARFIELD-AF)[70], from 18% to 8% (in the ORBIT-AF program)[71], from 6.1 to 2.5%[80], from 36,5% to 10,5%[59], and from 36% to 25%[58]. It is increasingly recognized that antiplatelet agents are of little benefit and have a not insignificant risk, although 2010 ESC guidelines still endorsed aspirin for patients at intermediate stroke risk according to CHADS2 risk stratification[18]; however, in the 2012 update[19] antiplatelet drugs were to be considered only in patients refusing any OAC. In the 2014 AHA/ACC/HRS treatment guidelines[24] aspirin was still considered an option for AF patients with moderate stroke risk. Conversely, the 2019 AHA/ACC/HRS guidelines[25] suggested that NVAF patients, regardless of their stroke risk, should not be treated with antiplatelet drugs monotherapy, unless an OAC is contraindicated; thus, high risk patients would be considered undertreated whenever only ASA is used. However, antiplatelet therapy continues in part to be inappropriately prescribed instead of OAC[68]. A reason of the persistence of antiplatelet agents use may be that anticoagulant prophylactic therapy is especially difficult in patients in whom a high thromboembolic risk coexists with contraindications for OAC treatment, such as the elderly. OAC underuse, associated with antiplatelet therapy prescription, regardless of a known atheromatous disease, has been reported by Averlant et al[94] in elderly AF patients. In addition, in the post-DOAC era, patients who are receiving antiplatelet drugs have more comorbidities[58].
We observed that DAPT also decreased over time from 13.72% to 2.12%. In ACS patients treated with percutaneous coronary intervention (PCI), a DAPT is recommended to reduce stent thrombosis risk[67]. This reduced use of DAPT is likely due to a greater prescription of DAT or TAT in a population with noticeable prevalence of coronary syndromes.
In our study, VKAs were prescribed in 29% of total antithrombotic drugs. Among VKAs, warfarin was undoubtedly the most prescribed drug (92.77% vs 7.23% of acenocoumarol). VKAs prescription decreased significantly from 35.63% in 2010-2012 to 11.12% in 2019-2021 (-24.48%, P < 0.0001). In other studies, warfarin was also the most prescribed OAC (from 24.2% to 88.8%[29,64,75,79,90] according to the period and to the country. The PINNACLE study, conducted by the National Cardiovascular Data Registry, showed that only 55% of warfarin-eligible patients actually received that drug[77]. A gradual decrease in warfarin use was also observed in GARFIELD-AF[70,79,80], mainly after DOAC introduction. Geographical differences exist, however, in VKA therapy, with a notably greater use of VKAs in China, where it was the fastest growing OAC used[45,46,64], likely for economic reasons. Age and comorbidities (in particular decreased renal function) may guide the choice of warfarin instead of OACs[95]. In the post-DOAC era, patients receiving VKA have more comorbidities[58,87] in comparison to the pre-DOAC era, and are frequently treated with polypharmacy[96]. VKA use is also common in patients with acute and chronic coronary syndromes requiring both OAC and antiplatelet therapy[68,72].
We observed that DOACs represented 20% of the total prescriptions. Apixaban was the most frequently prescribed (39% of all DOACs). The more relevant data from this study are the sharp, statistically significant increase in patients treated with DOAC, from 0.78% in 2013 to 52.38% in 2021, P < 0.0001, and from 3.04% in 2013-2015 to 50.06% in 2019-2021, +47.02%, P < 0.0001. In 2018, DOAC use surpassed that of warfarin. Our study confirms apixaban as the most used DOAC[52,87,97]; however, other authors have observed the prevalent use of dabigatran[29,65,68] or rivaroxaban[43]. It is difficult to explain these differences, which are likely a consequence of local preferences.
The prescription rate of DOACs for NVAF, after their release in 2011, has increased significantly in recent years, as demonstrated by many other studies, which reported a substantial increase from 14.5% to 70.1%[68,71,80,98,99]. Some of these studies, however, used data from registries of cardiovascular care practices, which may favor enrolment of highly motivated patients under specialist care, and the applicability of these results to the general population may be limited[65]. Actually, DOAC adoption trends are quite variable, with slow integration into clinical practice reported in most countries[98]. A systematic literature review indicates that suboptimal OACs use is a persisting challenge, despite the availability of DOACs[100]. After the launch of the first DOAC in 2011, the proportion of DOACs as OAC increased from 3% in 2012 to 42% in 2016 (P < 0.0001 for the trend)[16]. A marked variability in NOAC use was observed between countries, ranging from 6.1% (in Thailand) to 87.5% (in Switzerland) of all OAC-treated patients[72]. Many countries have some limitations on DOAC usage due to its costs. In Italy, in particular, reimbursement was possible only after mid-2013 for dabigatran, late 2013 for rivaroxaban, early 2014 for apixaban and late 2016 for edoxaban. In Italy, management with VKAs was better than in other European countries, allowing higher TTR[62]. Some studies aimed to determine the preference criteria in DOACs use. At patient level, prior stroke, transient ischemic attack, thromboembolism, thyroid disease, dyslipidemia, cancer, HAS-BLED ≥ 5, paroxysmal or non-permanent AF, and the presence of comorbidities were positive predictors of DOACs use over VKAs, whereas young age (≤ 64 years) and renal dysfunction (as they must be used with caution in this latter category of patients) were negative predictors of DOACs use over VKAs[57,68,99]. GARFIELD-AF showed that DOACs seemed to be favored for the management of patients with a low stroke risk (CHA2DS2-VASc 0 or 1)[72]. However, the extent of anticoagulant selection driven independently by ischemic stroke risk (predictions of treatment benefit) and bleeding risk (prediction of treatment harm) was marginal, as neither score explained much variation in the multivariable adjusted regression model[72,79]. Clinicians may be choosing warfarin in real-world clinical practice for patients with both high stroke risk and bleeding risk, indicating possible concerns about the lack of a reversal agent for the DOACs[29]. This contrasts with the reduced use of dabigatran in our study. DOAC use was more frequent than VKA in men and in the elderly[66,68,70,72], particularly apixaban[97], as they have fewer potential drug interactions in elderly patients. In the ANAFIE Registry, 72% of elderly AF patients receiving anticoagulant treatment were treated with DOACs[86]. However, in some studies DOACs were more frequently prescribed in female and young patients[55]. The rate of DOAC, rather than warfarin, was increased (P < 0.0001 for the trend) in patients with AF undergoing PCI[52]. OAC therapy at discharge was prescribed in approximately 30% of patients with AF and ACS requiring PCI (DOACs accounted for approximately half of them)[67].
In our study, both DAT (SAPT plus OAC) and TAT (the combination of OAC and DAPT to prevent both systemic embolism or stroke and coronary thrombosis, especially in the acute phase of the disease) increased over time (respectively from 4.90% in 2010 to 10.58% in 2021 and from 2.94% in 2010 to 12.69% in 2021). These data are in agreement with many other studies[52,59,63,68]. However, our data refer to a general population admitted to a cardiology unit with various diagnoses, while different results have been observed in selected samples such as patients with both AF and coronary artery disease (in particular those with ACS and/or undergoing PCI). Combined antithrombotic regimens present a great challenge in these real-world clinical scenarios. Previous studies have shown that warfarin alone was not sufficient to avoid stent thrombosis, and SAPT or DAPT alone was not adequate to prevent AF-related thromboembolic events; therefore, patients with AF undergoing PCI are typically prescribed multiple antithromboembolic drugs. Before the introduction of DOACs, from 2013 to 2014, only 1.7% of patients were treated using both warfarin and DAPT in the China acute myocardial infarction (CAMI) registry[92]. After DOAC introduction, in patients with ACS the rate of DAT prescription increased over the years (from 41% in 2010 to 59% in 2016, P = 0.012 for the trend) whereas TAT prescription decreased (from 14% in 2010 to 5% in 2016, P = 0.010 for the trend)[16] and the co-prescription of DOACs and antiplatelet drugs did not change much in recent years[59]. ‘Triple therapy’ is likely less prescribed by physicians due to concerns regarding bleeding risk. A greater risk-to-benefit ratio of DAT (DOAC plus a P2Y12 inhibitor) in comparison to a VKA-based TAT has been shown in randomized controlled trials[101,102]. The Danish nationwide administrative registries showed that DOACs use exceeded that of warfarin, in any combination with antiplatelet drugs, by 2016[103]. These studies influenced current international guidelines, now favoring, in this setting, a DAT with a DOAC and a P2Y12 inhibitor (especially clopidogrel).
From 2010 to 2021, globally, we observed that OAC was prescribed in 49% of all antithrombotic therapy, but even more we showed an increase in patients treated with OACs (from 34.31% in 2010 to 80.95% in 2021, P < 0.0001 and from 35.63% in 2010-2012 to 61.18% in 2019-2021, +25.55%, P < 0.0001). In a similar study, Mai[67] reviewed 3813 electronic medical records of patients aged ≥ 18 years, who were hospitalized from 2013 to 2018, which showed that prescription of OACs in patients with AF was low (29.7%). Another study, from 2014 to 2017, showed that 90.1% of patients received an OAC (either as a monotherapy or combined with antiplatelet drugs[68]. In other studies conducted in different temporal and geographical settings the rate of prescription of OACs, both in monotherapy and in association with antiplatelet drugs, varied from 41.2% to 92%[15,60,64,73,103]. These data, on the one hand, indicate how guidelines can be successfully applied in the real world, but, on the other hand, they suggest that OAC underuse persists, despite the growing awareness of anticoagulation benefits in AF[71,80]. OAC use seems especially low in Italy, as reported by the PREFER-AF registry[62]. Our results are in line with previous studies from different populations, demonstrating a recent increase in OAC use[44,46,60,70,104], particularly after the introduction of DOAC [15,59,72,89,91]. The proportion of patients treated with OAC monotherapy increased slowly, but gradually[52]. DOACs availability, together with comprehension of the reduced efficacy of antiplatelet drugs in comparison to OACs, have likely driven, at least in part, this paradigm shift in prescribing practice, notwithstanding an initial reluctance of healthcare payers due to the greater DOAC costs[70]. The prescription of OACs, as well as its temporal trend, is also related to various geographic and clinical patterns. In patients who underwent PCI, the OAC prescription rate increased from 56% in 2010 to 74% in 2016 (P = 0.041 for the trend) and OAC monotherapy gradually increased from 2% in 2010 to 9% in 2016 (P = 0.041 for the trend)[16]. Another study showed that OAC treatment was prescribed at discharge only in about 30% of patients with AF and ACS requiring PCI[67]. Among the factors playing a role in OACS underuse, we should consider demographic patterns (i.e., elderly and women), concomitant diseases such as hepatic or renal disease, lack of adherence, physicians’ and patients’ treatment fears, and lack of access to the healthcare system. With regard to age, for example, patients prescribed OACs at discharge were younger than those not prescribed OACs (mean age 71.7+/-10.6 vs 74.6+/-10.2 years)[64]. In the RAMSES prevention strategies trial[85], a national observational registry on Turkish adults with NVAF, OAC therapy was prescribed for 74.8% of participants younger than 80 years and 63% of those aged 80 and older (P < 0.001). Comorbidities and other individual-level characteristics may explain this difference in the elderly. Higher CHA2DS2-VASc score and lower HAS-BLED score were independent predictors of OAC prescription in participants aged 80 years and older[85]. OAC treatment was prescribed in only half of elderly patients in the Fushimi AF Registry[89]. A retrospective Chinese study showed that OACs were prescribed in only 41.1% of AF patients aged ≥ 65 years[15]. The overall OACs rate in older people, notwithstanding the higher risk of bleeding, was greater (87.3%) in another study[87], and 92% of patients ≥ 75 years old received OAC treatment in the All Nippon AF in the Elderly (ANAFIE) Registry[86], as well as 92% of patients ≥ 80 years old in the OCTOFA study[81]. Other factors, such as lower levels of education, lower income, prior antiplatelet use, having several cardiovascular comorbid conditions (including stroke or transient ischemic attack, hypertension, diabetes, dyslipidemia, valvular heart disease, heart failure, coronary syndromes, carotid stenosis, and peripheral vascular disease) were associated with not being prescribed an OAC[64,75]. OACs use was greater in low bleeding risk patients than in those with both high stroke and high bleeding risk (94.2% vs 91.3%, P < 0.0001)[88]. However, patients with contraindications to OACS are a minority. For example, in the elderly, only 4% of patients had such contraindications (primarily, active cancer and anemia)[68]. In 86,671 elderly AF patients, only 2% were ineligible for OAC therapy due to absolute contraindications (most often previous intracranial bleeding)[71]; also, OACs were contraindicated in less than 13% of 10130 patients in the ORBIT-AF trial[78].
In our study, LMWH and fondaparinux were used in approximately 6% of total antithrombotic drugs, but they were used in very low percentages as unique treatment throughout the 12-year period. Another study showed that LMWH, not endorsed just from the 2012 ESC guidelines[19], was used in 2.5% of patients[68].
Finally, we showed that the percentage of patients without any antithrombotic therapy, including antiplatelets and LMWH/fondaparinux, significantly decreased from 16.67% in 2010 to 4.23% in 2021 (P < 0.0003) and from 14.58% in 2010-2012 to about 1.5% in 2019-2021. These data are consistent with other studies, showing that a total varying from 21.9% to 30.2% did not receive any prophylactic antithrombotic therapy[46,60,70,75,90] with substantial variations across countries. A recent meta-analysis reviewed a total of 11,231 publications, demonstrating in patients with high stroke risk a rate of non-treatment of 23.3% (7.9%-51.1%)[100]. Undertreatment is frequent in female and older patients, notwithstanding their great stroke risk[36,87,93,105]. However, in the DOAC era, non-treatment rates in high-risk patients are lower than in the pre-DOAC era (11.1%, 95%CI 7.9%-40.2% vs 33.6%, 95%CI 13.4%-51.1%)[100]. Patients receiving no treatment are generally younger and healthier[70]. However, patients who received no treatment in the post-DOAC era had more comorbidities (P < 0.01, respectively)[58].
Our study has several strengths, including the analysis of clinicians’ preferences on antithrombotic treatment in a broad spectrum, consecutive, geographically defined population over a long period of time, providing a novel contribution by characterizing OAC prescriptions pattern among patients with AF.
However, our results should be interpreted in the context of the limitations of this study, whose purpose was restricted to the review of analyses of observational data collected through clinical databases, reflecting real-world clinical practice, which presented some limitations.
First, although efforts were made to standardize definitions and reduce missing data, this was a retrospective study with the limitations inherent to observational study design such as selection biases due to residual or not measured confounding factors (i.e. sociodemographic, patient preferences, biochemical parameters, and/or clinical confounding variables unavailable in the data, which would have likely impacted on the choice of treatment), all of which may restrict the interpretation of study results. In particular, data on CHA2DS2-VASc and HAS-BLED score were not available in the present analysis.
Second, data on detailed OAC types and the quality control of OAC use prior to hospitalization were not collected, likewise no follow-up was investigated, and therefore the effects of quality of warfarin control and of OAC adherence on outcome could not be evaluated.
Third, as all patients were discharged from a secondary center, the current registry is not free from referral bias. In addition, we studied patients managed only by cardiologists and discharged from a single center. The GARFIELD-AF registry found that patients who are managed in the outpatient setting are more likely to receive DOAC therapy than patients treated in emergency care or in the hospital setting[72].
Finally, we did not exclude valvular AF patients, in which VKA use is mandatory; however, only about 5% of all AF patients had mechanical heart valves or moderate-to-severe mitral stenosis[6] and an even lower proportion was observed in our study.
Thus, the results and conclusions of this study should be interpreted cautiously, as transferability to different contexts is limited.
AF has a negative impact on many cardiovascular diseases[106,107], but its most challenging is thromboprophylaxis. Although anticoagulation provides a net clinical benefit in patients with AF, a noticeable gap in antithrombotic prescription between real world and guideline recommendations was shown even in recent studies[108-111]. The long-awaited introduction of DOACs in the field of anticoagulation brought physicians a safer option, and in the last years, several real-world studies have confirmed their effectiveness and safety. The prescription trend of antithrombotic therapy in AF patients has noticeably changed over very recent years.
The main aim of our study was to describe patterns of OAC prescription for stroke prevention in a real-world population of Italian AF patients discharged by a cardiology ward. We demonstrated a significant increase from 2010 to 2021 in the proportion of OAC prescriptions, regardless of antiplatelet drugs use. This increase appears to be the consequence of greater DOACs use, mainly FXa inhibitors. Contextually, VKA use declined gradually regardless of antiplatelet drugs use, and the same pheno
These findings, in line with findings from other European and global datasets, appear consistent with recent changes in AF management guidelines; this suggests, in Italy, an improvement in adherence to guidelines clinical recommendations. Despite this significant improvement, we should highlight, however, that OAC prescription remains suboptimal over time; thus, a significant proportion of patients with AF still do not receive appropriate treatments for stroke prevention, suggesting that the increasing use of DOACs is not yet closing the gap between scientific evidence, recommendations from academic guidelines and clinical practice in the general population. Thus, an unmet medical need remains among patients with AF. Due to the nature of this study, we cannot, however, provide explanations as to the decision-making processes that underlie these apparent changes in prescriptions.
Improving adherence to AF guideline recommendations regarding OACs treatment requires still further efforts. Clinicians and policy makers should develop more specific educational intervention programs for physicians, to ensure that OACs, especially DOACs, are appropriately prescribed to eligible patients, in particular to vulnerable subgroups by age, socioeconomic status, and presence of comorbid conditions, in order to optimize health resources.
As the burden of disease continues to increase, it remains imperative to implement appropriate use of anticoagulation among AF patients with elevated stroke risk, targeted to local care delivery models, aiming to decrease both the risk of death and potentially preventable cardiovascular events, and associated medical costs for the healthcare systems. We need further studies investigating why OAC treatment in AF patients remains suboptimal, intervening on the relative barriers.
International guidelines extended prescription criteria for oral antithrombotic therapy, in particular for direct oral anticoagulants (DOACs) in atrial fibrillation (AF). However, oral anticoagulant (OAC) prescription is still suboptimal, mainly for DOACs.
Considering the huge clinical impact and healthcare economic burden (in terms of both direct medical costs and indirect productivity losses), there are a number of reasons why it is important to complement experimental data with real-life or observational data, investigating OAC treatment in the real world, and the potential nonadherence to AF treatment guidelines. It is, in fact, important that AF guidelines are followed, as non-adherence to OACs is associated with increased ischemic stroke and mortality in high-risk patients.
We aimed to evaluate temporal prescription patterns of antithrombotic agents in a cohort of patients hospitalized with AF in a Cardiology Department. This should be useful in determining how AF guidelines are followed in the real-world.
This was a retrospective, single-center, observational study conducted in the Cardiology Unit of S. Antonio Abate Hospital of Trapani (Western Sicily, Italy). We reviewed the database of medical records of all patients aged ≥ 18 years who were consecutively discharged from January 2010 to 2021. We collected data on demographic and clinical characteristics, including age and sex, main and secondary diagnosis at discharge, diagnostic and therapeutic procedures, and prescribed antithrombotic treatments from the discharge medication list.
From 2010 to 2021, we showed a significant increase in the proportion of AF patients on OAC therapy, regardless of antiplatelet agent use. The main reason for this increase was due to greater DOACs use, mainly FXa inhibitors. Contextually, VKA use, as well as antiplatelet therapy, alone or in double combination, declined; however, the proportion of patients not receiving any antithrombotic therapy globally decreased.
These findings, in line with findings from other European and global datasets, appear consistent with recent changes in AF management guidelines; this suggests, in Italy, an improvement in adherence to guidelines clinical recommendations. Despite this, we should highlight, however, that OAC prescription remains suboptimal over time; thus, a significant proportion of patients with AF still do not receive appropriate treatments for stroke prevention, suggesting that the increasing use of DOACs is not yet closing the gap between scientific evidence, recommendations from academic guidelines and clinical practice in the general population.
Improving the adherence to AF guideline recommendations for stroke prevention with OAC therapy requires further efforts. Clinicians and policy health makers need to develop more specific educational intervention programs for physicians to ensure that OACs, especially DOACs, are appropriately prescribed to eligible patients, in particular to vulnerable subgroups, in order to optimize health resources. We need further studies investigating why OAC treatment in AF patients remains suboptimal, intervening on the relative barriers.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gupta P, United States; Ong H, Malaysia S-Editor: Liu JH L-Editor: Webster JR P-Editor: Liu JH
| 1. | Ceornodolea AD, Bal R, Severens JL. Epidemiology and Management of Atrial Fibrillation and Stroke: Review of Data from Four European Countries. Stroke Res Treat. 2017;2017:8593207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 2. | Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, Seward JB, Tsang TS. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1763] [Cited by in F6Publishing: 1873] [Article Influence: 104.1] [Reference Citation Analysis (0)] |
| 3. | Berge T, Lyngbakken MN, Ihle-Hansen H, Brynildsen J, Pervez MO, Aagaard EN, Vigen T, Kvisvik B, Christophersen IE, Steine K, Omland T, Smith P, Røsjø H, Tveit A. Prevalence of atrial fibrillation and cardiovascular risk factors in a 63-65 years old general population cohort: the Akershus Cardiac Examination (ACE) 1950 Study. BMJ Open. 2018;8:e021704. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ. Worldwide epidemiology of atrial fibrillation: a Global Burden of Disease 2010 Study. Circulation. 2014;129:837-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2658] [Cited by in F6Publishing: 2973] [Article Influence: 270.3] [Reference Citation Analysis (0)] |
| 5. | Lip GY, Lane DA. Stroke prevention in atrial fibrillation: a systematic review. JAMA. 2015;313:1950-1962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 213] [Cited by in F6Publishing: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 6. | Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370-2375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4468] [Cited by in F6Publishing: 4436] [Article Influence: 192.9] [Reference Citation Analysis (0)] |
| 7. | Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4767] [Cited by in F6Publishing: 4774] [Article Influence: 144.7] [Reference Citation Analysis (0)] |
| 8. | Pandian JD, Gall SL, Kate MP, Silva GS, Akinyemi RO, Ovbiagele BI, Lavados PM, Gandhi DBC, Thrift AG. Prevention of stroke: a global perspective. Lancet. 2018;392:1269-1278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 9. | Rohla M, Weiss TW, Pecen L, Patti G, Siller-Matula JM, Schnabel RB, Schilling R, Kotecha D, Lucerna M, Huber K, De Caterina R, Kirchhof P. Risk factors for thromboembolic and bleeding events in anticoagulated patients with atrial fibrillation: the prospective, multicentre observational PREvention oF thromboembolic events - European Registry in Atrial Fibrillation (PREFER in AF). BMJ Open. 2019;9:e022478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4381] [Cited by in F6Publishing: 4804] [Article Influence: 320.3] [Reference Citation Analysis (0)] |
| 11. | Hannon N, Sheehan O, Kelly L, Marnane M, Merwick A, Moore A, Kyne L, Duggan J, Moroney J, McCormack PM, Daly L, Fitz-Simon N, Harris D, Horgan G, Williams EB, Furie KL, Kelly PJ. Stroke associated with atrial fibrillation--incidence and early outcomes in the north Dublin population stroke study. Cerebrovasc Dis. 2010;29:43-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Patel NJ, Deshmukh A, Pant S, Singh V, Patel N, Arora S, Shah N, Chothani A, Savani GT, Mehta K, Parikh V, Rathod A, Badheka AO, Lafferty J, Kowalski M, Mehta JL, Mitrani RD, Viles-Gonzalez JF, Paydak H. Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation. 2014;129:2371-2379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 311] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 13. | Chen ST, Patel MR. Comparison of Anticoagulant Therapy for Atrial Fibrillation - Novel Oral Anticoagulants Versus Vitamin K Antagonists. Prog Cardiovasc Dis. 2018;60:514-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Han TS, Fry CH, Fluck D, Affley B, Gulli G, Barrett C, Kakar P, Patel T, Sharma S, Sharma P. Anticoagulation therapy in patients with stroke and atrial fibrillation: a registry-based study of acute stroke care in Surrey, UK. BMJ Open. 2018;8:e022558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Yu LJ, Chen S, Xu Y, Zhang ZX. Clinical analysis of antithrombotic treatment and occurrence of stroke in elderly patients with nonvalvular persistent atrial fibrillation. Clin Cardiol. 2018;41:1353-1357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Horiguchi A, Fukaya H, Oikawa J, Shirakawa Y, Kobayashi S, Arakawa Y, Nishinarita R, Nakamura H, Ishizue N, Igarashi G, Satoh A, Kishihara J, Niwano S, Ako J. Real-World Antithrombotic Therapy in Atrial Fibrillation Patients with a History of Percutaneous Coronary Intervention. Int Heart J. 2019;60:1321-1327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Kotalczyk A, Gue YX, Potpara TS, Lip GYH. Current trends in the use of anticoagulant pharmacotherapy in the United Kingdom are changes on the horizon? Expert Opin Pharmacother. 2021;22:1061-1070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | European Heart Rhythm Association; European Association for Cardio-Thoracic Surgery, Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369-2429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3227] [Cited by in F6Publishing: 3267] [Article Influence: 233.4] [Reference Citation Analysis (0)] |
| 19. | Camm AJ, Lip GY, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33:2719-2747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2342] [Cited by in F6Publishing: 2362] [Article Influence: 196.8] [Reference Citation Analysis (0)] |
| 20. | Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4706] [Cited by in F6Publishing: 4664] [Article Influence: 583.0] [Reference Citation Analysis (0)] |
| 21. | Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373-498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3176] [Cited by in F6Publishing: 4761] [Article Influence: 1587.0] [Reference Citation Analysis (0)] |
| 22. | Skanes AC, Healey JS, Cairns JA, Dorian P, Gillis AM, McMurtry MS, Mitchell LB, Verma A, Nattel S; Canadian Cardiovascular Society Atrial Fibrillation Guidelines Committee. Focused 2012 update of the Canadian Cardiovascular Society atrial fibrillation guidelines: recommendations for stroke prevention and rate/rhythm control. Can J Cardiol. 2012;28:125-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 366] [Cited by in F6Publishing: 394] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 23. | National Institute for Health and Clinical Excellence (NICE). Nice Clinical Guideline 180; Atrial Fibrillation: the management of atrial fibrillation. 2014. Available from: https://www.nice.org.uk/guidance/cg180. Accessed March 7th, 2022.. [Cited in This Article: ] |
| 24. | January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199-e267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 784] [Cited by in F6Publishing: 900] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 25. | Writing Group Members; January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, Heidenreich PA, Murray KT, Shea JB, Tracy CM, Yancy CW. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2019;16:e66-e93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 26. | Wilke T, Bauer S, Mueller S, Kohlmann T, Bauersachs R. Patient Preferences for Oral Anticoagulation Therapy in Atrial Fibrillation: A Systematic Literature Review. Patient. 2017;10:17-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Lee SL, Ong TJ, Mazlan-Kepli W, Mageswaran A, Tan KH, Abd-Malek AM, Cronshaw R. Patients' time in therapeutic range on warfarin among atrial fibrillation patients in Warfarin Medication Therapy Adherence Clinic. World J Cardiol. 2021;13:483-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Connolly SJ, Pogue J, Eikelboom J, Flaker G, Commerford P, Franzosi MG, Healey JS, Yusuf S; ACTIVE W Investigators. Benefit of oral anticoagulant over antiplatelet therapy in atrial fibrillation depends on the quality of international normalized ratio control achieved by centers and countries as measured by time in therapeutic range. Circulation. 2008;118:2029-2037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 639] [Cited by in F6Publishing: 677] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 29. | Lauffenburger JC, Farley JF, Gehi AK, Rhoney DH, Brookhart MA, Fang G. Factors driving anticoagulant selection in patients with atrial fibrillation in the United States. Am J Cardiol. 2015;115:1095-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Mekaj YH, Mekaj AY, Duci SB, Miftari EI. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag. 2015;11:967-977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 259] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 31. | Hale ZD, Kong X, Haymart B, Gu X, Kline-Rogers E, Almany S, Kozlowski J, Krol GD, Kaatz S, Froehlich JB, Barnes GD. Prescribing trends of atrial fibrillation patients who switched from warfarin to a direct oral anticoagulant. J Thromb Thrombolysis. 2017;43:283-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139-1151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7917] [Cited by in F6Publishing: 7713] [Article Influence: 514.2] [Reference Citation Analysis (0)] |
| 33. | Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6519] [Cited by in F6Publishing: 6483] [Article Influence: 498.7] [Reference Citation Analysis (2)] |
| 34. | Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L; ARISTOTLE Committees and Investigators. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981-992. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6075] [Cited by in F6Publishing: 6100] [Article Influence: 469.2] [Reference Citation Analysis (0)] |
| 35. | Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, Waldo AL, Ezekowitz MD, Weitz JI, Špinar J, Ruzyllo W, Ruda M, Koretsune Y, Betcher J, Shi M, Grip LT, Patel SP, Patel I, Hanyok JJ, Mercuri M, Antman EM; ENGAGE AF-TIMI 48 Investigators. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093-2104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3447] [Cited by in F6Publishing: 3484] [Article Influence: 316.7] [Reference Citation Analysis (0)] |
| 36. | Ng KH, Hart RG, Eikelboom JW. Anticoagulation in Patients Aged ≥75 years with Atrial Fibrillation: Role of Novel Oral Anticoagulants. Cardiol Ther. 2013;2:135-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 37. | Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3181] [Cited by in F6Publishing: 3296] [Article Influence: 329.6] [Reference Citation Analysis (0)] |
| 38. | Kim IS, Kim HJ, Kim TH, Uhm JS, Joung B, Lee MH, Pak HN. Non-vitamin K antagonist oral anticoagulants have better efficacy and equivalent safety compared to warfarin in elderly patients with atrial fibrillation: A systematic review and meta-analysis. J Cardiol. 2018;72:105-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Li WJ, Archontakis-Barakakis P, Palaiodimos L, Kalaitzoglou D, Tzelves L, Manolopoulos A, Wang YC, Giannopoulos S, Faillace R, Kokkinidis DG. Dabigatran, rivaroxaban, and apixaban are superior to warfarin in Asian patients with non-valvular atrial fibrillation: An updated meta-analysis. World J Cardiol. 2021;13:82-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Camm AJ, Amarenco P, Haas S, Hess S, Kirchhof P, Kuhls S, van Eickels M, Turpie AG; XANTUS Investigators. XANTUS: a real-world, prospective, observational study of patients treated with rivaroxaban for stroke prevention in atrial fibrillation. Eur Heart J. 2016;37:1145-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 321] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 41. | Larsen TB, Skjøth F, Nielsen PB, Kjældgaard JN, Lip GY. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 307] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 42. | Yao X, Abraham NS, Sangaralingham LR, Bellolio MF, McBane RD, Shah ND, Noseworthy PA. Effectiveness and Safety of Dabigatran, Rivaroxaban, and Apixaban Versus Warfarin in Nonvalvular Atrial Fibrillation. J Am Heart Assoc. 2016;5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 242] [Cited by in F6Publishing: 294] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 43. | Guerriero F, Orlando V, Monetti VM, Colaccio FM, Sessa M, Scavone C, Capuano A, Menditto E. Predictors of new oral anticoagulant drug initiation as opposed to warfarin in elderly adults: a retrospective observational study in Southern Italy. Ther Clin Risk Manag. 2018;14:1907-1914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Kirchhof P, Ammentorp B, Darius H, De Caterina R, Le Heuzey JY, Schilling RJ, Schmitt J, Zamorano JL. Management of atrial fibrillation in seven European countries after the publication of the 2010 ESC Guidelines on atrial fibrillation: primary results of the PREvention oF thromboemolic events--European Registry in Atrial Fibrillation (PREFER in AF). Europace. 2014;16:6-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 287] [Cited by in F6Publishing: 303] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 45. | Huisman MV, Ma CS, Diener HC, Dubner SJ, Halperin JL, Rothman KJ, Teutsch C, Schoof N, Kleine E, Bartels DB, Lip GY; GLORIA-AF Investigators. Antithrombotic therapy use in patients with atrial fibrillation before the era of non-vitamin K antagonist oral anticoagulants: the Global Registry on Long-Term Oral Antithrombotic Treatment in Patients with Atrial Fibrillation (GLORIA-AF) Phase I cohort. Europace. 2016;18:1308-1318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 46. | Huisman MV, Rothman KJ, Paquette M, Teutsch C, Diener HC, Dubner SJ, Halperin JL, Ma CS, Zint K, Elsaesser A, Bartels DB, Lip GY; GLORIA-AF Investigators. The Changing Landscape for Stroke Prevention in AF: Findings From the GLORIA-AF Registry Phase 2. J Am Coll Cardiol. 2017;69:777-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 215] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 47. | Ogilvie IM, Newton N, Welner SA, Cowell W, Lip GY. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123:638-645.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 672] [Cited by in F6Publishing: 695] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 48. | Baczek VL, Chen WT, Kluger J, Coleman CI. Predictors of warfarin use in atrial fibrillation in the United States: a systematic review and meta-analysis. BMC Fam Pract. 2012;13:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 49. | Cowan C, Healicon R, Robson I, Long WR, Barrett J, Fay M, Tyndall K, Gale CP. The use of anticoagulants in the management of atrial fibrillation among general practices in England. Heart. 2013;99:1166-1172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 50. | Wilke T, Groth A, Pfannkuche M, Harks O, Fuchs A, Maywald U, Krabbe B. Real life anticoagulation treatment of patients with atrial fibrillation in Germany: extent and causes of anticoagulant under-use. J Thromb Thrombolysis. 2015;40:97-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Lopes RD, Shah BR, Olson DM, Zhao X, Pan W, Bushnell CD, Peterson ED. Antithrombotic therapy use at discharge and 1 year in patients with atrial fibrillation and acute stroke: results from the AVAIL Registry. Stroke. 2011;42:3477-3483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Monelli M, Molteni M, Cassetti G, Bagnara L, De Grazia V, Zingale L, Zilli F, Bussotti M, Totaro P, De Maria B, Dalla Vecchia LA. Non-vitamin K oral anticoagulant use in the elderly: a prospective real-world study - data from the REGIstry of patients on Non-vitamin K oral Anticoagulants (REGINA). Vasc Health Risk Manag. 2019;15:19-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Kodani E, Atarashi H, Inoue H, Okumura K, Yamashita T, Origasa H; J-RHYTHM Registry Investigators. Use of warfarin in elderly patients with non-valvular atrial fibrillation -- subanalysis of the J-RHYTHM Registry. Circ J. 2015;79:2345-2352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Sholzberg M, Gomes T, Juurlink DN, Yao Z, Mamdani MM, Laupacis A. The Influence of Socioeconomic Status on Selection of Anticoagulation for Atrial Fibrillation. PLoS One. 2016;11:e0149142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Dalmau Llorca MR, Aguilar Martín C, Carrasco-Querol N, Hernández Rojas Z, Forcadell Drago E, Rodríguez Cumplido D, Castro Blanco E, Pepió Vilaubí JM, Gonçalves AQ, Fernández-Sáez J. Gender and Socioeconomic Inequality in the Prescription of Direct Oral Anticoagulants in Patients with Non-Valvular Atrial Fibrillation in Primary Care in Catalonia (Fantas-TIC Study). Int J Environ Res Public Health. 2021;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Lunde ED, Joensen AM, Fonager K, Lundbye-Christensen S, Johnsen SP, Larsen ML, Lip GYH, Riahi S. Socioeconomic inequality in oral anticoagulation therapy initiation in patients with atrial fibrillation with high risk of stroke: a register-based observational study. BMJ Open. 2021;11:e048839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 57. | García-Sempere A, Bejarano-Quisoboni D, Librero J, Rodríguez-Bernal CL, Peiró S, Sanfélix-Gimeno G. A Multilevel Analysis of Real-World Variations in Oral Anticoagulation Initiation for Atrial Fibrillation in Valencia, a European Region. Front Pharmacol. 2017;8:576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Esposti LD, Briere JB, Bowrin K, Diego S, Perrone V, Pasquale GD. Antithrombotic treatment patterns in patients with atrial fibrillation in Italy pre- and post-DOACs: the REPAIR study. Future Cardiol. 2019;15:109-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 59. | Apenteng PN, Gao H, Hobbs FR, Fitzmaurice DA; UK GARFIELD-AF Investigators and GARFIELD-AF Steering Committee. Temporal trends in antithrombotic treatment of real-world UK patients with newly diagnosed atrial fibrillation: findings from the GARFIELD-AF registry. BMJ Open. 2018;8:e018905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 60. | Durham TA, Hassmiller Lich K, Viera AJ, Fine JP, Mukherjee J, Weinberger M, Dusetzina SB. Utilization of Standard and Target-Specific Oral Anticoagulants Among Adults in the United Kingdom With Incident Atrial Fibrillation. Am J Cardiol. 2017;120:1820-1829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Deambrosis P, Bettiol A, Bolcato J, Pirolo R, Franchin G, Themistoclakis S, Pellizzari M, Chinellato A, Giusti P. Real-practice thromboprophylaxis in atrial fibrillation. Acta Pharm. 2017;67:227-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Renda G, Patti G, Sangiuolo R, Attena E, Malpezzi MG, De Caterina R; a nome dello Steering Committee del Registro Europeo PREFER in AF. [Management of thromboembolic risk in patients with atrial fibrillation in Italy: follow-up data from the PREFER in AF European Registry]. G Ital Cardiol (Rome). 2016;17:922-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 63. | Ermini G, Perrone V, Veronesi C, Degli Esposti L, Di Pasquale G. Antithrombotic prophylaxis of atrial fibrillation in an Italian real-world setting: a retrospective study. Vasc Health Risk Manag. 2017;13:239-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Gu HQ, Yang X, Wang CJ, Zhao XQ, Wang YL, Liu LP, Meng X, Jiang Y, Li H, Liu C, Xiong YY, Fonarow GC, Wang D, Xian Y, Li ZX, Wang YJ. Assessment of Trends in Guideline-Based Oral Anticoagulant Prescription for Patients With Ischemic Stroke and Atrial Fibrillation in China. JAMA Netw Open. 2021;4:e2118816. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 65. | Yu AYX, Malo S, Svenson LW, Wilton SB, Hill MD. Temporal Trends in the Use and Comparative Effectiveness of Direct Oral Anticoagulant Agents Versus Warfarin for Nonvalvular Atrial Fibrillation: A Canadian Population-Based Study. J Am Heart Assoc. 2017;6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 66. | Kováčová B, Červeňová J. Direct oral anticoagulants in real clinical practice: analysis of patient characteristics and prescribing patterns in a large teaching hospital. Pharmazie. 2017;72:555-560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 67. | Mai L, Wu Y, Luo J, Liu X, Zhu H, Zheng H, Liang G, Zhang Y, Huang Y. A retrospective cohort study of oral anticoagulant treatment in patients with acute coronary syndrome and atrial fibrillation. BMJ Open. 2019;9:e031180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 68. | Gorczyca I, Jelonek O, Michalska A, Chrapek M, Wałek P, Wożakowska-Kapłon B. Stroke prevention and guideline adherent antithrombotic treatment in elderly patients with atrial fibrillation: A real-world experience. Medicine (Baltimore). 2020;99:e21209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Alam M, Bandeali SJ, Shahzad SA, Lakkis N. Real-life global survey evaluating patients with atrial fibrillation (REALISE-AF): results of an international observational registry. Expert Rev Cardiovasc Ther. 2012;10:283-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 70. | Camm AJ, Accetta G, Ambrosio G, Atar D, Bassand JP, Berge E, Cools F, Fitzmaurice DA, Goldhaber SZ, Goto S, Haas S, Kayani G, Koretsune Y, Mantovani LG, Misselwitz F, Oh S, Turpie AG, Verheugt FW, Kakkar AK; GARFIELD-AF Investigators. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart. 2017;103:307-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 71. | Steinberg BA, Gao H, Shrader P, Pieper K, Thomas L, Camm AJ, Ezekowitz MD, Fonarow GC, Gersh BJ, Goldhaber S, Haas S, Hacke W, Kowey PR, Ansell J, Mahaffey KW, Naccarelli G, Reiffel JA, Turpie A, Verheugt F, Piccini JP, Kakkar A, Peterson ED, Fox KAA; GARFIELD-AF; ORBIT-AF Investigators. International trends in clinical characteristics and oral anticoagulation treatment for patients with atrial fibrillation: Results from the GARFIELD-AF, ORBIT-AF I, and ORBIT-AF II registries. Am Heart J. 2017;194:132-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 72. | Haas S, Camm AJ, Bassand JP, Angchaisuksiri P, Cools F, Corbalan R, Gibbs H, Jacobson B, Koretsune Y, Mantovani LG, Misselwitz F, Panchenko E, Ragy HI, Stepinska J, Turpie AG, Sawhney JP, Steffel J, Lim TW, Pieper KS, Virdone S, Verheugt FW, Kakkar AK; GARFIELD-AF Investigators. Predictors of NOAC versus VKA use for stroke prevention in patients with newly diagnosed atrial fibrillation: Results from GARFIELD-AF. Am Heart J. 2019;213:35-46. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 73. | Nieuwlaat R, Capucci A, Lip GY, Olsson SB, Prins MH, Nieman FH, López-Sendón J, Vardas PE, Aliot E, Santini M, Crijns HJ; Euro Heart Survey Investigators. Antithrombotic treatment in real-life atrial fibrillation patients: a report from the Euro Heart Survey on Atrial Fibrillation. Eur Heart J. 2006;27:3018-3026. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 74. | Lip GY, Laroche C, Dan GA, Santini M, Kalarus Z, Rasmussen LH, Ioachim PM, Tica O, Boriani G, Cimaglia P, Diemberger I, Hellum CF, Mortensen B, Maggioni AP. 'Real-world' antithrombotic treatment in atrial fibrillation: The EORP-AF pilot survey. Am J Med. 2014;127:519-29.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 75. | Lip GY, Laroche C, Ioachim PM, Rasmussen LH, Vitali-Serdoz L, Petrescu L, Darabantiu D, Crijns HJ, Kirchhof P, Vardas P, Tavazzi L, Maggioni AP, Boriani G. Prognosis and treatment of atrial fibrillation patients by European cardiologists: one year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase (EORP-AF Pilot registry). Eur Heart J. 2014;35:3365-3376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 177] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 76. | Proietti M, Laroche C, Opolski G, Maggioni AP, Boriani G, Lip GYH; AF Gen Pilot Investigators. 'Real-world' atrial fibrillation management in Europe: observations from the 2-year follow-up of the EURObservational Research Programme-Atrial Fibrillation General Registry Pilot Phase. Europace. 2017;19:722-733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 77. | Chan PS, Maddox TM, Tang F, Spinler S, Spertus JA. Practice-level variation in warfarin use among outpatients with atrial fibrillation (from the NCDR PINNACLE program). Am J Cardiol. 2011;108:1136-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 78. | O'Brien EC, Holmes DN, Ansell JE, Allen LA, Hylek E, Kowey PR, Gersh BJ, Fonarow GC, Koller CR, Ezekowitz MD, Mahaffey KW, Chang P, Peterson ED, Piccini JP, Singer DE. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J. 2014;167:601-609.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 79. | Ten Cate V, Ten Cate H, Verheugt FW. The Global Anticoagulant Registry in the FIELD-Atrial Fibrillation (GARFIELD-AF) : Exploring the changes in anticoagulant practice in patients with non-valvular atrial fibrillation in the Netherlands. Neth Heart J. 2016;24:574-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 80. | Seelig J, Verheugt FWA, Hemels MEW, Illingworth L, Lucassen A, Adriaansen H, Bongaerts MCM, Pieterse M, Herrman JPR, Hoogslag P, Hermans W, Groenemeijer BE, Boersma LVA, Pieper K, Ten Cate H; GARFIELD-AF Investigators. Changes in anticoagulant prescription in Dutch patients with recent-onset atrial fibrillation: observations from the GARFIELD-AF registry. Thromb J. 2020;18:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 81. | Blacher J, Sorbets E, Guedj Meynier D, Huberman JP, Gauthier J, Cohen S, Hoffman O. Determinants of Antithrombotic Treatment for Atrial Fibrillation in Octogenarians: Results of the OCTOFA Study. Clin Drug Investig. 2019;39:891-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Haeusler KG, Gerth A, Limbourg T, Tebbe U, Oeff M, Wegscheider K, Treszl A, Ravens U, Meinertz T, Kirchhof P, Breithardt G, Steinbeck G, Nabauer M; AFNET registry investigators. Use of vitamin K antagonists for secondary stroke prevention depends on the treating healthcare provider in Germany - results from the German AFNET registry. BMC Neurol. 2015;15:129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Di Pasquale G, Mathieu G, Maggioni AP, Fabbri G, Lucci D, Vescovo G, Pirelli S, Chiarella F, Scherillo M, Gulizia MM, Gussoni G, Colombo F, Panuccio D, Nozzoli C, Berisso MZ; ATA-AF Investigators. Current presentation and management of 7148 patients with atrial fibrillation in cardiology and internal medicine hospital centers: the ATA AF study. Int J Cardiol. 2013;167:2895-2903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Basaran O, Filiz Basaran N, Cekic EG, Altun I, Dogan V, Mert GO, Mert KU, Akin F, Soylu MO, Memic Sancar K, Biteker M. PRescriptiOn PattERns of Oral Anticoagulants in Nonvalvular Atrial Fibrillation (PROPER study). Clin Appl Thromb Hemost. 2017;23:384-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 85. | Biteker M, Başaran Ö, Doğan V, Altun İ, Özpamuk Karadeniz F, Tekkesin Aİ, Çakıllı Y, Türkkan C, Hamidi M, Demir V, Gürsoy MO, Tek Öztürk M, Aksan G, Seyis S, Ballı M, Alıcı MH, Bozyel S. Real-World Clinical Characteristics and Treatment Patterns of Individuals Aged 80 and Older with Nonvalvular Atrial Fibrillation: Results from the ReAl-life Multicenter Survey Evaluating Stroke Study. J Am Geriatr Soc. 2017;65:1684-1690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Koretsune Y, Yamashita T, Akao M, Atarashi H, Ikeda T, Okumura K, Shimizu W, Tsutsui H, Toyoda K, Hirayama A, Yasaka M, Yamaguchi T, Teramukai S, Kimura T, Kaburagi J, Takita A, Inoue H. Baseline Demographics and Clinical Characteristics in the All Nippon AF in the Elderly (ANAFIE) Registry. Circ J. 2019;83:1538-1545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 87. | Hiasa KI, Kaku H, Inoue H, Yamashita T, Akao M, Atarashi H, Koretsune Y, Okumura K, Shimizu W, Ikeda T, Toyoda K, Hirayama A, Yasaka M, Yamaguchi T, Teramukai S, Kimura T, Kaburagi J, Takita A, Tsutsui H. Age-Related Differences in the Clinical Characteristics and Treatment of Elderly Patients With Atrial Fibrillation in Japan - Insight From the ANAFIE (All Nippon AF In Elderly) Registry. Circ J. 2020;84:388-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Yasaka M, Yamashita T, Akao M, Atarashi H, Ikeda T, Koretsune Y, Okumura K, Shimizu W, Tsutsui H, Toyoda K, Hirayama A, Yamaguchi T, Teramukai S, Kimura T, Kaburagi J, Takita A, Inoue H. Background characteristics and anticoagulant usage patterns of elderly non-valvular atrial fibrillation patients in the ANAFIE registry: a prospective, multicentre, observational cohort study in Japan. BMJ Open. 2021;11:e044501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 89. | Yamashita Y, Uozumi R, Hamatani Y, Esato M, Chun YH, Tsuji H, Wada H, Hasegawa K, Ogawa H, Abe M, Morita S, Akao M. Current Status and Outcomes of Direct Oral Anticoagulant Use in Real-World Atrial Fibrillation Patients - Fushimi AF Registry. Circ J. 2017;81:1278-1285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 90. | Kim H, Kim TH, Cha MJ, Lee JM, Park J, Park JK, Kang KW, Shim J, Uhm JS, Kim J, Park HW, Choi EK, Kim JB, Kim C, Lee YS, Joung B. A Prospective Survey of Atrial Fibrillation Management for Real-world Guideline Adherence: COmparison study of Drugs for symptom control and complication prEvention of Atrial Fibrillation (CODE-AF) Registry. Korean Circ J. 2017;47:877-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 91. | Lee H, Kim TH, Baek YS, Uhm JS, Pak HN, Lee MH, Joung B. The Trends of Atrial Fibrillation-Related Hospital Visit and Cost, Treatment Pattern and Mortality in Korea: 10-Year Nationwide Sample Cohort Data. Korean Circ J. 2017;47:56-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 92. | Dai Y, Yang J, Gao Z, Xu H, Sun Y, Wu Y, Gao X, Li W, Wang Y, Gao R, Yang Y; CAMI Registry study group. Atrial fibrillation in patients hospitalized with acute myocardial infarction: analysis of the china acute myocardial infarction (CAMI) registry. BMC Cardiovasc Disord. 2017;17:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 93. | Li YM, Jiang C, He L, Li XX, Hou XX, Chang SS, Lip GYH, Du X, Dong JZ, Ma CS. Sex Differences in Presentation, Quality of Life, and Treatment in Chinese Atrial Fibrillation Patients: Insights from the China Atrial Fibrillation Registry Study. Med Sci Monit. 2019;25:8011-8018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 94. | Averlant L, Ficheur G, Ferret L, Boulé S, Puisieux F, Luyckx M, Soula J, Georges A, Beuscart R, Chazard E, Beuscart JB. Underuse of Oral Anticoagulants and Inappropriate Prescription of Antiplatelet Therapy in Older Inpatients with Atrial Fibrillation. Drugs Aging. 2017;34:701-710. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 95. | Hart RG, Pearce LA, Asinger RW, Herzog CA. Warfarin in atrial fibrillation patients with moderate chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2599-2604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 160] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 96. | Spillane S, Bennett K, Barry M. Initiation of Oral Anticoagulant Drugs: Identification of Drivers of Prescribing of New Agents Versus Warfarin. Value Health. 2014;17:A499-A500. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 97. | Kattoor AJ, Pothineni NV, Goel A, Syed M, Syed S, Paydak H, Mehta JL. Prescription Patterns and Outcomes of Patients With Atrial Fibrillation Treated With Direct Oral Anticoagulants and Warfarin: A Real-World Analysis. J Cardiovasc Pharmacol Ther. 2019;24:428-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Alamneh EA, Chalmers L, Bereznicki LR. Suboptimal Use of Oral Anticoagulants in Atrial Fibrillation: Has the Introduction of Direct Oral Anticoagulants Improved Prescribing Practices? Am J Cardiovasc Drugs. 2016;16:183-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 99. | Song HY, Son KB, Shin JY, Bae S. Utilization of oral anticoagulants in Korean nonvalvular atrial fibrillation patients. Int J Clin Pharm. 2019;41:1434-1441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 100. | Sussman M, Barnes GD, Guo JD, Tao CY, Gillespie JA, Ferri M, Adair N, Cato MS, Shirkhorshidian I, Di Fusco M. The burden of undertreatment and non-treatment among patients with non-valvular atrial fibrillation and elevated stroke risk: a systematic review. Curr Med Res Opin. 2022;38:7-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 101. | Zhao S, Hong X, Cai H, Liu M, Li B, Ma P. Antithrombotic Management for Atrial Fibrillation Patients Undergoing Percutaneous Coronary Intervention or With Acute Coronary Syndrome: An Evidence-Based Update. Front Cardiovasc Med. 2021;8:660986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 102. | Sindet-Pedersen C, Staerk L, Lamberts M, Gerds TA, Berger JS, Nissen Bonde A, Langtved Pallisgaard J, Hansen ML, Torp-Pedersen C, Gislason GH, Bjerring Olesen J. Use of oral anticoagulants in combination with antiplatelet(s) in atrial fibrillation. Heart. 2018;104:912-920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 103. | Mazzaglia G, Filippi A, Alacqua M, Cowell W, Shakespeare A, Mantovani LG, Bianchi C, Cricelli C. A national survey of the management of atrial fibrillation with antithrombotic drugs in Italian primary care. Thromb Haemost. 2010;103:968-975. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 104. | Urbaniak AM, Strøm BO, Krontveit R, Svanqvist KH. Prescription Patterns of Non-Vitamin K Oral Anticoagulants Across Indications and Factors Associated with Their Increased Prescribing in Atrial Fibrillation Between 2012-2015: A Study from the Norwegian Prescription Database. Drugs Aging. 2017;34:635-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 105. | Essien UR, Magnani JW, Chen N, Gellad WF, Fine MJ, Hernandez I. Race/Ethnicity and Sex-Related Differences in Direct Oral Anticoagulant Initiation in Newly Diagnosed Atrial Fibrillation: A Retrospective Study of Medicare Data. J Natl Med Assoc. 2020;112:103-108. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 106. | Patel NJ, Patel A, Agnihotri K, Pau D, Patel S, Thakkar B, Nalluri N, Asti D, Kanotra R, Kadavath S, Arora S, Patel N, Sheikh A, Badheka AO, Deshmukh A, Paydak H, Viles-Gonzalez J. Prognostic impact of atrial fibrillation on clinical outcomes of acute coronary syndromes, heart failure and chronic kidney disease. World J Cardiol. 2015;7:397-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 107. | Gigli L, Ameri P, Secco G, De Blasi G, Miceli R, Lorenzoni A, Torre F, Chiarella F, Brunelli C, Canepa M. Clinical characteristics and prognostic impact of atrial fibrillation in patients with chronic heart failure. World J Cardiol. 2016;8:647-656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 108. | Jortveit J, Sandberg EL, Pripp AH, Halvorsen S. Time trends in adherence to guideline recommendations for anticoagulation therapy in patients with atrial fibrillation and myocardial infarction. Open Heart. 2022;9. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 109. | Gorczyca-Głowacka I, Bielecka B, Wałek P, Chrapek M, Ciba-Stemplewska A, Jelonek O, Kot A, Czyżyk A, Pióro M, Major A, Wożakowska-Kapłon B. Temporal Trends in Oral Anticoagulant Prescription in Atrial Fibrillation Patients between 2004 and 2019. Int J Environ Res Public Health. 2022;19. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 110. | Bayer V, Kotalczyk A, Kea B, Teutsch C, Larsen P, Button D, Huisman MV, Lip GYH, Olshansky B. Global Oral Anticoagulation Use Varies by Region in Patients With Recent Diagnosis of Atrial Fibrillation: The GLORIA-AF Phase III Registry. J Am Heart Assoc. 2022;11:e023907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 10] [Reference Citation Analysis (0)] |
| 111. | Xia X, Wang L, Lin T, Yue J, Yang Z, Mi C, Liao Z, Chen Y, Ge N, Wu C. Barriers to prescribing oral anticoagulants to inpatients aged 80 years and older with nonvalvular atrial fibrillation: a cross-sectional study. BMC Geriatr. 2022;22:263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |