Published online Jun 26, 2018. doi: 10.4330/wjc.v10.i6.41
Peer-review started: January 31, 2018
First decision: February 27, 2018
Revised: March 1, 2018
Accepted: March 18, 2018
Article in press: March 18, 2018
Published online: June 26, 2018
To investigate if patent foramen ovale (PFO) closure device reduces the risk of recurrent stroke in patients with cryptogenic stroke.
We searched five databases - PubMed, EMBASE, Cochrane, CINAHL and Web-of-Science and clinicaltrials.gov from January 2000 to September 2017 for randomized trials comparing PFO closure to medical therapy in cryptogenic stroke. Heterogeneity was determined using Cochrane’s Q statistics. Random effects model was used.
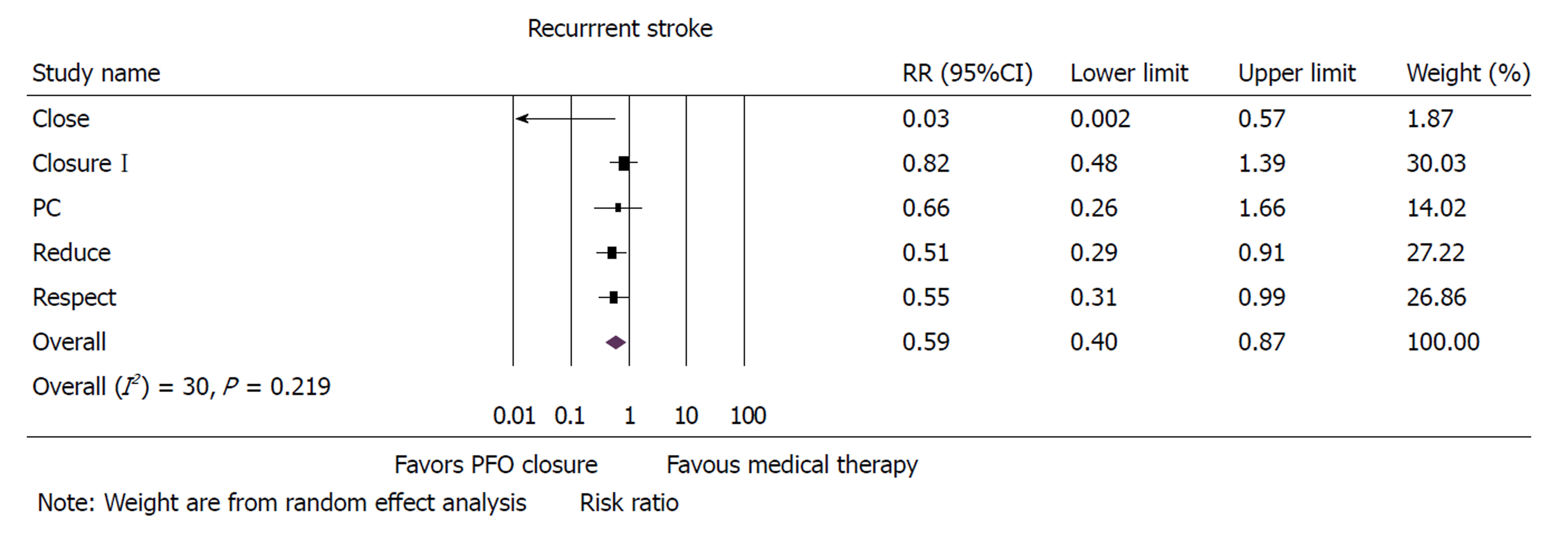
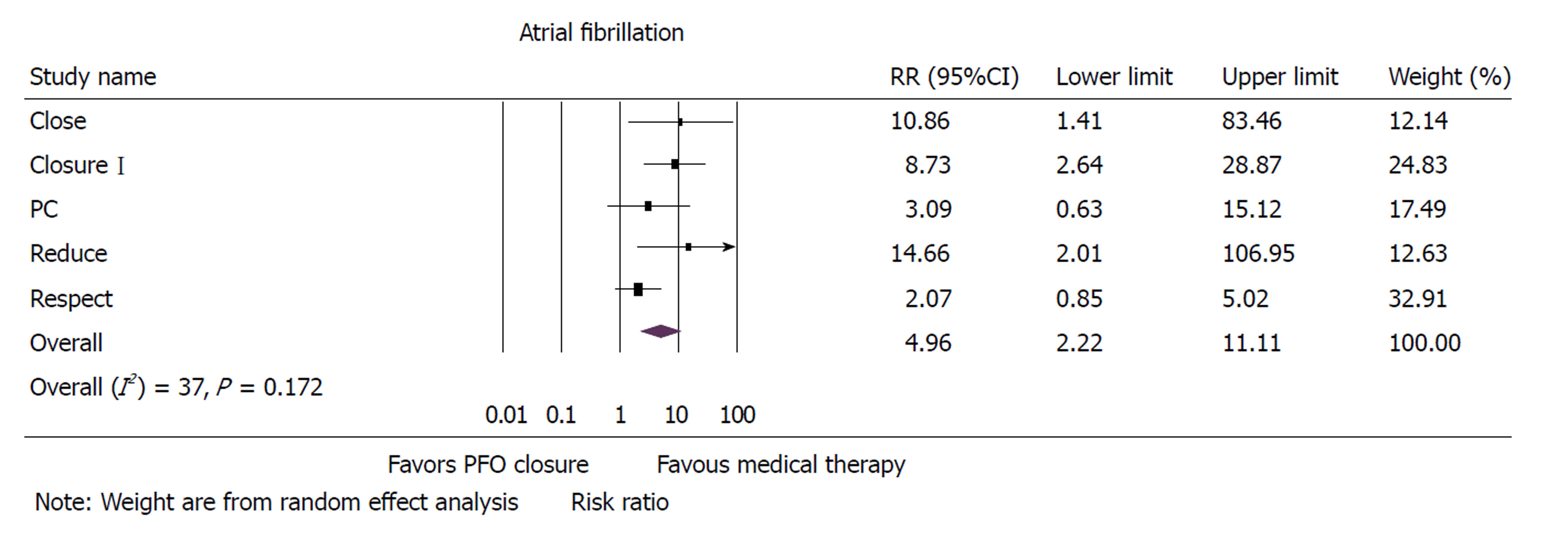
Five randomized controlled trials with 3440 patients were included in the analysis. Mean follow-up was 50 ± 20 mo. PFO closure was associated with a 41% reduction in incidence of recurrent strokes when compared to medical therapy alone in patients with cryptogenic stroke [risk ratio (RR): 0.59, 95%CI: 0.40-0.87, P = 0.008]. Atrial fibrillation was higher with device closure when compared to medical therapy alone (RR: 4.97, 95%CI: 2.22-11.11, P < 0.001). There was no difference between the two groups with respect to all-cause mortality, major bleeding or adverse events.
PFO device closure in appropriately selected patients with moderate to severe right-to-left shunt and/or atrial septal aneurysm shows benefit with respect to recurrent strokes, particularly in younger patients. Further studies are essential to evaluate the impact of higher incidence of atrial fibrillation seen with the PFO closure device on long-term mortality and stroke rates.
Core tip: The American Association of Neurology guidelines focused update recommended against routine patent foramen ovale (PFO) closure in patients with cryptogenic stroke; but two recent randomized trials showed a significant reduction in recurrent stroke events in patients who had a PFO closure device when compared to patients on medical therapy alone. We therefore, performed a systematic review & meta-analysis of five available randomized controlled clinical trials that addressed the efficacy of PFO closure in patients with cryptogenic stroke. Our analysis shows PFO closure device in appropriately selected patient population is associated with reduction in recurrent stroke events but at the cost of increase in incidence of atrial fibrillation.
- Citation: Anantha-Narayanan M, Anugula D, Das G. Patent foramen ovale closure reduces recurrent stroke risk in cryptogenic stroke: A systematic review and meta-analysis of randomized controlled trials. World J Cardiol 2018; 10(6): 41-48
- URL: https://www.wjgnet.com/1949-8462/full/v10/i6/41.htm
- DOI: https://dx.doi.org/10.4330/wjc.v10.i6.41
The prevalence of patent foramen ovale (PFO) in the general population is approximately 15%-25%[1-3]. Cryptogenic stroke accounts for 25% of the strokes in the United States[4,5]. Although PFO is strongly associated with cryptogenic stroke, the incidence of PFO is approximately only 40%-60% in patients with cryptogenic stroke suggesting all cryptogenic strokes are not essentially secondary to a PFO[6,7]. The issue with selecting patients for PFO closure is that in patients with PFO, the overall incidence of recurrence of PFO related cryptogenic strokes is much lower than the incidence of non-PFO related strokes[8,9]. In the PFO related cryptogenic stroke population, one third of the recurrent stroke risk is not related to the PFO itself and closure of PFO would not prevent the risk of recurrent stroke in this population[10,11]. Previously published randomized controlled trials (RCTs) evaluated device closure of PFO as compared to medical therapy but results were limited by very low event rates, lack of appropriate patient selection and large dropout rates at follow up[12,13]. Considering this, we performed a systematic review and meta-analysis of all the published trials including the three recent RCTs[14-16] to compare device closure to medical therapy for PFO in patients with cryptogenic stroke.
We performed a search of five databases-EMBASE, Pub-Med, Cochrane, WOS and CINAHL for RCTs between January 2000 and September 2017 using the following: “cerebrovascular disease”, “patent foramen ovale”, “atrial septal defect”, “cryptogenic strokes”, “anti coagulation therapy”, “anti platelet therapy” and their various combinations. Our search was limited to English language and we included adult population only. We also searched clinicaltrials.gov and performed a secondary search of the references of the relevant articles. The methodology has been validated and published in previous studies[17].
Studies need to meet the following criteria to be eligible for inclusion: (1) RCT; (2) age > 18 years of age; (3) compare PFO device closure to medical therapy in patients with cryptogenic stroke; and (4) report the estimate of relative-risk (RR) with a 95% confidence interval (CI), or any other equivalent measures of RR including odds ratio, hazard ratio or provide other forms of data from which effect size could be calculated. After relevant exclusions, the final study population was extracted from five studies. Our search strategy is shown in Supplementary Figure 1.
Two reviewers independently reviewed the abstracts, titles of the individual studies and selected full-length articles identified by the above-mentioned search strategy to include/exclude studies. The reviewers also independently extracted and abstracted the data from these studies including design, methods, study characteristics, and other relevant outcomes. Any discrepancy between the first and second authors was resolved by consensus or by consulting with the third author.
Our study included patients with cryptogenic stroke and PFO, who received either PFO device closure or medical therapy (anti platelet therapy). Age criteria of the individual trials are shown in Table 1. Trials enrolled patients with cryptogenic stroke in the 3 to 6 mo prior to randomization. While CLOSE[14], REDUCE[15] and RESPECT[16] only included cryptogenic ischemic strokes, CLOSURE I[12] and PC[13] included transient ischemic attacks (TIAs) as well.
| Variables | Treatment groups | CLOSE(mean ± SD) or N | CLOSURE I(mean ± SD) or N | PC(mean ± SD) or N | REDUCE(mean ± SD) or N | RESPECT(mean ± SD) or N |
| Age (yr) | PFO Closure | 42.9 ± 10.1 | 46.3 ± 9.6 | 44.3 ± 10.2 | 45.4 ± 9.3 | 45.7 ± 9.7 |
| Medical therapy | 43.8 ± 10.5 | 45.7 ± 9.1 | 44.6 ± 10.1 | 44.8 ± 9.6 | 46.2 ± 10 | |
| Age range (yr) | PFO Closure | 16-60 | 18-60 | < 60 | 18-59 | 18-60 |
| Medical therapy | 16-60 | 18-60 | < 60 | 18-59 | 18-60 | |
| Male | PFO Closure | 137 | 233 | 92 | 261 | 268 |
| Medical therapy | 142 | 238 | 114 | 138 | 268 | |
| Smoker | PFO Closure | 68 | 96 | 52 | 63 | 75 |
| Medical therapy | 69 | 104 | 47 | 25 | 55 | |
| Hypertension | PFO Closure | 27 | 151 | 49 | 112 | 160 |
| Medical therapy | 24 | 131 | 58 | 58 | 163 | |
| Hyper | PFO Closure | 30 | 212 | 50 | - | 196 |
| lipidemia | Medical therapy | 36 | 189 | 62 | - | 195 |
| Diabetes mellitus | PFO Closure | 3 | - | 5 | 18 | 33 |
| Medical therapy | 9 | - | 6 | 10 | 41 | |
| CAD | PFO Closure | - | 6 | 4 | - | 19 |
| Medical therapy | - | 4 | 4 | - | 9 | |
| Family hx of CAD or stroke | PFO Closure | - | 247 | 53 | - | 136 |
| Medical therapy | - | 257 | 40 | - | 109 | |
| CHF | PFO Closure | - | 2 | - | 3 | |
| Medical therapy | - | 0 | - | 0 | ||
| MI | PFO Closure | 0 | 7 | 3 | - | 5 |
| Medical therapy | 0 | 5 | 1 | - | 2 | |
| Cardiac catheterization | PFO Closure | - | 23 | - | - | - |
| Medical therapy | - | 17 | - | - | - | |
| Valvular disease | PFO Closure | - | 49 | 8 | - | - |
| Medical therapy | - | 45 | 5 | - | - | |
| Arrhythmia | PFO Closure | - | 26 | - | - | - |
| Medical therapy | - | 19 | - | - | - | |
| PTCA | PFO Closure | - | 6 | - | - | - |
| Medical therapy | - | 2 | - | - | - | |
| PVD | PFO Closure | - | 5 | 3 | - | 5 |
| Medical therapy | - | 7 | 2 | - | 1 | |
| Stokes-adams syndrome | PFO Closure | - | 4 | - | - | - |
| Medical therapy | - | 3 | - | - | - | |
| DVT or PE | PFO Closure | 5 | 0 | - | - | - |
| Medical therapy | 4 | 4 | - | - | - | |
| Migraine | PFO Closure | 67 | - | 47 | - | 195 |
| Medical therapy | 78 | - | 38 | - | 186 | |
| Pericarditis | PFO Closure | - | 2 | - | - | - |
| Medical therapy | - | 3 | - | - | - | |
| Cardio | PFO Closure | - | 1 | - | - | - |
| myopathy | Medical therapy | - | 0 | - | - | - |
| Index cryptogenic stroke | PFO Closure | 238 | 324 | 165 | 441 | - |
| Medical therapy | 235 | 329 | 163 | 223 | - | |
| Index TIA | PFO Closure | - | 122 | 33 | - | - |
| Medical therapy | - | 132 | 42 | - | - | |
| TEE with moderate- severe shunt | PFO Closure | - | 250 | 135 | 348 | 385 |
| Medical therapy | - | 231 | 112 | 173 | 352 | |
| Atrial septal aneurysm > 10 mm | PFO Closure | - | 158 | 47 | 86 | 180 |
| Medical therapy | - | 165 | 45 | - | 169 | |
| > 1 previous TIA or stroke | PFO Closure | 10 | - | 76 | 68 | 111 |
| Medical therapy | 7 | - | 79 | 24 | 112 |
Our primary outcome of interest was incidence of recurrent ischemic stroke. Secondary outcomes included incidence of atrial fibrillation, all-cause mortality, major bleeding and adverse events. We also compared TIA events between the device closure and the medical therapy group and reported outcomes from the available studies.
Random effects model was used to pool categorical data. Analysis of risk ratio (RR) with 95%CI limits was performed. Cochrane’s Q statistics was used to assess heterogeneity of the included studies for outcomes of interest. I2 values of < 25%, 25%-50%, and 50%-75% represented low, moderate and high heterogeneity respectively. Publication bias was visually assessed by using funnel plot. Whenever necessary, we included an exclusion-sensitivity analysis to minimize heterogeneity. We performed meta-regression when necessary study the impact of moderator variables on outcomes of interest. A P-value of < 0.05 was considered to be statistically significant. Analyses were performed by Mahesh Anantha-Narayanan using the software Comprehensive Meta-analysis (version 3.3)[18].
Five RCTs were included[12-16] in the final analysis. Table 1 shows the baseline characteristics of the included studies and patients groups used in the analysis.
The overall study population consisted of 3440 patients extracted from 5 RCTs and 1991 were males. Mean follow up time was 50 ± 20 mo. Mean age of the entire cohort was 45 ± 1.1 years. In CLOSE, 238 patients were assigned to the PFO closure group, 187 patients were assigned to the anti-coagulation group and 409 patients received anti-platelet therapy alone. Details of the included trials are listed in Table 2.
| Study name | Type of study | Devices used | Follow-up (median or mean), (mo) | PFO closure device | Medical therapy | Primary composite end point |
| CLOSE | Randomized multicenter | Amplatz PFO Occluder or Cribriform; Starflex; CardioSeal; Intrasept PFO; PFOStar; Helex; Premere; PFO occluder OCCLUTECH; PFO occluder GORE (GSO) | 64 | 238 | 235 | Fatal or nonfatal stroke |
| CLOSURE I | Prospective, multicenter, randomized, open-label, two-group superiority trial | STARFlex device (NMT Medical) | 22 | 447 | 462 | Composite of stroke or TIA during 2 yr of follow-up, death from any cause during the first 30 d, and death from neurologic causes between 31 d and 2 yr |
| PC | Multicenter, multinational, randomized, clinical trial | Amplatzer PFO Occluder (St. Jude Medical) | 54 | 204 | 210 | Composite of death, nonfatal stroke, TIA, or peripheral embolism |
| REDUCE | Multinational, randomized, clinical trial | Gore Helex or Gore Cardioform (WL Gore and Associates) septal occluders | 38 | 441 | 223 | Freedom from recurrent clinical ischemic stroke through at least 24 mo and incidence of new brain infarct |
| RESPECT | Prospective, multicenter, controlled, randomized, open-label clinical trial | Amplatzer PFO Occluder | 71 | 499 | 481 | Recurrent nonfatal ischemic stroke, fatal ischemic stroke, or early death |
Risk of recurrent ischemic stroke was 41% lower in patients who received PFO device closure when compared to patients who received medical therapy alone (RR: 0.59, 95%CI: 0.40-0.87, P = 0.008) (Figure 1). Sensitivity analysis with exclusion of the study with the maximum strength[12] did not alter the results (RR: 0.51, 95%CI: 0.33-0.81, P = 0.004). Funnel plot showed very minimal bias (Supplementary Figure 2) and heterogeneity within the included studies was found to be moderate (I2 = 30). A meta-regression of incidence of recurrent stroke on follow-up time was insignificant (Supplementary Figure 3) (P = 0.408). Incidence of TIA was not different between PFO closure and medical therapy (RR: 0.78, 95%CI: 0.48-1.25, P = 0.301).
Atrial fibrillation was significantly higher in the PFO closure group when compared to group that received medical therapy (RR: 4.97, 95% CI: 2.22-11.11, P < 0.001) (Figure 2). Sensitivity analysis with exclusion of study with maximum strength[12] did not change the results (RR: 7.57, 95%CI: 3.42-16.72, P < 0.001). Heterogeneity within the included studies was moderate (I2 = 37).
All-cause mortality was similar between the groups (RR: 1.09, 95%CI: 0.47-2.53, P = 0.839) (Supplementary Figure 4). Sensitivity analysis performed with exclusion of study with maximum strength[16] did not change the results (RR: 1.04, 95%CI: 0.27-4.02, P = 0.952). Heterogeneity of the included studies was low (I2 = 0).
Major bleeding events were similar between PFO closure and medical therapy groups (RR: 0.76, 95%CI: 0.36-1.58, P = 0.466) (Supplementary Figure 5). Sensitivity analysis performed with exclusion of study with the maximum strength[15] did not alter the results (RR: 0.75, 95%CI: 0.27-2.11, P = 0.587). Heterogeneity within the included studies was moderate (I2 = 28).
Rate of adverse events did not differ between the PFO device closure and the medical therapy groups (RR: 0.94, 95%CI: 0.83-1.07, P = 0.343) (Supplementary Figure 6). Sensitivity analysis excluding the study with maximum strength[13] did not affect the results (RR: 0.90, 95%CI: 0.74-1.10, P = 0.299). Heterogeneity of the included studies was low (I2 = 0).
Results from our current meta-analysis show that PFO device closure reduces the risk of recurrent stroke in appropriately selected patients with cryptogenic stroke. There was a higher incidence of atrial fibrillation associated with PFO closure but there was no significant difference in all-cause mortality, major bleeding or adverse events between the groups.
The usage of PFO closure devices, especially the Amplatzer device, increased steadily since 1998 until 2006 when the device was voluntarily withdrawn by the dealer[19]. The device did not qualify for Humanitarian device exemption (HDE) related to its increased usage without supporting evidence[19]. The American Association of Neurology issued a practice update in August 2016 recommending against the routine use of PFO closure devices in cryptogenic stroke patients[20]. Following this update, the Amplatzer closure device regained its approval for use in patients with PFO by the Center for Devices and Radiological Health of the Food and Drug Administration. The approval comes with an advisory stating that the decision to implant the device should be determined by a cardiologist and a neurologist after excluding the other common etiologies of stroke.
Guidelines for PFO closure in cryptogenic stroke patients come from two major RCTs[12,13] along with the 2 year follow-up data of the third RCT, the RESPECT trial[21]. CLOSURE I compared STARFlex device to medical therapy in patients with cryptogenic stroke. The trial reported no difference in recurrent stroke events between PFO device closure and medical therapy[12]. Also, there were significantly higher numbers of device related procedural complications. Interpretation of results from the trial was limited by lower number of events when compared to number of patients lost at follow up. Following this came PC[13] and RESPECT[21] that compared Amplatzer closure device with medical therapy for prevention of cryptogenic stroke. Though results from the individual trials did not report any meaningful difference with respect to recurrent ischemic strokes, previous meta analyses and pooled analysis including these trials showed a significant reduction in incidence of recurrent ischemic stroke events with the use of Amplatzer device[22,23]. Combined analysis also showed significantly higher rates of atrial fibrillation in PFO device closure group[22,23]. Recently the long-term follow-up data from RESPECT showed a significant reduction in recurrent ischemic stroke events in the PFO device closure group compared to medical therapy[16] as well as the two other major RCTs - CLOSE and REDUCE.
It is worthwhile discussing the inclusion criteria of the previously published trials. CLOSURE I, PC and RESPECT included patients with any PFO size with or without atrial septal aneurysm. In these initial trials, there was an overall higher dropout rate for the very low event rate at follow up. Notably, there were issues with patient selection. For example, patients with small PFO and patients with concomitant atrial fibrillation were included in these trials. In these patients, atrial fibrillation and concomitant coronary disease increases the risk of arterial stroke and these patients may not essentially benefit from PFO closure.
The CLOSE, REDUCE and RESPECT trials recently reported long term outcomes in patients with large sized PFO or patients with atrial septal aneurysm. Whereas REDUCE used GORE Helex or GORE Cardioform device, CLOSE included multiple devices as listed in Table 2. CLOSE showed a significant reduction in recurrent ischemic stroke events in patients with PFO closure device when compared to medical therapy alone. The trial also compared anti platelet to anti-coagulation therapy and reported no meaningful difference in recurrent ischemic strokes between the two groups. We did not have similar data from the other trials to compare efficacy of anti-coagulation therapy to anti-platelet therapy. REDUCE showed a 77% reduction in the incidence of recurrent ischemic strokes with PFO closure therapy. The trial also showed a 49% reduction in new brain infarcts on MRI. CLOSE and REDUCE differ significantly from the previously published RCTs as these trials employed very strict exclusion criteria to exclude patients with other source of emboli including patients with atrial fibrillation, coronary artery disease and small vessel disease. CLOSE only studied patients with a large PFO or an atrial septal aneurysm whereas REDUCE included patients mostly with moderate to severe right-to-left shunt. Also, the definitions employed were considerably strict to avoid including symptoms that could mimic a TIA.
Another interesting result in CLOSE was the higher incidence of recurrent ischemic strokes in patients with concurrent PFO and atrial septal aneurysm when compared to patients with a PFO alone suggesting that aneurysmal atrial septum is associated with higher risk of recurrent strokes. We could not analyze this effect as shunt sizes were not reported in the other studies. The diagnosis of PFO can be challenging in some patients. The presence of an eustachian valve (EV) can potentially lead to a false negative arm based echocardiographic bubble study in the presence of PFO as the valve redirects contrast free blood from inferior vena cava to atrial septum, thereby preventing the contrast rich superior vena cava blood from reaching the left side. Previous investigators demonstrated that the detection of atrial septal defect was enhanced when contrast agent was delivered into inferior vena cava rather than the superior vena cava[24-26]. CLOSE investigators recommended looking for an EV during TEE but none of the included trials compared superior vs inferior vena caval injection techniques.
The strength of our meta-analysis is the inclusion of only RCTs to avoid potential patient selection bias. The previous RCTs including RESPECT, CLOSURE I and PC suffered slow recruitment which could introduce a potential recruitment bias. Previous studies included patients with small to medium PFOs and combining these trials with the REDUCE and CLOSE trial with strict inclusion criteria for PFO may create bias. Trials did not differentiate between cryptogenic and non-cryptogenic strokes at follow up. Also, studies did not have a long term atrial fibrillation follow up data. The variable definitions used across the studies for major bleeding may create bias. We did not have patient level data to assess outcomes for sub-groups with different shunt sizes. Studies used different PFO closure devices (Amplatzer in PC and RESPECT, GORE Helex/Cardioform in REDUCE, STARFLEX in CLOSURE I whereas CLOSE used multiple closure devices) and so we did not have enough power to compare outcomes between various PFO closure devices. Studies reporting device complications were limited in number making it difficult to draw strong conclusions. Finally, publication bias is a limitation of any meta-analysis.
In summary, this systematic review and meta-analysis of the published RCTs supports PFO device closure in selected patients with cryptogenic stroke, especially with moderate and large sized shunt and/or with atrial septal aneurysm. PFO closure is associated with a lower incidence of recurrent ischemic strokes but carries a higher risk of atrial fibrillation. Further RCTs to study the long-term effect of atrial fibrillation on recurrent stroke events are essential.
Cryptogenic stroke accounts for one-fourth of the ischemic strokes and the presumed mechanism is venous thromboembolisms entering systemic circulation via patent foramen ovale (PFO). Percutaneous device closure of PFO has been shown to reduce stroke rates but there is lack of evidence on whether percutaneous closure of PFO is better when compared to medical therapy with antiplatelet and/or anticoagulation. Previously published randomized controlled trials (RCTs) comparing PFO closure to medical therapy lacked appropriate patient selection and had large dropout rates at follow up. Based on this available data, the American Association of Neurology (AAN) guidelines recommended against PFO device closure
Though current guidelines do not support PFO device closure, two recently published RCTs showed reduction in incidence of recurrent strokes with PFO closure in appropriately selected patient population with cryptogenic stroke. We therefore performed a systematic review and meta-analysis to evaluate if PFO closure is superior to medical therapy alone including all published RCTs to date.
The purpose of the study is to analyze if PFO closure device is superior to medical therapy alone to prevent recurrent strokes in appropriately selected patient population with cryptogenic stroke.
We searched five databases for studies comparing PFO device closure to medical therapy in patients with cryptogenic stroke. To qualify for inclusion, trials must have a randomized design, include patients > 18 years of age and compare PFO closure to medical therapy in patients with cryptogenic stroke. We obtained a total of five randomized controlled trials for inclusion and performed a meta-analysis. Our primary outcome was incidence of recurrent ischemic stroke. We also looked at secondary outcomes including incidence of atrial fibrillation, all-cause mortality, major bleeding and adverse events.
PFO device closure in appropriately selected patient population with cryptogenic stroke is superior to medical therapy alone in reducing incidence of recurrent strokes. There was no difference between the PFO device closure and the medical therapy groups in terms of overall mortality, major bleeding and adverse events but there was a significant increase in incidence of atrial fibrillation in the closure device group.
Our current meta-analysis including all published randomized controlled trials comparing PFO closure device to medical therapy alone supports PFO device closure in appropriately selected patient population. PFO closure in younger patients with moderate to large PFO and with atrial septal aneurysm is clearly associated with reduction in incidence of recurrent strokes without increasing mortality, major bleeding or adverse events. There is an increase in atrial fibrillation with PFO closure compared to medical therapy alone but this was mostly in the immediate post-operative period.
From this meta-analysis, it could be seen that PFO closure device reduces risk of recurrent stroke in appropriately selected patient population with cryptogenic stroke. PFO closure is associated with increase in atrial fibrillation but this could likely be an organic phenomenon related to atrial irritation from the device itself. Further studies are essential to address whether this increase in atrial fibrillation rates with PFO closure device is associated with adverse outcomes on long term follow up.
Manuscript source: Unsolicited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Vermeersch P S- Editor: Wang XJ L- Editor: A E- Editor: Huang Y
| 1. | Schneider B, Zienkiewicz T, Jansen V, Hofmann T, Noltenius H, Meinertz T. Diagnosis of patent foramen ovale by transesophageal echocardiography and correlation with autopsy findings. Am J Cardiol. 1996;77:1202-1209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 169] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 2. | Meissner I, Khandheria BK, Heit JA, Petty GW, Sheps SG, Schwartz GL, Whisnant JP, Wiebers DO, Covalt JL, Petterson TM. Patent foramen ovale: innocent or guilty? Evidence from a prospective population-based study. J Am Coll Cardiol. 2006;47:440-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 299] [Cited by in F6Publishing: 265] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 3. | Hagen PT, Scholz DG, Edwards WD. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clin Proc. 1984;59:17-20. [PubMed] [Cited in This Article: ] |
| 4. | Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, Sacco RL, Connolly SJ; Cryptogenic Stroke/ESUS International Working Group. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2014;13:429-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 980] [Cited by in F6Publishing: 1046] [Article Influence: 104.6] [Reference Citation Analysis (0)] |
| 5. | Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, Glahn J, Brandt T, Hacke W, Diener HC. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke. 2001;32:2559-2566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 534] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 6. | Lechat P, Mas JL, Lascault G, Loron P, Theard M, Klimczac M, Drobinski G, Thomas D, Grosgogeat Y. Prevalence of patent foramen ovale in patients with stroke. N Engl J Med. 1988;318:1148-1152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1167] [Cited by in F6Publishing: 1055] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 7. | Job FP, Ringelstein EB, Grafen Y, Flachskampf FA, Doherty C, Stockmanns A, Hanrath P. Comparison of transcranial contrast Doppler sonography and transesophageal contrast echocardiography for the detection of patent foramen ovale in young stroke patients. Am J Cardiol. 1994;74:381-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 135] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Homma S, Sacco RL. Patent foramen ovale and stroke. Circulation. 2005;112:1063-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 274] [Cited by in F6Publishing: 264] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, Coste J; Patent Foramen Ovale and Atrial Septal Aneurysm Study Group. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001;345:1740-1746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 969] [Cited by in F6Publishing: 853] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 10. | Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke. 2009;40:2349-2355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 243] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 11. | Mono ML, Geister L, Galimanis A, Jung S, Praz F, Arnold M, Fischer U, Wolff S, Findling O, Windecker S. Patent foramen ovale may be causal for the first stroke but unrelated to subsequent ischemic events. Stroke. 2011;42:2891-2895. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Furlan AJ, Reisman M, Massaro J, Mauri L, Adams H, Albers GW, Felberg R, Herrmann H, Kar S, Landzberg M. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012;366:991-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 730] [Cited by in F6Publishing: 674] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 13. | Meier B, Kalesan B, Mattle HP, Khattab AA, Hildick-Smith D, Dudek D, Andersen G, Ibrahim R, Schuler G, Walton AS. Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med. 2013;368:1083-1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 633] [Cited by in F6Publishing: 597] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 14. | Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, Arquizan C, Béjot Y, Vuillier F, Detante O. Patent Foramen Ovale Closure or Anticoagulation vs. Antiplatelets after Stroke. N Engl J Med. 2017;377:1011-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 660] [Cited by in F6Publishing: 648] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 15. | Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, Settergren M, Sjöstrand C, Roine RO, Hildick-Smith D. Patent Foramen Ovale Closure or Antiplatelet Therapy for Cryptogenic Stroke. N Engl J Med. 2017;377:1033-1042. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 654] [Cited by in F6Publishing: 654] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 16. | Saver JL, Carroll JD, Thaler DE, Smalling RW, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Stroke. N Engl J Med. 2017;377:1022-1032. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 628] [Cited by in F6Publishing: 605] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 17. | Anantha Narayanan M, Mahfood Haddad T, Kalil AC, Kanmanthareddy A, Suri RM, Mansour G, Destache CJ, Baskaran J, Mooss AN, Wichman T. Early versus late surgical intervention or medical management for infective endocarditis: a systematic review and meta-analysis. Heart. 2016;102:950-957. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 18. | Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2715] [Cited by in F6Publishing: 3205] [Article Influence: 228.9] [Reference Citation Analysis (1)] |
| 19. | Slottow TL, Steinberg DH, Waksman R. Overview of the 2007 Food and Drug Administration Circulatory System Devices Panel meeting on patent foramen ovale closure devices. Circulation. 2007;116:677-682. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Messé SR, Gronseth G, Kent DM, Kizer JR, Homma S, Rosterman L, Kasner SE. Practice advisory: Recurrent stroke with patent foramen ovale (update of practice parameter): Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2016;87:815-821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 21. | Carroll JD, Saver JL, Thaler DE, Smalling RW, Berry S, MacDonald LA, Marks DS, Tirschwell DL; RESPECT Investigators. Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med. 2013;368:1092-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 682] [Cited by in F6Publishing: 626] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 22. | Rengifo-Moreno P, Palacios IF, Junpaparp P, Witzke CF, Morris DL, Romero-Corral A. Patent foramen ovale transcatheter closure vs. medical therapy on recurrent vascular events: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J. 2013;34:3342-3352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Kent DM, Dahabreh IJ, Ruthazer R, Furlan AJ, Reisman M, Carroll JD, Saver JL, Smalling RW, Jüni P, Mattle HP. Device Closure of Patent Foramen Ovale After Stroke: Pooled Analysis of Completed Randomized Trials. J Am Coll Cardiol. 2016;67:907-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 157] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 24. | Gin KG, Huckell VF, Pollick C. Femoral vein delivery of contrast medium enhances transthoracic echocardiographic detection of patent foramen ovale. J Am Coll Cardiol. 1993;22:1994-2000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 76] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Hamann GF, Schätzer-Klotz D, Fröhlig G, Strittmatter M, Jost V, Berg G, Stopp M, Schimrigk K, Schieffer H. Femoral injection of echo contrast medium may increase the sensitivity of testing for a patent foramen ovale. Neurology. 1998;50:1423-1428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 97] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Agmon Y, Khandheria BK, Meissner I, Gentile F, Sicks JD, O’Fallon WM, Whisnant JP, Wiebers DO, Seward JB. Comparison of frequency of patent foramen ovale by transesophageal echocardiography in patients with cerebral ischemic events versus in subjects in the general population. Am J Cardiol. 2001;88:330-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 1.7] [Reference Citation Analysis (0)] |