Published online Jan 26, 2020. doi: 10.4330/wjc.v12.i1.44
Peer-review started: July 17, 2019
First decision: August 20, 2019
Revised: November 15, 2019
Accepted: November 25, 2019
Article in press: November 25, 2019
Published online: January 26, 2020
ST-elevation myocardial infarction (STEMI) remains a major cause of mortality despite early revascularization and optimal medical therapy. Tailoring individual management by considering patients’ specificities may help in improving post-STEMI survival.
To evaluate whether in-hospital bleeding complications may be involved in post STEMI prognosis among overweight patients.
We prospectively included 2070 patients with a STEMI between January 2005 and December 2012 in the French observational cohort, “Registre d’Infarctus Maine-Anjou”. Bleeding Academic Research Consortium (BARC) in-hospital bleeding complications were recorded.
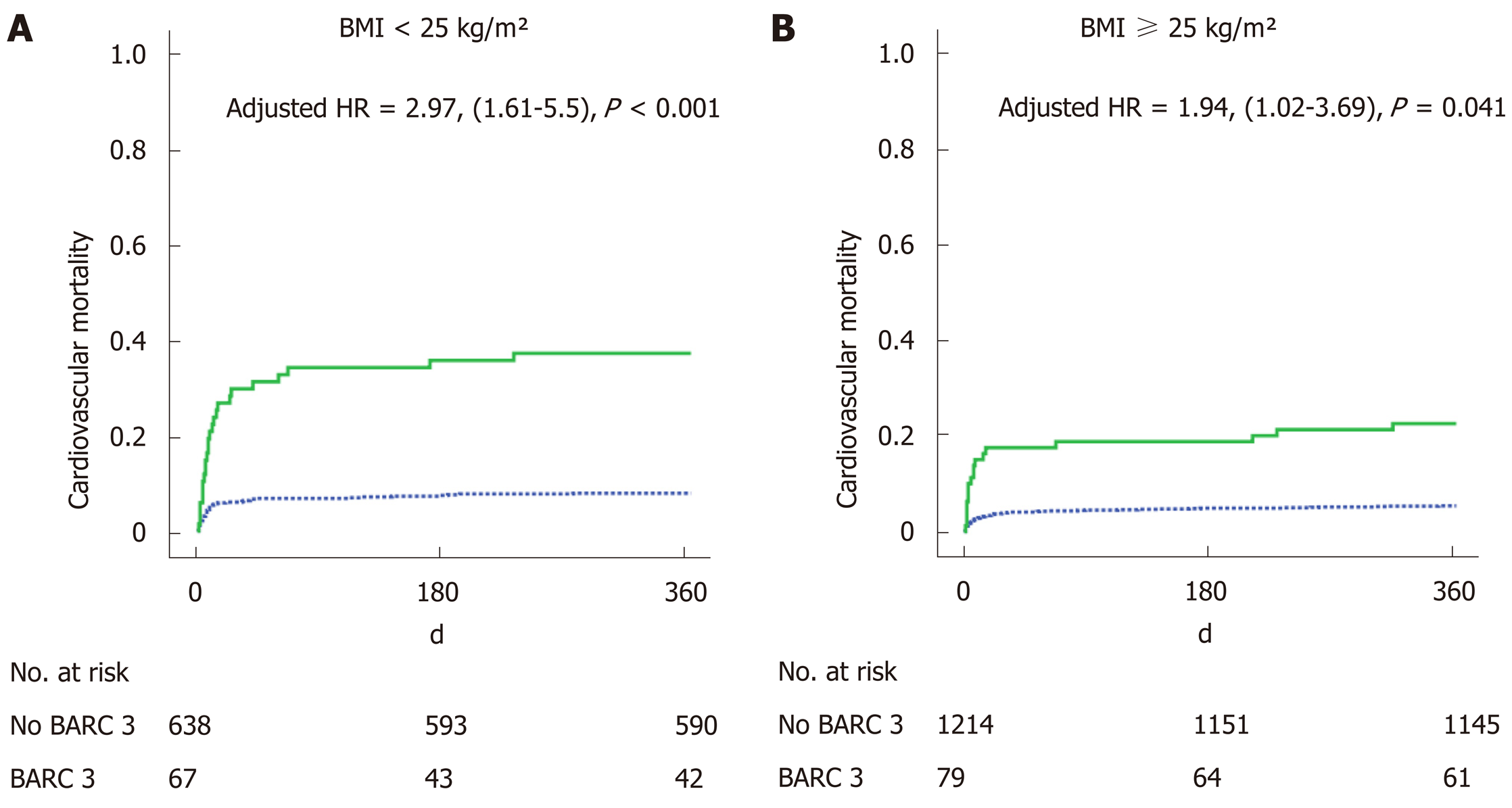
Of 705 patients (35.3%) were presented as being of normal weight, defined as a body mass index (BMI) < 25 kg/m², 877 (43.9%) had a BMI between 25 and 30 kg/m² and 416 (20.8%) had a BMI ≥ 30 kg/m². One-year cardiovascular mortality was lower for BMI ≥ 25 kg/m² (5.3% and 7.1%) patients than for normal weight patients (10.8%) (P = 0.001). We found an interaction between the effect of BARC 3 on mortality and BMI groups. While a BARC 3 was related to a higher 1-year mortality in general (HR: 2.58, 95%CI: 1.44-4.64, P ≤ 0.001), prognosis was even worse in normal weight patients (HR: 2.97, 95%CI: 1.61-5.5, P < 0.001) than for patients with a BMI ≥ 25 kg/m² (HR: 1.94, 95%CI: 1.02-3.69, P = 0.041).
Normal weight patients presented higher rates of in-hospital bleeding complications and lower survival after a STEMI. Excess mortality might be due to greater vulnerability to bleeding amongst normal weight patients.
Core tip: There was an obesity paradox, with body mass index (BMI) ≥ 25 kg/m² ST-elevation myocardial infarction patients presenting better survival. Normal weight patients presented more in-hospital bleeding than others. In-hospital bleeding was related to 1-year cardiovascular mortality. Presenting a normal BMI increased the effect of bleeding on mortality.
- Citation: Ingremeau D, Grall S, Valliet F, Desprets L, Prunier F, Furber A, Bière L. Prognostic impact of body mass index on in-hospital bleeding complications after ST-segment elevation myocardial infarction. World J Cardiol 2020; 12(1): 44-54
- URL: https://www.wjgnet.com/1949-8462/full/v12/i1/44.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i1.44
The prevalence of overweight people in France [Body Mass Index (BMI) between 25 and 30 kg/m²] in 2012 was 32% and obesity rates (BMI greater than 30 kg/m²) were at 15% according to the “National Epidemiological Study on excess weight and obesity” (ObÉpi)[1]. In the United States of America, the obesity rate more than doubled from 15% in 1980 to 34% in 2006[2]. It is an independent risk factor for cardiovascular (CV) disease, conducive to the occurrence of ST-segment elevation myocardial infarction (STEMI)[3].
Yet, increasingly, studies also suggest that obesity might also have a protective role in some chronic diseases once they are established, including coronary artery disease. Recent studies have demonstrated an “obesity paradox” after percutaneous coronary intervention, whereby overweight and obese patients seem to have better outcomes than normal weight individuals[4]. There are multiple explanations. Obese patients may be more likely to receive full-dose guideline-based medical therapy during hospital admission and at discharge[4]. They present greater coronary diameters[5]. Thin patients may present higher rates of comorbidities such as cancer[6], but also a higher rate of bleeding[7].
In parallel, the unfavourable impact on the prognosis of bleeding complications after STEMI was recently highlighted in various studies. A meta-analysis based on 133597 patients presenting an acute coronary syndrome pointed to major bleeding as a strong predictor of in-hospital or 30-d death and acute myocardial infarction[8]. Evaluating the relationship between bleeding events and BMI could explain the obesity paradox[7]. The objective of this study is to evaluate the impact of BMI on the occurrence of bleeding complications and its further relationship with prognosis after a STEMI.
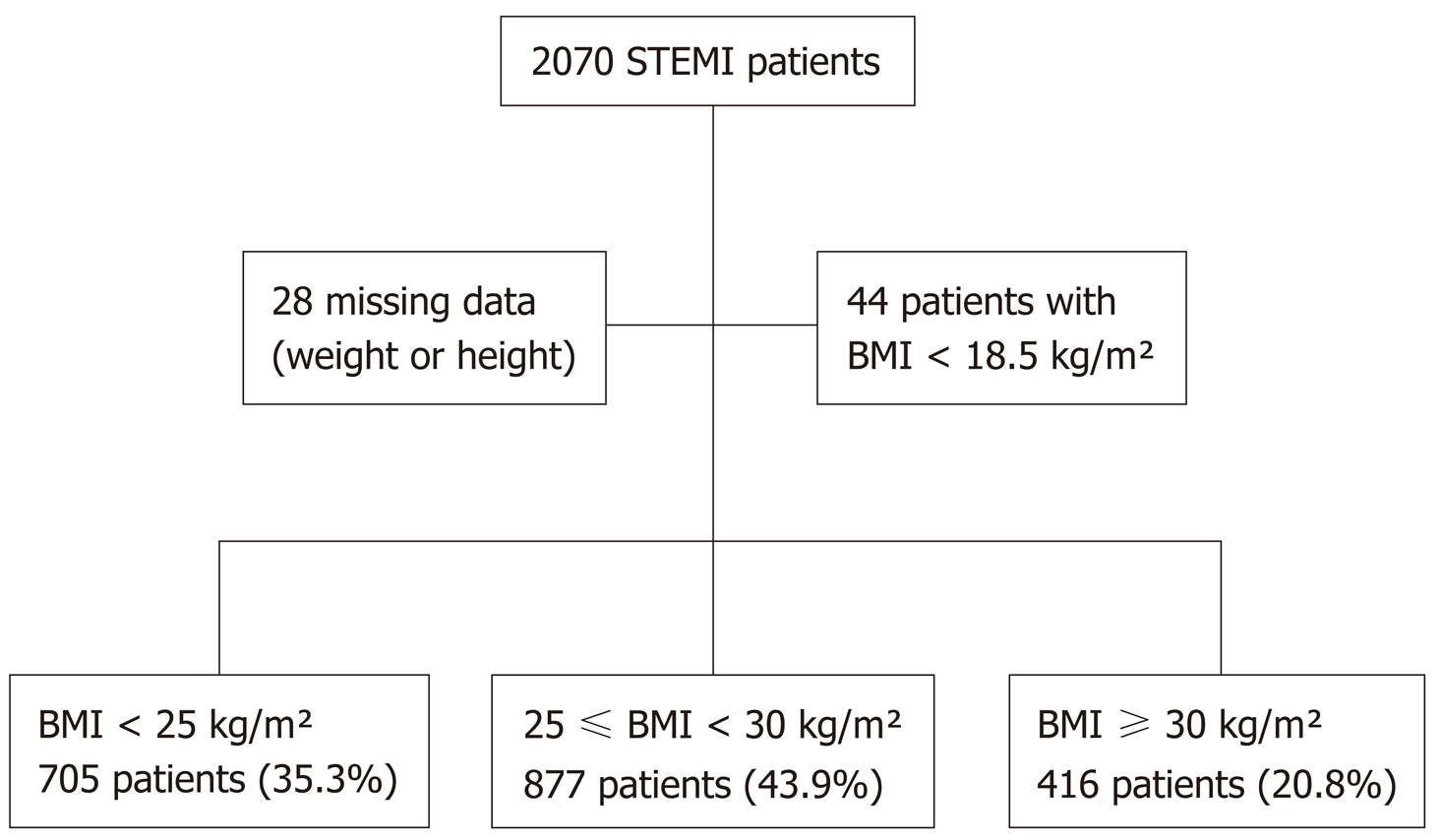
We studied 2070 patients consecutively from the “Registre d’Infarctus Maine-Anjou” (RIMA) survey[9]. We prospectively included all patients presenting with a STEMI in a Western region of France, in which the only available 24 h-7 d coronary angiography service was in Angers University Hospital. Patients were recruited between January 2005 and December 2012. Comparisons were carried out between 3 groups: Normal weight (BMI < 25 kg/m²) (n = 705, 35.3%), overweight (25 kg/m² ≤ BMI < 30 kg/m²) (n = 877, 43.9%), and obese (BMI ≥ 30 kg/m²) (n = 416, 20.8%) (Figure 1).
Patients with missing weight or height data (n = 28), or a BMI < 18.5 kg/m² (n = 44) were not considered for analysis. This study was carried out in accordance with the Declaration of Helsinki and the protocol was approved by the ethics committee of Angers University Hospital.
STEMI was defined as the presence of symptoms attributed to myocardial ischemia for at least 30 min accompanied with ST-segment elevation of ≥ 1 mm in at least two contiguous limb leads or ≥ 2 mm in precordial leads, or new or undetermined left bundle-branch block, as well as elevation of cardiac biomarkers[10].
In-hospital bleeding complications were defined according to the Bleeding Academic Research Consortium (BARC)[11] bleeding classification. The BARC classification identified STEMI patients at risk of 1-year mortality[12]. We considered type 3 and 5 for analysis: BARC 3a was defined as haemorrhage (haematoma ≥ 4 cm at the site of vascular puncture, or gastrointestinal blood loss, or retroperitoneal bleeding verified by either ultrasound or computed tomography imaging, or intracranial bleeding[10]) plus haemoglobin drop equal to between 3 g/dL and < 5 g/dL or any transfusion with bleeding; BARC 3b was defined as haemorrhage plus haemoglobin drop ≥ 5g/dL, cardiac tamponade, haemorrhage requiring surgical intervention for control (excluding dental/nasal/skin/haemorrhoids) or haemorrhage requiring intravenous vasoactive agents; BARC 3c was defined as intracranial haemorrhage; BARC 5 bleeding was defined as fatal haemorrhage[12].
Data on demographics (age, sex, BMI), CV risk factors (smoker, diabetes history, hypertension, dyslipidaemia, family history of premature coronary vascular disease[13], obstructive sleep apnoea), medical history (prior MI, stroke, peripheral vascular disease, renal failure, cancer) and admission characteristics (systolic blood pressure) were prospectively recorded for each patient at admission. The CRUSADE[14] and HEMORR2HAGES[15] bleeding risk scores were calculated based on the patient’s initial characteristics.
We collected data concerning acute stage management, including thrombolytic use, time from symptom onset to first medical contact or to admission in the coronary angiography room, coronary angiogram findings [infarct location, vessel disease, puncture access, duration of the procedure and final thrombolysis in myocardial infarction (TIMI) flow], and the medical therapy given before and in the first 24 h after the first medical contact (antiplatelet therapy, antithrombotic therapy). Anticoagulants and their doses were mandated by a single protocol (see supplementary file). During the hospital stay, an echocardiography was obtained for the evaluation of left ventricular ejection fraction (LVEF) by the biplane Simpson method. Blood samples were collected to measure serum haemoglobin at admission and during hospitalisation to determine the haemoglobin drop, haematocrit and creatine phosphokinase peak. In-hospital complications were examined by two physicians and included death, bleeding event, transfusion, necrosis recurrence, heart failure and stroke. The 1-year follow-up for survival was available for all patients who were initially included.
Patient characteristics, in-hospital and 1-year events were compared relating to the BMI group. Continuous variables are expressed as the median (interquartile range). Continuous variables were analysed using the Mann-Whitney U-test for 2-group comparisons and the Kruskal-Wallis test for 3-group comparisons. Qualitative variables were expressed as frequencies and percentages. The Pearson χ² test or Fisher’s exact test were used to carry out comparisons between qualitative variables and in-hospital event rates.
Binomial logistic regression was carried out to determine correlates of in-hospital BARC 3 or 5 bleeding. The variables entered into the model were gender, age, history of hypertension, BMI, smoking, renal failure, puncture access, duration of the procedure, number of diseased vessel(s), final TIMI flow, creatine phosphokinase peak, LVEF and pre-use of antivitamin K. Interactions between BMI and procedure duration and puncture access were evaluated.
Univariate Cox analysis was made to identify predictors of 1-year CV mortality. Overweight and obese patients were grouped for analysis, and variables demonstrating a P < 0.05 were included in an exploratory Cox proportional hazards model to assess 1-year CV mortality. Interactions between the effect of BMI groups and independent variables on mortality (BARC 3, in-hospital heart failure, history of cancer, stroke) were calculated and included in the multivariate model when significant. P < 0.05 was considered significant. Statistical tests were performed using SPSS software (Version 17; SPSS, Inc., Illinois, United States).
Obese patients were younger than overweight and normal weight patients [respectively 61 (51, 74) years old vs 64 (53, 76) and 67 (53, 79)]. They presented more CV risk factors such as hypertension, diabetes, dyslipidaemia and obstructive sleep apnoea. The main characteristics are summarised in Table 1. There was no difference in medical history and infarct characteristics among the three groups. There was less use of femoral access for obese patients (51.5%) than for the other groups (60.1% and 59.5%) (P = 0.021).
| Total | BMI < 25 kg/m² | 25 ≤ BMI < 30 kg/m² | BMI ≥ 30 kg/m² | P value | |
| n = 1998 | n = 705 | n = 877 | n = 416 | ||
| Cardiovascular risk factors | |||||
| Age (yr) | 64 (53-77) | 67 (53-79)ae | 64 (53-76)ac | 61 (51-74)ce | < 0.001 |
| Male | 1485 (74.3) | 493 (69.6)a | 693 (79.0)ac | 302 (72.6)c | < 0.001 |
| Hypertension | 986 (49.3) | 308 (43.6)e | 422 (48.2)c | 257 (61.9)ce | < 0.001 |
| Diabetes mellitus | 492 (24.6) | 134 (19.1)ae | 211 (24.3)ac | 147 (35.6)ce | < 0.001 |
| Dyslipidaemia | 1001 (50.1) | 323 (45.8)ae | 442 (50.8)ac | 236 (57.0)ce | 0.001 |
| Smoker | 708 (35.4) | 261 (37)ae | 299 (34.2)a | 148 (35.8)e | 0.006 |
| Family history of coronary artery disease | 442 (22.1) | 134 (19.1)ae | 210 (24.4)a | 98 (24.0)e | 0.032 |
| Prior myocardial infarction | 171 (8.6) | 53 (7.5) | 83 (9.5) | 35 (8.5) | 0.36 |
| Obstructive sleep apnoea | 22 (1.1) | 0 (0.0)ae | 10 (1.5)ac | 12 (3.7)ce | < 0.001 |
| History | |||||
| Stroke | 86 (4.3) | 34 (4.8) | 37 (4.2) | 15 (3.6) | 0.4 |
| Renal failure | 59 (3.0) | 21 (3.0) | 29 (3.3) | 9 (2.2) | 0.52 |
| Cancer | 158 (7.9) | 61 (8.7) | 71 (8.1) | 27 (6.5) | 0.41 |
| Clinical presentation | |||||
| Systolic blood pressure (mmHg) | 139 (120-159) | 135 (116-155)ae | 140 (120-160)ac | 141 (127-162)ce | < 0.001 |
| HEMORR2HAGES score | 1 (0-2) | 1 (0-2) | 1 (0-2) | 1 (0-2) | 0.09 |
| CRUSADE score | 24 (14-37) | 29 (18-42)ae | 21 (12-33)ac | 18 (9-33)ce | < 0.001 |
| Infarct characteristics | |||||
| Time from symptoms to first medical contact (h) | 2.25 (1-5.5) | 2.3 (1.2-5.7) | 2 (1-4)c | 2 (1-6)c | 0.024 |
| Time from symptoms to admission (h) | 3 (1.9-6.5) | 3.1 (2-6.3) | 2 (1-6)c | 3 (2-7)c | 0.025 |
| Anterior infarction | 820 (41) | 294 (41.6) | 362 (41.3) | 166 (40.2) | 0.57 |
| Multivessel disease | 1003 (53) | 356 (53.2) | 433 (52.5) | 217 (54.5) | 0.75 |
| Femoral access | 1017 (57.5) | 365 (59.5)e | 463 (58.7)c | 191 (51.5)ce | 0.021 |
| Duration of the angioplasty procedure (min) | 39 (22-60) | 40 (22-59) | 38 (22-60) | 40 (21-60) | 0.8 |
| Fibrinolysis | 307 (15.4) | 100 (14.1) | 153 (17.4) | 55 (13.3) | 0.06 |
| Final TIMI 3 flow | 1508 (92.8) | 527 (92.5) | 670 (93.3) | 314 (92.4) | 0.7 |
| LVEF (%) | 50 (40-55) | 47 (36-58) | 49 (38-59) | 48 (38-59) | 0.09 |
| Creatine phosphokinase peak (UI/L) | 1404 (606-2675) | 1374 (520-2565)e | 1367 (626-2711) | 1526 (728-2683)e | 0.048 |
| Prior medication | |||||
| Antivitamin K | 62 (3.1) | 21 (3) | 29 (3.3) | 12 (2.9) | 0.91 |
| In-hospital medication | |||||
| Aspirin | 1964 (98.3) | 696 (98.9) | 863 (98.6) | 408 (98.8) | 0.95 |
| Clopidogrel | 1777 (88.9) | 627 (89.1) | 786 (89.9) | 367 (88.9) | 0.74 |
| Prasugrel | 223 (11.2) | 75 (11.5) | 95 (11.4) | 53 (13.6) | 0.48 |
| Antivitamin K | 35 (1.8) | 13 (1.9) | 12 (1.4) | 10 (2.4) | 0.4 |
| LMWH | 1481 (74.1) | 511 (72.7) | 671 (76.7) | 302 (73.3) | 0.06 |
| UFH | 454 (22.7) | 166 (23.6) | 180 (20.6) | 108 (26.2) | 0.06 |
| LMWH + UFH | 198 (9.9) | 71 (10.1) | 78 (8.9) | 49 (11.9) | 0.25 |
| Bivalirudin (since 2010, for information) | 136 | 55 | 55 | 26 | 0.41 |
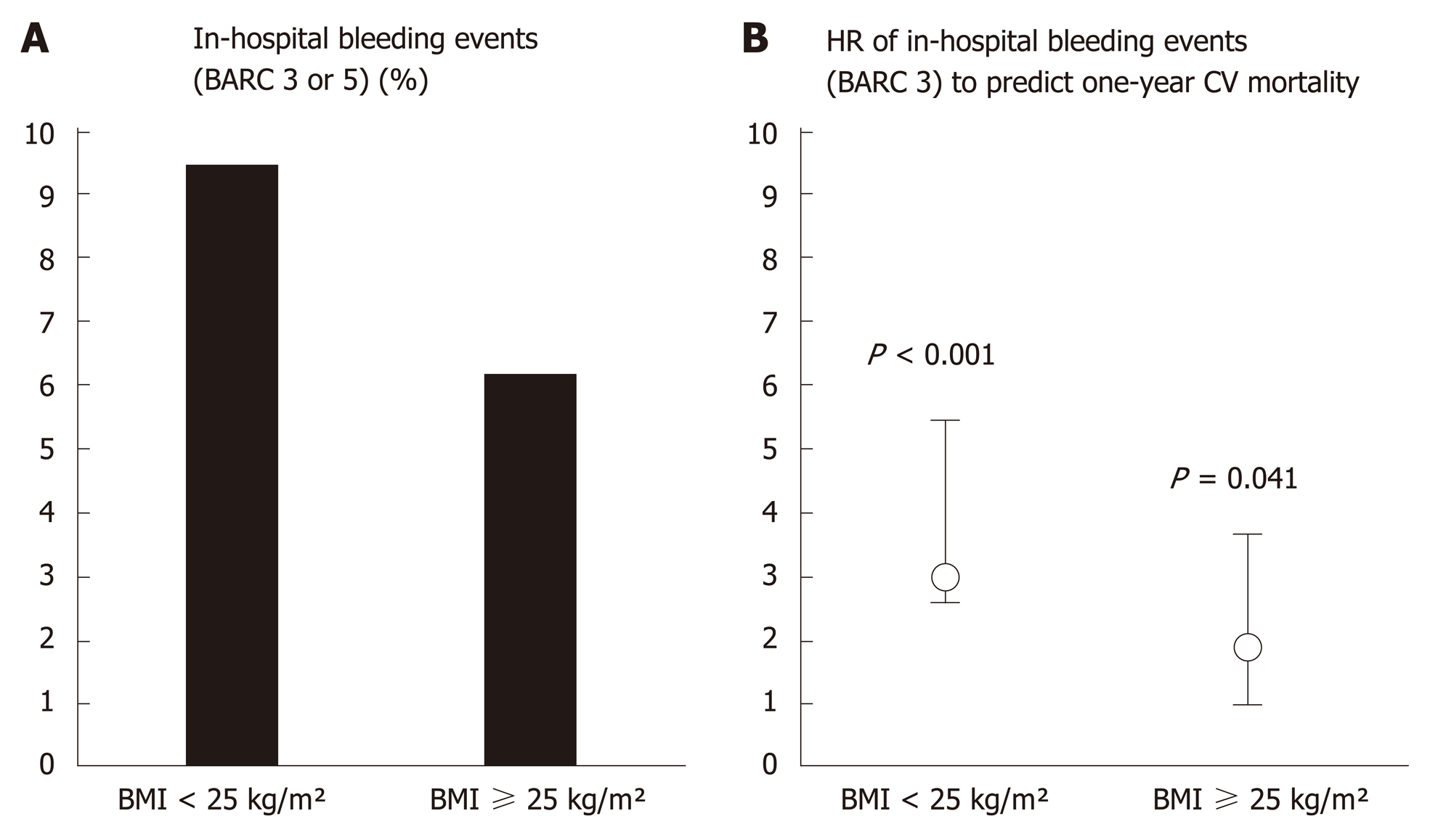
Among the population, 150 patients (7.5%) presented a BARC 3 or 5 bleeding event. These patients were older (70.2 ± 13.9 vs 64.1 ± 14.73, P < 0.001) and the use of femoral access was higher (71.5% vs 56.6%, P = 0.001). Normal weight patients had higher rates of BARC 3 and 5 bleeding than overweight and obese patients (9.5% vs 6.2% and 6.2%, P = 0.031) (Table 2). Independent variables associated with in-hospital BARC 3 or 5 bleeding were duration of the procedure, puncture access and LVEF (Table 3). The other in-hospital outcomes were not different among the three groups.
| Total | BMI < 25 kg/m² | 25 ≤ BMI < 30 kg/m² | BMI ≥ 30 kg/m² | P value | |
| n = 1998 | n = 705 | n = 877 | n = 416 | ||
| In-hospital events | |||||
| Duration of hospitalisation in intensive care (d) | 5 (3-7) | 5 (4-7) | 5 (3-7) | 5 (3-6) | 0.21 |
| Heart failure | 417 (20.9) | 153 (21.7) | 176 (20.1) | 89 (21.5) | 0.56 |
| Stent thrombosis | 38 (1.9) | 19 (2.5) | 11 (1.3) | 8 (1.9) | 0.16 |
| Atrial fibrillation | 196 (9.8) | 75 (10.6) | 92 (10.5) | 29 (7.0) | 0.06 |
| Stroke | 39 (2.0) | 14 (2) | 19 (2.2) | 6 (1.4) | 0.65 |
| Tamponade | 22 (1.1) | 10 (1.4) | 7 (0.8) | 5 (1.2) | 0.56 |
| Mortality | 115 (5.8) | 54 (7.6)ae | 43 (4.9)a | 18 (4.3)e | 0.018 |
| Cardiovascular mortality | 112 (5.6) | 54 (7.6)ae | 40 (4.6)a | 18 (4.3)e | 0.013 |
| In-hospital bleeding events | |||||
| Input Haemoglobin (g/dL) | 14.4 (13.3-15.5) | 14.1 (13-15.3)ae | 14.6 (13.5-15.6)a | 14.7 (13.5-15.7)e | < 0.001 |
| Haemoglobin drop (g/dL) | 1.9 (0.9-2.9) | 2 (1-3.2)ae | 1.7 (0.8-2.9)a | 1.8 (0.9-2.7)e | 0.016 |
| Input haematocrit (%) | 42.6 (39.6-45.5) | 42 (38.9-44.9)ae | 42.8 (40-45.8)a | 43.3 (40.2-45.6)e | < 0.001 |
| Haematocrit drop (%) | 5.3 (2.8-8.4) | 6.1 (3.1-9.3)ae | 5 (2.6-8.1)a | 4.9 (2.8-7.8)e | < 0.001 |
| Haemorrhagic stroke | 9 (0.5) | 6 (0.8) | 3 (0.3) | 0 (0.0) | 0.07 |
| Gastrointestinal bleeding | 25 (1.3) | 12 (1.7) | 8 (0.9) | 5 (1.2) | 0.45 |
| Haematoma at the puncture site | 138 (6.9) | 56 (7.9) | 58 (6.6) | 24 (5.8) | 0.22 |
| Surgical repair of the haematoma | 9 (0.5) | 3 (0.4) | 4 (0.5) | 2 (0.5) | 0.98 |
| Use of amines in bleeding | 33 (1.7) | 18 (2.5) | 10 (1.1) | 6 (1.4) | 0.13 |
| Transfusion | 82 (4.1) | 42 (5.9)a | 25 (2.9)a | 15 (3.6) | 0.011 |
| Fatal bleeding (BARC 5) | 9 (0.5) | 6 (0.8) | 2 (0.2) | 1 (0.2) | 0.17 |
| BARC 3 or 5 | 146 (7.3) | 67 (9.5)ae | 54 (6.2)a | 26 (6.2)e | 0.031 |
| One-year events | |||||
| Mortality | 198 (9.9) | 93 (13.1)ae | 82 (9.4)ac | 23 (5.5)ce | < 0.001 |
| CV mortality | 160 (8.0) | 76 (10.8)ae | 62 (7.1)a | 22 (5.3)e | 0.001 |
| Univariate analysis | Multivariate analysis | Odds ratio (95%CI) | |
| P value | P value | ||
| Male | 0.001 | 0.19 | |
| Age (per decade) | < 0.001 | 0.08 | |
| Hypertension | 0.001 | 0.18 | |
| BMI < 25 kg/m² | 0.006 | 0.75 | |
| Smoker | 0.005 | 0.31 | |
| Renal failure | 0.032 | 0.77 | |
| Radial puncture access | 0.001 | 0.036 | 0.54 (0.3-0.96) |
| Duration of the procedure | < 0.001 | 0.004 | 1 (1-1.01) |
| Multivessel disease | 0.003 | 0.76 | |
| Final TIMI flow | 0.005 | 0.46 | |
| Creatine phosphokinase peak (per 500 UI/L) | < 0.001 | 0.15 | |
| Left ventricular ejection fraction | < 0.001 | 0.017 | 0.97 (0.94-0.99) |
| Antivitamin K use before | 0.016 | 0.48 |
One-year CV mortality was significantly lower for BMI ≥ 25 kg/m² (5.3% and 7.1%) patients than for normal weight patients (10.8%) with P = 0.001 (Table 2). Independent variables associated with 1-year CV mortality were age, prior myocardial infarction, prior stroke, cancer, creatine phosphokinase peak, in-hospital heart failure and BARC 3 bleeding (Table 4). BMI was not an independent variable in this multivariate analysis although there was an interaction between BARC 3 and BMI (HR: 2.58, 95%CI: 1.44-4.64, P = 0.001), demonstrating BARC 3 bleeding to have a stronger clinical impact among normal weight patients (HR: 2.97, 95%CI: 1.61-5.5, P < 0.001) than for BMI ≥ 25 kg/m² patients (HR: 1.94, 95%CI: 1.02-3.69, P = 0.041) (Figure 2).
| Univariate analysis | Multivariate analysis | Hazard ratio (95%CI) | |
| P value | P value | ||
| Male | < 0.001 | 0.5 | |
| Age (per decade) | < 0.001 | < 0.001 | 1.56 (1.31-1.87) |
| Hypertension | < 0.001 | 0.28 | |
| BMI < 25 kg/m² | < 0.001 | 0.5 | |
| Smoker | < 0.001 | 0.62 | |
| Diabetes mellitus | 0.042 | 0.71 | |
| Prior myocardial infarction | 0.011 | 0.015 | 1.91 (1.13-3.22) |
| Stroke history | < 0.001 | 0.011 | 2.07 (1.17-3.64) |
| Renal failure | < 0.001 | 0.43 | |
| Cancer | 0.002 | 0.02 | 1.83 (1.09-3.06) |
| Radial puncture access | < 0.001 | 0.06 | |
| Creatine phosphokinase peak (per 500 UI/L) | 0.003 | < 0.001 | 1.05 (1.03-1.07) |
| In-hospital heart failure | < 0.001 | < 0.001 | 5.29 (3.44-8.13) |
| BARC 3 among BMI < 25 kg/m² | < 0.001 | < 0.001 | 2.97 (1.61-5.5) |
| BARC 3 among BMI ≥ 25 kg/m² | < 0.001 | 0.041 | 1.94 (1.02-3.69) |
The 1-year cardio-vascular mortality of patients with BMI ≥ 25 kg/m² in the RIMA cohort was lower than for normal weight patients, yet BMI was not an independent predictor for mortality, while an in-hospital bleeding event was. We found a BMI ≥ 25 kg/m² to be related to the bleeding event have less of an effect on prognosis.
The 416 patients in our study with BMI ≥ 30 kg/m² were younger, predominantly male and their prevalence of diabetes, hypertension and dyslipidaemia was higher. These are typical characteristics as identified in populations with acute coronary syndrome[16], including STEMI[17]. Nevertheless, there was no difference in regard of infarct location, reperfusion success, and LVEF.
Bucholz et al[18] studied 2334 patients with an infarction in the PREMIER registry. The 4-year mortality was 24.1% for normal weight patients, 17.2% for overweight patients and 12.4% for obese patients. The increase of 1 BMI point in the RICO cohort led to a reduction of 5% in the risk of death after one-year [OR: 0.95, (0.93-0.98), P < 0.001][19]. Despite a higher coronary risk, in-hospital and one-year mortality was lower for patients with BMI ≥ 25 kg/m².
Several theories have been developed to explain this phenomenon. One hypothesis is that thin patients are older and have more comorbidities than overweight patients, notably in term of higher prevalence of cancer. This hypothesis was developed by Witassek et al[20] in the Swiss AMIS Plus registry. The prevalence of history of cancer among the three groups in the RIMA appeared to be similar, despite the fact that normal weight patients were older. Other explanations have been put forward, such as obtaining targeted and adapted therapeutic doses in overweight and obese patients, which was not the case for thin patients[4,9], particularly regarding anticoagulants[16].
In our study, factors conducive to 1-year cardio-vascular mortality were age, history of infarction or stroke; history of cancer, creatine phosphokinase peak, in-hospital heart failure, and a BARC 3 in-hospital haemorrhagic event. While BMI did not appear to be an independent factor for mortality per se, BMI was an effect modifier of the impact of haemorrhagic complications. Of note, bleeding events were more frequent among BMI < 25 kg/m² patients compared to others (Table 2).
The prevalence for bleeding events varies in the literature from 3.9% in a GRACE-derived report[21] to 22% in a STEMI cohort[22]. In our study, we defined bleeding events by the BARC scoring system, which is robust and takes into account quantitative parameters such as a haemoglobin drop. Consequently, we reported a bleeding prevalence of 7.3% in the RIMA cohort; and we showed this prevalence to be higher among normal weight patients than among the other groups. Das et al[17] found the same relationship in 49329 patients with STEMI, as already reported by other sources[4,6,19,23-26]. The main location of bleeding events was at the puncture site.
According to Hamon et al[8], predictive factors for haemorrhagic complications are being female, low weight (BMI < 19 kg/m²), aged 75 or more, severe renal failure, history of stroke and uncontrolled arterial hypertension. Indeed, pharmacokinetics of anticoagulants may be driven by body composition, as patients with a lower ratio of lean body mass are exposed to supra-therapeutic dose of anticoagulants. Moreover, the management of antiplatelet therapy being mostly unadjusted to body weight, normal weight patients may receive relatively greater doses. In the present study, independent correlates to bleeding events were longer procedure duration, lower LVEF and a femoral puncture site. Of note, a femoral puncture site was responsible for higher rates of bleeding events BARC 3 or 5 (Figure 2). It should be noted that a femoral puncture site was responsible for higher rates of BARC 3 or 5 bleeding events (Table 3)[27].
These bleeding complications are also associated with a more severe prognosis in normal weight patients than for other patients (Figure 2). The originality of this report was the evidence of lower prevalence and a poorer prognostic impact of bleeding complications in normal weight patients (Figure 3). Special concerns should be given to the management of antithrombotic therapies, including following drug requirements, stopping anticoagulants early after effective angioplasty[28], avoiding changing anticoagulants several times[29], and encouraging low-risk strategies when invasive management is uncertain[30]. Even though the benefits of newer antiplatelet therapy is unquestionable in terms of prevention of stent thrombosis, a switch to clopidogrel – as soon as one month after angioplasty - may lower bleeding events[31].
However, there are limits to this real-life cohort study. It only included hospitalised patients, leading to a bias in the recruitment of survivors. The BMI is not the best criterion for evaluating abdominal obesity, our study did not include a measurement of the waist size and the waist-to-hip ratio, and did not take into account for confounders like chronic inflammatory disease or coagulation disorder in the database.
In conclusion, in the present study, we show in-hospital bleeding to be more prevalent amongst normal weight patients (BMI < 25 kg/m²) with greater impact on prognosis. Normal weight did not impact on one-year CV mortality per se, but increased the effect of bleeding on mortality. Here, we raise one explanatory hypothesis of the obesity paradox, with in-hospital bleeding in BMI ≥ 25 kg/m² patients related to lower impact on one-year mortality after STEMI.
ST-segment elevation myocardial infarction (STEMI) remains a major cause of mortality despite early revascularization and optimal medical therapy. Tailoring individual management by considering patients’ specificities may help in improving post-STEMI survival.
While overweight and obesity are correlated with common cardiovascular (CV) risk factors and outcomes, overweight and obese patients present better survival after suffering from myocardial infarction. This obesity paradox is not elucidated.
To assess whether the obesity paradox might be explained by bleeding events after a first STEMI.
We studied 2070 patients consecutively from the “Registre d’Infarctus Maine-Anjou” survey, that prospectively included all patients presenting with a STEMI in a Western region of France, in which the only available 24 h-7 d coronary angiography service was in Angers University Hospital. Median age was 64 (interquartile range 53-77) years, 74.3% were male, 41% presented with anterior infarction and 81% underwent primary percutaneous coronary intervention. Outcomes were gathered during the year following MI. Bleeding Academic Research Consortium (BARC) 3 and 5 bleeding events were used to assess in-hospital bleeding complications. Cox regression analyses were performed to assess correlates for 1-year mortality.
One-year CV mortality was significantly lower for body mass index (BMI) ≥ 25 kg/m² (5.3% and 7.1%) patients than for normal weight patients (10.8%) with P = 0.001. Independent variables associated with 1-year CV mortality were age, prior myocardial infarction, prior stroke, cancer, creatine phosphokinase peak, in-hospital heart failure and BARC 3 bleeding. BMI was not an independent variable in this multivariate analysis although there was an interaction between BARC 3 and BMI (HR: 2.58, 95%CI: 1.44-4.64, P = 0.001), demonstrating BARC 3 bleeding to have a stronger clinical impact among normal weight patients (HR: 2.97, 95%CI: 1.61-5.5, P < 0.001) than for BMI ≥ 25 kg/m² patients (HR: 1.94, 95%CI: 1.02-3.69, P = 0.041).
We show in the present study the role that in-hospital bleeding may play in the obesity paradox. Indeed, not only in-hospital bleeding events were lower among overweight patients, but also presented a weaker impact on 1-year CV mortality. The results of this study first suggest a need to adjust antithrombotic therapies in normal weight patients. Lowering doses to lower bleeding events must be balanced with anti-ischemic efficacy. Second, the reasons why intra-hospital bleeding presents a lower impact on overweight patients raise question and need further investigation.
Randomized control trials are needed to better monitor anti-thrombotic therapies in STEMI patients. Beside age, gender and clinical presentation, BMI might be a valuable feature to assess.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: France
Peer-review report classification
Grade A (Excellent):
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hussain SAR, Schulten HJ, Su G S-Editor: Yan JP L-Editor: A E-Editor: Zhang YL
| 1. | Inserm/Kantar Health/Roch. [cited 2018 May 10]. Obépi 2012: Enquête épidémiologique nationale sur le surpoids et l’obésité [Internet]. Available from: http://www.roche.fr/innovation-recherche-medicale/decouverte-scientifique-medicale/cardio-metabolisme/enquete-nationale-obepi-2012.html. [Cited in This Article: ] |
| 2. | Nguyen DM, El-Serag HB. The epidemiology of obesity. Gastroenterol Clin North Am. 2010;39:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 364] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 3. | Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation. 1983;67:968-977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2601] [Cited by in F6Publishing: 2393] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 4. | Lancefield T, Clark DJ, Andrianopoulos N, Brennan AL, Reid CM, Johns J, Freeman M, Charter K, Duffy SJ, Ajani AE, Proietto J, Farouque O; MIG (Melbourne Interventional Group) Registry. Is there an obesity paradox after percutaneous coronary intervention in the contemporary era? An analysis from a multicenter Australian registry. JACC Cardiovasc Interv. 2010;3:660-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Schunkert H, Harrell L, Palacios IF. Implications of small reference vessel diameter in patients undergoing percutaneous coronary revascularization. J Am Coll Cardiol. 1999;34:40-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Herrmann J, Gersh BJ, Goldfinger JZ, Witzenbichler B, Guagliumi G, Dudek D, Kornowski R, Brener SJ, Parise H, Fahy M, McAndrew TC, Stone GW, Mehran R. Body mass index and acute and long-term outcomes after acute myocardial infarction (from the Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction Trial). Am J Cardiol. 2014;114:9-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Nikolsky E, Stone GW, Grines CL, Cox DA, Garcia E, Tcheng JE, Griffin JJ, Guagliumi G, Stuckey T, Turco M, Negoita M, Lansky AJ, Mehran R. Impact of body mass index on outcomes after primary angioplasty in acute myocardial infarction. Am Heart J. 2006;151:168-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Hamon M, Filippi-Codaccioni E, Riddell JW, Lepage O. Prognostic impact of major bleeding in patients with acute coronary syndromes.A systematic review and meta-analysis. EuroIntervention. 2007;3:400-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Grall S, Biere L, Le Nezet M, Bouvier JM, Lucas-Chauvelon P, Richard C, Abi-Khalil W, Delepine S, Prunier F, Furber A. Relationship between beta-blocker and angiotensin-converting enzyme inhibitor dose and clinical outcome following acute myocardial infarction. Circ J. 2015;79:632-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Fuchs S, Kornowski R, Teplitsky I, Brosh D, Lev E, Vaknin-Assa H, Ben-Dor I, Iakobishvili Z, Rechavia E, Battler A, Assali A. Major bleeding complicating contemporary primary percutaneous coronary interventions-incidence, predictors, and prognostic implications. Cardiovasc Revasc Med. 2009;10:88-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736-2747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2595] [Cited by in F6Publishing: 3040] [Article Influence: 233.8] [Reference Citation Analysis (0)] |
| 12. | Kikkert WJ, van Geloven N, van der Laan MH, Vis MM, Baan J, Koch KT, Peters RJ, de Winter RJ, Piek JJ, Tijssen JG, Henriques JP. The prognostic value of bleeding academic research consortium (BARC)-defined bleeding complications in ST-segment elevation myocardial infarction: a comparison with the TIMI (Thrombolysis In Myocardial Infarction), GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries), and ISTH (International Society on Thrombosis and Haemostasis) bleeding classifications. J Am Coll Cardiol. 2014;63:1866-1875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 13. | Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37:2315-2381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4739] [Cited by in F6Publishing: 4358] [Article Influence: 544.8] [Reference Citation Analysis (1)] |
| 14. | Ariza-Solé A, Sánchez-Elvira G, Sánchez-Salado JC, Lorente-Tordera V, Salazar-Mendiguchía J, Sánchez-Prieto R, Romaguera-Torres R, Ferreiro-Gutiérrez JL, Gómez-Hospital JA, Cequier-Fillat A. CRUSADE bleeding risk score validation for ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Thromb Res. 2013;132:652-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Gage BF, Yan Y, Milligan PE, Waterman AD, Culverhouse R, Rich MW, Radford MJ. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). Am Heart J. 2006;151:713-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 689] [Cited by in F6Publishing: 673] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 16. | Mehta L, Devlin W, McCullough PA, O'Neill WW, Skelding KA, Stone GW, Boura JA, Grines CL. Impact of body mass index on outcomes after percutaneous coronary intervention in patients with acute myocardial infarction. Am J Cardiol. 2007;99:906-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 93] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Das SR, Alexander KP, Chen AY, Powell-Wiley TM, Diercks DB, Peterson ED, Roe MT, de Lemos JA. Impact of body weight and extreme obesity on the presentation, treatment, and in-hospital outcomes of 50,149 patients with ST-Segment elevation myocardial infarction results from the NCDR (National Cardiovascular Data Registry). J Am Coll Cardiol. 2011;58:2642-2650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 18. | Bucholz EM, Rathore SS, Reid KJ, Jones PG, Chan PS, Rich MW, Spertus JA, Krumholz HM. Body mass index and mortality in acute myocardial infarction patients. Am J Med. 2012;125:796-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | Zeller M, Steg PG, Ravisy J, Lorgis L, Laurent Y, Sicard P, Janin-Manificat L, Beer JC, Makki H, Lagrost AC, Rochette L, Cottin Y; RICO Survey Working Group. Relation between body mass index, waist circumference, and death after acute myocardial infarction. Circulation. 2008;118:482-490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Witassek F, Schwenkglenks M, Erne P, Radovanovic D. Impact of Body Mass Index on mortality in Swiss hospital patients with ST-elevation myocardial infarction: does an obesity paradox exist? Swiss Med Wkly. 2014;144:w13986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Moscucci M, Fox KA, Cannon CP, Klein W, López-Sendón J, Montalescot G, White K, Goldberg RJ. Predictors of major bleeding in acute coronary syndromes: the Global Registry of Acute Coronary Events (GRACE). Eur Heart J. 2003;24:1815-1823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 667] [Cited by in F6Publishing: 649] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 22. | Boden H, Velders MA, van der Hoeven BL, Cannegieter SC, Schalij MJ. In-hospital major bleeding and its clinical relevance in patients with ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Am J Cardiol. 2013;112:1533-1539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, Ahmed LM, Kent KM, Pichard AD, Suddath WO, Satler LF, Lindsay J. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39:578-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 464] [Cited by in F6Publishing: 482] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 24. | Hastie CE, Padmanabhan S, Slack R, Pell AC, Oldroyd KG, Flapan AD, Jennings KP, Irving J, Eteiba H, Dominiczak AF, Pell JP. Obesity paradox in a cohort of 4880 consecutive patients undergoing percutaneous coronary intervention. Eur Heart J. 2010;31:222-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 25. | Buettner HJ, Mueller C, Gick M, Ferenc M, Allgeier J, Comberg T, Werner KD, Schindler C, Neumann FJ. The impact of obesity on mortality in UA/non-ST-segment elevation myocardial infarction. Eur Heart J. 2007;28:1694-1701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 89] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Kosuge M, Kimura K, Kojima S, Sakamoto T, Ishihara M, Asada Y, Tei C, Miyazaki S, Sonoda M, Tsuchihashi K, Yamagishi M, Shirai M, Hiraoka H, Honda T, Ogata Y, Ogawa H; Japanese Acute Coronary Syndrome Study (JACSS) Investigators. Impact of body mass index on in-hospital outcomes after percutaneous coronary intervention for ST segment elevation acute myocardial infarction. Circ J. 2008;72:521-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Jolly SS, Amlani S, Hamon M, Yusuf S, Mehta SR. Radial versus femoral access for coronary angiography or intervention and the impact on major bleeding and ischemic events: a systematic review and meta-analysis of randomized trials. Am Heart J. 2009;157:132-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 736] [Cited by in F6Publishing: 767] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 28. | Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimský P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5364] [Cited by in F6Publishing: 5941] [Article Influence: 990.2] [Reference Citation Analysis (0)] |
| 29. | Feldman DN, Wang TY, Chen AY, Swaminathan RV, Kim LK, Wong SC, Minutello RM, Bergman G, Singh HS, Madias C. In-Hospital Bleeding Outcomes of Myocardial Infarction in the Era of Warfarin and Direct Oral Anticoagulants for Atrial Fibrillation in the United States: A Report From the National Cardiovascular Data Registry Acute Coronary Treatment and Intervention Outcomes Network Registry. J Am Heart Assoc. 2019;8:e011606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Yusuf S, Mehta SR, Chrolavicius S, Afzal R, Pogue J, Granger CB, Budaj A, Peters RJ, Bassand JP, Wallentin L, Joyner C, Fox KA; OASIS-6 Trial Group. Effects of fondaparinux on mortality and reinfarction in patients with acute ST-segment elevation myocardial infarction: the OASIS-6 randomized trial. JAMA. 2006;295:1519-1530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 631] [Cited by in F6Publishing: 485] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 31. | Cuisset T, Deharo P, Quilici J, Johnson TW, Deffarges S, Bassez C, Bonnet G, Fourcade L, Mouret JP, Lambert M, Verdier V, Morange PE, Alessi MC, Bonnet JL. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017;38:3070-3078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 256] [Article Influence: 42.7] [Reference Citation Analysis (0)] |