Published online Jul 26, 2018. doi: 10.4330/wjc.v10.i7.52
Peer-review started: February 19, 2018
First decision: March 9, 2018
Revised: March 24, 2018
Accepted: June 25, 2018
Article in press: June 27, 2018
Published online: July 26, 2018
Processing time: 157 Days and 13.7 Hours
Ventricular tachycardia (VT) is a crucial cause of sudden cardiac death (SCD) and a primary cause of mortality and morbidity in patients with structural cardiac disease. VT includes clinical disorders varying from benign to life-threatening. Most life-threatening episodes are correlated with coronary artery disease, but the risk of SCD varies in certain populations, with various underlying heart conditions, specific family history, and genetic variants. The targets of VT management are symptom alleviation, improved quality of life, reduced implantable cardioverter defibrillator shocks, prevention of reduction of left ventricular function, reduced risk of SCD, and improved overall survival. Antiarrhythmic drug therapy and endocardial catheter ablation remains the cornerstone of guideline-endorsed VT treatment strategies in patients with structural cardiac abnormalities. Novel strategies such as epicardial ablation, surgical cryoablation, transcoronary alcohol ablation, pre-procedural imaging, and stereotactic ablative radiotherapy are an appealing area of research. In this review, we gathered all recent advances in innovative therapies as well as experimental evidence focusing on different aspects of VT treatment that could be significant for future favorable clinical applications.
Core tip: Antiarrhythmic drug therapy and endocardial catheter ablation remains the cornerstone of ventricular tachycardia (VT) treatment management, but both treatments have limited efficacy and important adverse effects. Catheter ablation for cardiomyopathic (scar-related) VT is associated with recurrence rates as high as 50% at 6 mo. Implantable cardioverter defibrillator provides a safety net; however, there is an increased need for more effective and safer methods to decrease VT recurrence episodes. We sought to review current literature in order to summarize data on innovative techniques for VT treatment.
- Citation: Spartalis M, Spartalis E, Tzatzaki E, Tsilimigras DI, Moris D, Kontogiannis C, Livanis E, Iliopoulos DC, Voudris V, Theodorakis GN. Novel approaches for the treatment of ventricular tachycardia. World J Cardiol 2018; 10(7): 52-59
- URL: https://www.wjgnet.com/1949-8462/full/v10/i7/52.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i7.52
Sudden cardiac death (SCD) is a vital public health issue, accountable for almost 50% of all cardiovascular deaths[1]. In the last three decades, SCD was the leading cause for almost 230000 to 350000 deaths per annum in the United States[1]. Ventricular arrhythmias account for 25% to 36% of witnessed sudden cardiac arrests (SCA) at home and 38% to 79% of witnessed SCA in public[2].
Ischemic heart disease, structural disorders, various forms of cardiomyopathy associated with myocardial fibrosis, cardiac channelopathies, myocarditis, congenital heart diseases, and other genetic rare disorders are associated with ventricular arrhythmias[1].
Even though treatment for heart failure lowers mortality and SCD, it was unsuccessful in lessening ventricular tachycardia (VT) recurrences[3]. Implantable cardioverter defibrillators (ICD) are very effective in eliminating VT episodes and in lowering the possibility of SCD, but they are not useful for arrhythmia prevention[4]. When the VT substrate manifests, anti-arrhythmic drug treatment or catheter ablation are the current choices to reduce VT episodes[5]. Catheter ablation and antiarrhythmic drug therapy though, are also limited by incomplete efficacy, unfavorable side effects, and procedural risk[5]. In this review, we outline the current advances in VT treatment options and describe the imaging modalities, progress, and novel strategies.
We have collected all experimental and clinical investigations focused on new aspects that could be essential for tailoring VT therapy according to underlying etiology, in order to achieve higher efficacy. The MEDLINE database was screened for studies with the medical term “ventricular tachycardia” and keywords “treatment”, or “ablation”, or “management”. We restricted our search to English literature.
Endocardial catheter ablation and antiarrhythmic drug treatment are currently the mainstays of VT treatment[1,3]. However, the procedural success rates of VT are quite variable due to the heterogeneous substrates that reflect the variety of pathophysiological processes[6,7]. The success rate of endocardial ablation in patients with outflow tract VT is 84%, in patients with papillary muscle VT is 60%, and in patients with idiopathic left ventricular VT is 70%[6]. Moreover, the VT recurrence rates of endocardial ablation in ischemic cardiomyopathy patients are between 23% and 49% and in dilated cardiomyopathy patients between 46% and 61%[7]. Non-ischemic cardiomyopathy patients have worse outcomes than ischemic cardiomyopathy patients due to scar patterns with epicardial and intramural sites[7].
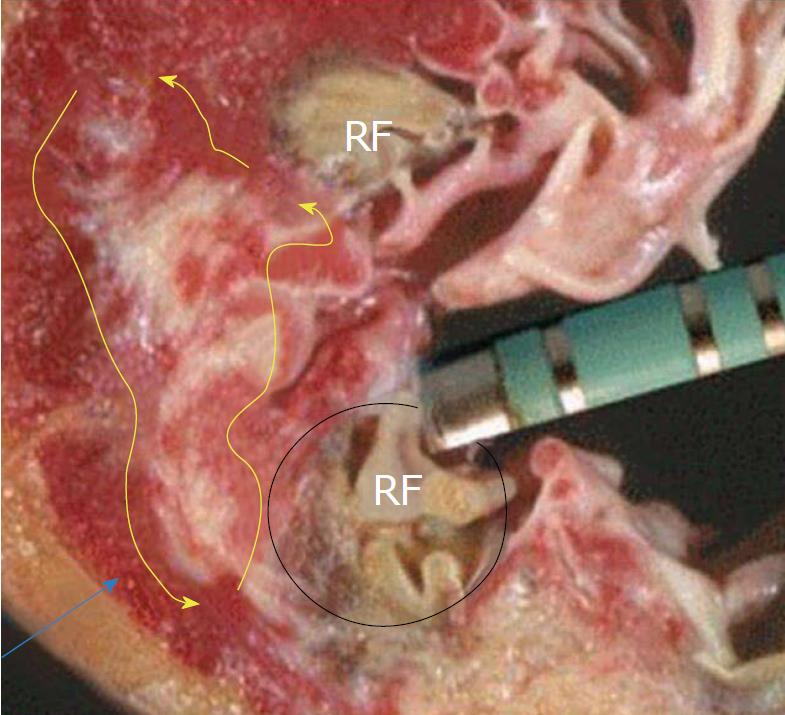
Epicardial ablation has emerged as a potential alternative ablation strategy in order to increase the success rate in complex substrates and to eliminate VT in patients with different cardiomyopathies and more recently in patients with Brugada syndrome[8-11]. Percutaneous approach to the pericardial area facilitates epicardial ablation when the VT substrate is situated in the subepicardium[8] (Figure 1). Adjacency to coronary circulation and the phrenic nerve may hinder the procedure in certain situations[9]. In patients with previous heart surgery or previous epicardial ablation attempts, percutaneous access may not be possible and as such, video-assisted thoracoscopy may be a good and minimally invasive alternative to open surgery[12].
Epicardial ablation is a safe procedure with low complication rates[13]. Pericardial effusion is the most common complication[13]. Damage to subdiaphragmatic organs and hemorrhage from diaphragmatic vessels have also been reported[13].
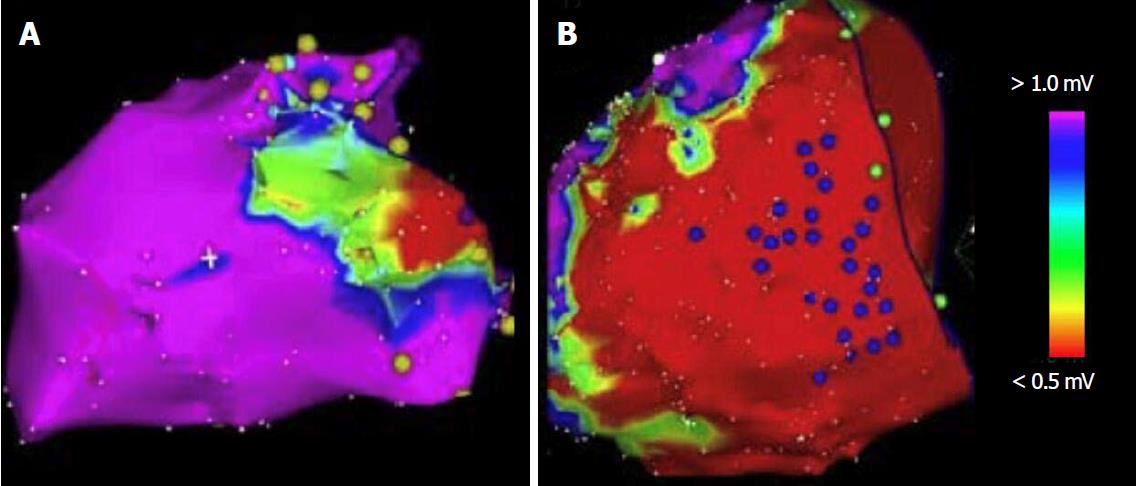
Della Bella et al[14] evaluated the possible benefit of endo-epicardial catheter ablation for the management of VT in 528 patients with any form of structural cardiac disorder (Figure 2). Endo-epicardial catheter ablation resulted in a VT recurrence rate of 34.1%, in comparison to a rate of up to 50% with the standard endocardial approach[14].
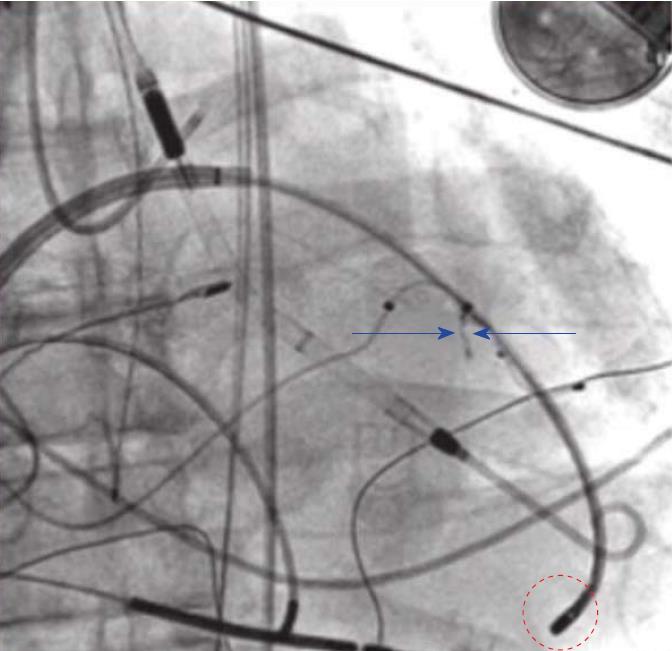
Transcoronary alcohol ablation has emerged to approach deep intramyocardial substrate in patients not amenable to endo-epicardial catheter ablation (mechanical valve, thrombus, significant comorbidities)[15,16]. Transcoronary alcohol ablation requires the injected dose of sterile pure ethanol with proximal balloon expansion to a culprit vessel with a target of abolishing perfusion[15,16] (Figure 3). This method can prevent recurrent VT, VT storm and can control incessant VT[15,16]. The exact recognition of the target vessel and the existence of collaterals may hinder the adoption of the method[15-17].
Kim et al[15] introduced the novel use of cardioplegia as a mapping technique in order to identify the critical VT isthmus to facilitate effective transcoronary ethanol ablation and avoid irreversible myocardial injury. Furthermore, Sapp et al[17] showed that intramyocardial needle mapping and ablation with saline infusion could create deep injuries and is a practical and efficient method. Intracoronary wire mapping and coil embolization have also been utilized just as alcohol ablation to target VT arising from intraventricular septum[18]. After intracoronary wire mapping and recognition of a culprit vessel, coils are directed to embolize the branch, eliminating the desired target perfusion[18].
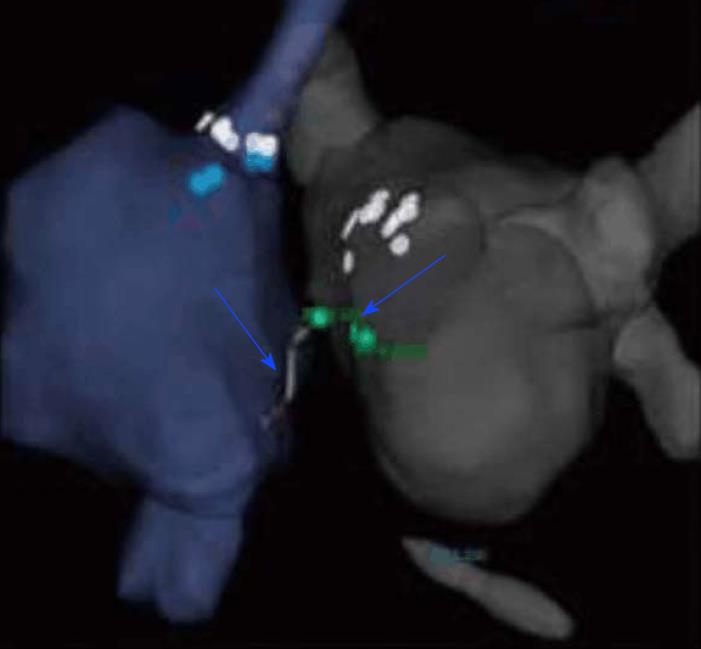
Bipolar ablation between two ablation catheters located on either position of the septum from both ventricles improves lesion transmurality because it depends less on catheter contact and alignment[19,20] (Figure 4). Bipolar ablation has the theoretical benefit of producing more powerful energy and providing deeper lesions, in comparison to two separate unipolar catheters[19,20]. Sakamoto et al[20] recently successfully eliminated the critical VT circuit in a patient with an arrhythmogenic substrate (cardiac sarcoidosis), utilizing bipolar ablation.
Accumulated evidence strongly suggests a role of sympathetic neuromodulation in controlling refractory VT[19-24]. Cardiac sympathetic denervation surgery has been proven to be useful for the management of congenital long QT syndrome and catecholaminergic polymorphic VT[21]. The procedure requires extraction of the lower fraction of the stellate ganglion and T2-T4 sympathetic thoracic ganglia[21]. Complications regarding the procedure were infrequent, with 4% developing acute ptosis or Horner syndrome[21]. Vaseghi et al[21] showed that cardiac sympathetic denervation has greater shock free survival as well as a considerable decline in shock burden in patients with recurrent VT or VT storm, regardless of antiarrhythmic drug therapy and catheter ablation. In addition, bilateral cardiac sympathetic denervation appeared to be more efficacious than left-sided denervation[21,22].
Augmented sympathetic activity leads to early and delayed afterdepolarization, enhances diffuse repolarization, leading to ventricular electrical susceptibility an–d increases the possibility of malignant VT[23]. Stellate ganglionectomy lengthens the ventricular refractory period and raises the VF threshold, decreasing VT or VF inducibility in the context of myocardial infarction[23,24]. Locally invasive sympathetic ganglion block could select individuals with greater possibilities of long-term clinical benefits prior to sympathetic denervation[23,24].
Enhanced sympathetic tone shortens the ventricular effective refractory period, enhances automaticity, and lowers the threshold for ventricular arrhythmias[25-27]. Feyz et al[25] performed bilateral renal denervation in a patient with polymorphic VT with excellent results. Aksu et al[27] also showed that catheter-based renal denervation was successful in a patient with an electrical storm due to catecholaminergic polymorphic VT. However, the microanatomy of human renal vessels has great variability. Accessory renal vessels that bifurcated early can also influence the result negatively, and there is still the absence of procedural endpoint during the technique[26].
As a result, renal sympathetic denervation is not currently recognized as an ideal or approved VT treatment method[25-27]. However, certain ventricular arrhythmias do not terminate after catheter ablation, thus making renal sympathetic denervation a possible option for patients in whom other ablative approaches were ineffective[25,27].
Despite catheter-oriented ablation, which applies radiofrequency or freezing to damage tissues, radiotherapy is based on photons from X-rays or gamma rays to injure the desired target, mainly cancer. Through novel distribution methods such as intensity-modulated radiotherapy, a dosage of radiotherapy can be precisely and accurately directed to the desired site, while diminishing dosage to adjoining healthy tissues[28,29].
Ablative radiotherapy is generally a noninvasive, outpatient method, which does not involve anesthesia[28,29]. Potential risks consist of damage to tissue next to the ablated site, such as brain edema for intracranial lesions, pneumonitis for chest therapies, myelopathy for spinal carcinomas, or bowel perforation for abdominal locations[28,29]. In comparison to radiofrequency or cryoenergy, the damage from ablative radiotherapy progresses over days to months, needing time for the total tissue damage to be shown[28,29].
The first patient was treated on a robotic radiosurgery system (CyberKnife®, Accuray, Sunnyvale, CA, United States) in 2012[28]. The follow-up revealed no definite acute or late adverse effects, and a seven-month reduction burden in VT on standard antiarrhythmic dr–ug therapy, suggesting a potential transient benefit of this method[28].
Cuculich et al[29] investigated five patients with increased-risk, refractory VT. The authors focused on arrhythmogenic scar sites by merging anatomical imaging with noninvasive electrocardiographic imaging during VT that was produced using an ICD[29]. Patients were treated with a single dose of 25 Gy while awake, using a noninvasive distribution of accurate ablative radiation with stereotactic body radiation treatment[29]. Cuculich et al[29] reported a reduction in events of VT in all five patients.
Catheter radiofrequency ablation of VT originating from the left ventricle’s papillary muscles has been linked to conflicting outcomes[30,31]. Rivera et al[31] investigated twenty-one patients with drug-refractory VT, who underwent catheter cryoablation or radiofrequency ablation. Cryoablation was correlated with greater success rates and smaller recurrence rates than radiofrequency procedures, superior catheter support, and smaller frequency of polymorphic arrhythmias[31]. Marai et al[30] recently used cryoablation guided by intracardiac echocardiography, 3-dimensional mapping system, and image integration to treat a patient with refractory VT originating from papillary muscle with excellent results.
Catheter ablation provides efficient outcomes for sustained monomorphic VT, but certain situations, such as the existence of mural thrombus and heavy calcification, can lead to adverse results[32,33]. Higuchi et al[33] successfully treated a 67-year-old patient with sustained monomorphic VT due to ventricular scar and resistant to endocardial radiofrequency ablation, by left ventricular reconstruction with cryoablation. Li et al[34] conducted a retrospective investigation of 38 consecutive surgical epicardial VT ablation procedures and compared the results with those of a propensity-matched percutaneous epicardial access control group. Surgical epicardial access after heart surgery for VT ablation showed no statistical difference in long-term results in comparison to the percutaneous epicardial group[34].
Recently, Berte et al[35] presented the first animal survey utilizing a more potent cryoablation system that can generate larger, transmural ventricular lesions from both the endocardium and the epicardium. Surgical cryoablation in sheep had no acute macroscopic vascular or extracardiac damage and resulted in 100% successful lesions at necropsy[35].
In some patients with non-ischemic cardiomyopathy and VT refractory to standard therapy or undergoing cardiac surgery, surgical ablation may be an alternative option for potentially reducing the burden of ICD shocks during long-term follow-up[36]. Liang et al[36] showed that detailed arrhythmogenic substrate in the electrophysiology lab before surgery, in conjunction with a direct scar and radiofrequency ablation lesions visualization in the operating room, is crucial for guiding surgical ablation.
Extracorporeal life support is a highly efficient bridging treatment in patients with refractory VT associated with cardiogenic shock[37]. Extracorporeal life support allows the usage of negative inotropic antiarrhythmic drug therapy, leads to the weaning of catecholamine delivery, thus resolving the dangerous period of the catecholamine driven electrical storm[37]. The utilization of extracorporeal life support maintains hemodynamic support during an ablation procedure, while mapping and induction of VT are commenced and provides sufficient vital organ perfusion in patients with refractory VT[37]. Current literature suggests the usage of extracorporeal life support, as it has proven to be a safe, practical and efficient therapeutic solution when traditional treatments have failed[37].
Okabe et al[38] successfully treated a patient with cardiac sarcoidosis associated with VT using steroid pulse therapy.
Catecholaminergic polymorphic VT (CPVT) is a rare cardiac ion channelopathy induced by anomalies in proteins that regulate Ca2+ transport in heart cells that can lead to SCD[39,40]. CPVT is associated with mutations in the gene encoding the cardiac RyR2, a cardiac ryanodine receptor protein which is involved in calcium homeostasis and mutations in the gene that encodes calsequestrin (CASQ2), a protein that interacts with RyR2[39-41].
Denegri et al[42] showed that viral gene transfer of wild-type CASQ2 into the heart of mice prevented and reverted severe manifestations of CPVT. Furthermore, Lodola et al[43] infected induced pluripotent stem cells with an adeno-associated viral vector serotype 9 (AAV9) encoding the human CASQ2 gene and noticed a significant decline in the percentage of delayed afterdepolarizations. Li et al[44] used tetracaine, a local anesthetic drug with known RyR2 inhibiting action, in mice and showed that the drug efficiently halted the induction of VT in a mouse model of CPVT.
Endo-epicardial ablation reduces VT recurrences, but not all patients have a VT substrate[45]. Contrast-enhanced magnetic resonance imaging (ceMRI) is utilized to identify VT substrate after myocardial infarction[45]. Arena et al[45] showed that ceMRI-based endo-epicardial signal intensity mapping in a porcine model allowed characterization of the epicardial VT substrate.
Klein et al[46] used 3D meta-iodobenzylguanidine (MIBG) imaging to guide VT ablation. MIBG innervation defects are greater than scars produced from bipolar voltage maps, and the investigation showed that 36% of successful ablative locations were situated in sections of irregular innervation and normal voltage, suggesting that innervation maps may identify additional VT ablation sites[46].
Zhang et al[47] investigated non-invasive high-resolution mapping and electrocardiographic imaging to provide epicardial substrate information. Electrocardiographic imaging identified scar electrophysiologic substrates in ischemic cardiomyopathy patients[47].
Cardiac ripple mapping for slow conducting channels is an innovative technique to integrate voltage and activation mapping[48,49]. Cardiac ripple mapping allows the concurrent vision of voltage and activation data and facilitates recognition of slow conduction channels within scar areas in the myocardium that could be probable VT ablation sites[48,49]. Table 1 summarizes the studies investigating novel approaches for the treatment of VT.
| Ref. | Type of study | No. of subjects | Focus of study | Complications |
| Della Bella et al[14] | Clinical | 528 | Endo-epicardial Ablation | Pericardial effusion, tamponade |
| Kim et al[15] | Clinical | 1 | Cardioplegia/Transcoronary alcohol ablation | Atrioventricular block, extensive myocardial damage, perforation |
| Sapp et al[17] | Clinical | 8 | Intramyocardial infusion-needle catheter ablation | Atrioventricular block, perforation, tamponade |
| Tholakanahalli et al[18] | Clinical | 2 | Intracoronary wire mapping and coil embolization | Atrioventricular block, coronary injury, embolization of unintended branches |
| Vaseghi et al[21] | Clinical | 121 | Cardiac sympathetic denervation | Hemothorax, pneumothorax, ptosis or Horner syndrome |
| Cuculich et al[29] | Clinical | 5 | Stereotactic radioablation therapy | Fatigue |
| Rivera et al[31] | Clinical | 21 | Cryoablation | - |
| Li et al[34] | Clinical | 38 | Surgical epicardial ablation | Ventricle laceration |
| Berte et al[35] | Experimental | 5 (sheep) | Surgical cryoablation | - |
| Liang et al[36] | Clinical | 20 | Surgical cryoablation | - |
| Denegri et al[42] | Experimental | 25 (mice) | Viral gene transfer of wild-type CASQ2 | - |
| Li et al[44] | Experimental | 9 (mice) | Tetracaine derivatives (RyR2 inhibitors) | - |
| Arenal et al[45] | Experimental | 31 (pigs) | MRI-based signal intensity mapping for epicardial substrate | Coronary occlusion |
| Klein et al[46] | Clinical | 15 | 3D meta-iodobenzyl-guanidine innervation maps to assess substrate and successful ablation sites | - |
| Zhang et al[47] | Clinical | 32 | Non-invasive high-resolution endocardial and epicardial mapping and electro-cardiographic imaging | - |
| Luther et al[48] | Clinical | 15 | Cardiac ripple mapping for slow conducting channels | - |
| Jamil-Copley et al[49] | Clinical | 21 | Cardiac ripple mapping for slow conducting channels | - |
The management of patients with VT can be demanding. ICD implant led to a considerable difference in the survival of subjects with VT, but the estimate of subjects with recurrent ICD shocks is still a growing issue. Antiarrhythmic drug treatment has reduced effectiveness and is correlated with serious adverse effects. Catheter ablation remains the cornerstone in the treatment of VT and efficiently lowers recurrent VT episodes but carries upfront procedural danger. Novel methods could enhance its future effectiveness. The final management strategy should be individualized utilizing clinical and imaging assessment, patient views and intentions, futility concerns, and operator’s catheter ablation experience.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aksu T S- Editor: Ji FF L- Editor: Filipodia E- Editor: Tan WW
| 1. | Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2017;pii:CIR.0000000000000548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 174] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 2. | Weisfeldt ML, Everson-Stewart S, Sitlani C, Rea T, Aufderheide TP, Atkins DL, Bigham B, Brooks SC, Foerster C, Gray R. Ventricular tachyarrhythmias after cardiac arrest in public versus at home. N Engl J Med. 2011;364:313-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 3. | Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793-2867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2447] [Cited by in RCA: 2645] [Article Influence: 264.5] [Reference Citation Analysis (0)] |
| 4. | Hohnloser SH, Israel CW. Current evidence base for use of the implantable cardioverter-defibrillator. Circulation. 2013;128:172-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Santangeli P, Muser D, Maeda S, Filtz A, Zado ES, Frankel DS, Dixit S, Epstein AE, Callans DJ, Marchlinski FE. Comparative effectiveness of antiarrhythmic drugs and catheter ablation for the prevention of recurrent ventricular tachycardia in patients with implantable cardioverter-defibrillators: A systematic review and meta-analysis of randomized controlled trials. Heart Rhythm. 2016;13:1552-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 131] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 6. | Dukkipati SR, Choudry S, Koruth JS, Miller MA, Whang W, Reddy VY. Catheter Ablation of Ventricular Tachycardia in Structurally Normal Hearts: Indications, Strategies, and Outcomes-Part I. J Am Coll Cardiol. 2017;70:2909-2923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Dukkipati SR, Koruth JS, Choudry S, Miller MA, Whang W, Reddy VY. Catheter Ablation of Ventricular Tachycardia in Structural Heart Disease: Indications, Strategies, and Outcomes-Part II. J Am Coll Cardiol. 2017;70:2924-2941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | MacIntyre CJ, Sapp JL. Treatment of persistent ventricular tachycardia: Drugs or ablation? Trends Cardiovasc Med. 2017;27:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Baldinger SH, Stevenson WG, John RM. Ablation of ischemic ventricular tachycardia: evidence, techniques, results, and future directions. Curr Opin Cardiol. 2016;31:29-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Aryana A, d’Avila A. Epicardial Catheter Ablation of Ventricular Tachycardia. Card Electrophysiol Clin. 2017;9:119-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Nademanee K, Hocini M, Haïssaguerre M. Epicardial substrate ablation for Brugada syndrome. Heart Rhythm. 2017;14:457-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 12. | Aksu T, Erdem Guler T, Yalin K. Successful ablation of an epicardial ventricular tachycardia by video-assisted thoracoscopy. Europace. 2015;17:1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Graham AJ, Orini M, Lambiase PD. Limitations and Challenges in Mapping Ventricular Tachycardia: New Technologies and Future Directions. Arrhythm Electrophysiol Rev. 2017;6:118-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Della Bella P, Baratto F, Tsiachris D, Trevisi N, Vergara P, Bisceglia C, Petracca F, Carbucicchio C, Benussi S, Maisano F. Management of ventricular tachycardia in the setting of a dedicated unit for the treatment of complex ventricular arrhythmias: long-term outcome after ablation. Circulation. 2013;127:1359-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 15. | Kim SM, Virgadamo S, Gurley J, Elayi CS. Use of Cardioplegia to Guide Alcohol Ablation for Incessant Ventricular Tachycardia. Pacing Clin Electrophysiol. 2017;40:213-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Markowitz SM, Minutello RM, Kim LK, Ip JE, Thomas G, Lerman BB. Treatment of intramural ventricular tachycardia in cardiac sarcoidosis with transcoronary ethanol ablation. Europace. 2017;19:1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Sapp JL, Beeckler C, Pike R, Parkash R, Gray CJ, Zeppenfeld K, Kuriachan V, Stevenson WG. Initial human feasibility of infusion needle catheter ablation for refractory ventricular tachycardia. Circulation. 2013;128:2289-2295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Tholakanahalli VN, Bertog S, Roukoz H, Shivkumar K. Catheter ablation of ventricular tachycardia using intracoronary wire mapping and coil embolization: description of a new technique. Heart Rhythm. 2013;10:292-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Gianni C, Mohanty S, Trivedi C, Di Biase L, Al-Ahmad A, Natale A, David Burkhardt J. Alternative Approaches for Ablation of Resistant Ventricular Tachycardia. Card Electrophysiol Clin. 2017;9:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Sakamoto K, Nozoe M, Tsutsui Y, Suematsu N, Kubota T, Okabe M, Yamamoto Y. Successful bipolar ablation for ventricular tachycardia with potential substrate identification by pre-procedural cardiac magnetic resonance imaging. Int Med Case Rep J. 2017;10:167-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Vaseghi M, Barwad P, Malavassi Corrales FJ, Tandri H, Mathuria N, Shah R, Sorg JM, Gima J, Mandal K, Sàenz Morales LC. Cardiac Sympathetic Denervation for Refractory Ventricular Arrhythmias. J Am Coll Cardiol. 2017;69:3070-3080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 22. | Kopecky K, Afzal A, Felius J, Hall SA, Mendez JC, Assar M, Mason DP, Bindra AS. Bilateral sympathectomy for treatment of refractory ventricular tachycardia. Pacing Clin Electrophysiol. 2018;41:93-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Amer M, Burrows WM, Dickfeld TM. Unilateral sympathetic ganglion denervation in the management of sustained ventricular tachycardia. HeartRhythm Case Rep. 2017;3:467-469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Rajesh MC, Deepa KV, Ramdas EK. Stellate Ganglion Block as Rescue Therapy in Refractory Ventricular Tachycardia. Anesth Essays Res. 2017;11:266-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Feyz L, Wijchers S, Daemen J. Renal denervation as a treatment strategy for vasospastic angina induced ventricular tachycardia. Neth Heart J. 2017;25:596-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Aksu T, Güler TE, Özcan KS, Bozyel S, Yalın K. Renal sympathetic denervation assisted treatment of electrical storm due to polymorphic ventricular tachycardia in a patient with cathecolaminergic polymorphic ventricular tachycardia. Turk Kardiyol Dern Ars. 2017;45:441-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Aksu T, Guler E. Percutaneous renal sympathetic denervation in catecholaminergic polymorphic ventricular tachycardia. J Arrhythm. 2017;33:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Wang L, Fahimian B, Soltys SG, Zei P, Lo A, Gardner EA, Maguire PJ, Loo BW Jr. Stereotactic Arrhythmia Radioablation (STAR) of Ventricular Tachycardia: A Treatment Planning Study. Cureus. 2016;8:e694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Cuculich PS, Schill MR, Kashani R, Mutic S, Lang A, Cooper D, Faddis M, Gleva M, Noheria A, Smith TW. Noninvasive Cardiac Radiation for Ablation of Ventricular Tachycardia. N Engl J Med. 2017;377:2325-2336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 491] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 30. | Marai I, Andria N, Gurevitz O. Cryoablation for Ventricular Tachycardia Originating from Anterior Papillary Muscle of Left Ventricle Guided by Intracardiac Echocardiography. Case Rep Cardiol. 2017;2017:9734795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Rivera S, Ricapito Mde L, Tomas L, Parodi J, Bardera Molina G, Banega R, Bueti P, Orosco A, Reinoso M, Caro M. Results of Cryoenergy and Radiofrequency-Based Catheter Ablation for Treating Ventricular Arrhythmias Arising From the Papillary Muscles of the Left Ventricle, Guided by Intracardiac Echocardiography and Image Integration. Circ Arrhythm Electrophysiol. 2016;9:e003874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Shenasa M, Miller JM, Callans DJ, Almendral JM, Marchlinski FE, Buxton AE. Conquest of Ventricular Tachycardia: Insights Into Mechanisms, Innovations in Management: Contribution of Mark E. Josephson, MD, to Clinical Electrophysiology. Circ Arrhythm Electrophysiol. 2017;10:pii: e005150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Higuchi T, Tsutsumi Y, Monta O, Asada S, Matsumoto R, Yamada S, Ohashi H. Surgical treatment for endocardial radiofrequency ablation-resistant sustained monomorphic ventricular tachycardia with mural thrombus including dense calcification in the left ventricle. Gen Thorac Cardiovasc Surg. 2017; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Li A, Hayase J, Do D, Buch E, Vaseghi M, Ajijola OA, Macias C, Krokhaleva Y, Khakpour H, Boyle NG. Hybrid surgical vs percutaneous access epicardial ventricular tachycardia ablation. Heart Rhythm. 2018;15:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Berte B, Sacher F, Wielandts JY, Mahida S, Pillois X, Weerasooriya R, Bernus O, Jaïs P. A new cryoenergy for ventricular tachycardia ablation: a proof-of-concept study. Europace. 2017;19:1401-1407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Liang JJ, Betensky BP, Muser D, Zado ES, Anter E, Desai ND, Callans DJ, Deo R, Frankel DS, Hutchinson MD. Long-term outcome of surgical cryoablation for refractory ventricular tachycardia in patients with non-ischemic cardiomyopathy. Europace. 2018;20:e30-e41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Bhandary SP, Joseph N, Hofmann JP, Saranteas T, Papadimos TJ. Extracorporeal life support for refractory ventricular tachycardia. Ann Transl Med. 2017;5:73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Okabe T, Yakushiji T, Hiroe M, Oyama Y, Igawa W, Ono M, Kido T, Ebara S, Yamashita K, Yamamoto MH. Steroid pulse therapy was effective for cardiac sarcoidosis with ventricular tachycardia and systolic dysfunction. ESC Heart Fail. 2016;3:288-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Roston TM, Cunningham TC, Sanatani S. Advances in the diagnosis and treatment of catecholaminergic polymorphic ventricular tachycardia. Cardiol Young. 2017;27:S49-S56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Arakawa J, Hamabe A, Aiba T, Nagai T, Yoshida M, Touya T, Ishigami N, Hisadome H, Katsushika S, Tabata H. A novel cardiac ryanodine receptor gene (RyR2) mutation in an athlete with aborted sudden cardiac death: a case of adult-onset catecholaminergic polymorphic ventricular tachycardia. Heart Vessels. 2015;30:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Kawata H, Ohno S, Aiba T, Sakaguchi H, Miyazaki A, Sumitomo N, Kamakura T, Nakajima I, Inoue YY, Miyamoto K. Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) Associated With Ryanodine Receptor (RyR2) Gene Mutations- Long-Term Prognosis After Initiation of Medical Treatment. Circ J. 2016;80:1907-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 42. | Denegri M, Bongianino R, Lodola F, Boncompagni S, De Giusti VC, Avelino-Cruz JE, Liu N, Persampieri S, Curcio A, Esposito F. Single delivery of an adeno-associated viral construct to transfer the CASQ2 gene to knock-in mice affected by catecholaminergic polymorphic ventricular tachycardia is able to cure the disease from birth to advanced age. Circulation. 2014;129:2673-2681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 43. | Lodola F, Morone D, Denegri M, Bongianino R, Nakahama H, Rutigliano L, Gosetti R, Rizzo G, Vollero A, Buonocore M. Adeno-associated virus-mediated CASQ2 delivery rescues phenotypic alterations in a patient-specific model of recessive catecholaminergic polymorphic ventricular tachycardia. Cell Death Dis. 2016;7:e2393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 44. | Li N, Wang Q, Sibrian-Vazquez M, Klipp RC, Reynolds JO, Word TA, Scott L Jr, Salama G, Strongin RM, Abramson JJ, Wehrens XHT. Treatment of catecholaminergic polymorphic ventricular tachycardia in mice using novel RyR2-modifying drugs. Int J Cardiol. 2017;227:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Arenal A, Pérez-David E, Avila P, Fernández-Portales J, Crisóstomo V, Báez C, Jiménez-Candil J, Rubio-Guivernau JL, Ledesma-Carbayo MJ, Loughlin G. Noninvasive identification of epicardial ventricular tachycardia substrate by magnetic resonance-based signal intensity mapping. Heart Rhythm. 2014;11:1456-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Klein T, Abdulghani M, Smith M, Huang R, Asoglu R, Remo BF, Turgeman A, Mesubi O, Sidhu S, Synowski S. Three-dimensional 123I-meta-iodobenzylguanidine cardiac innervation maps to assess substrate and successful ablation sites for ventricular tachycardia: feasibility study for a novel paradigm of innervation imaging. Circ Arrhythm Electrophysiol. 2015;8:583-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 47. | Zhang J, Cooper DH, Desouza KA, Cuculich PS, Woodard PK, Smith TW, Rudy Y. Electrophysiologic Scar Substrate in Relation to VT: Noninvasive High-Resolution Mapping and Risk Assessment with ECGI. Pacing Clin Electrophysiol. 2016;39:781-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Luther V, Linton NW, Jamil-Copley S, Koa-Wing M, Lim PB, Qureshi N, Ng FS, Hayat S, Whinnett Z, Davies DW. A Prospective Study of Ripple Mapping the Post-Infarct Ventricular Scar to Guide Substrate Ablation for Ventricular Tachycardia. Circ Arrhythm Electrophysiol. 2016;9:pii: e004072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | Jamil-Copley S, Vergara P, Carbucicchio C, Linton N, Koa-Wing M, Luther V, Francis DP, Peters NS, Davies DW, Tondo C. Application of ripple mapping to visualize slow conduction channels within the infarct-related left ventricular scar. Circ Arrhythm Electrophysiol. 2015;8:76-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |