Copyright
©The Author(s) 2018.
World J Biol Chem. Oct 18, 2018; 9(1): 1-15
Published online Oct 18, 2018. doi: 10.4331/wjbc.v9.i1.1
Published online Oct 18, 2018. doi: 10.4331/wjbc.v9.i1.1
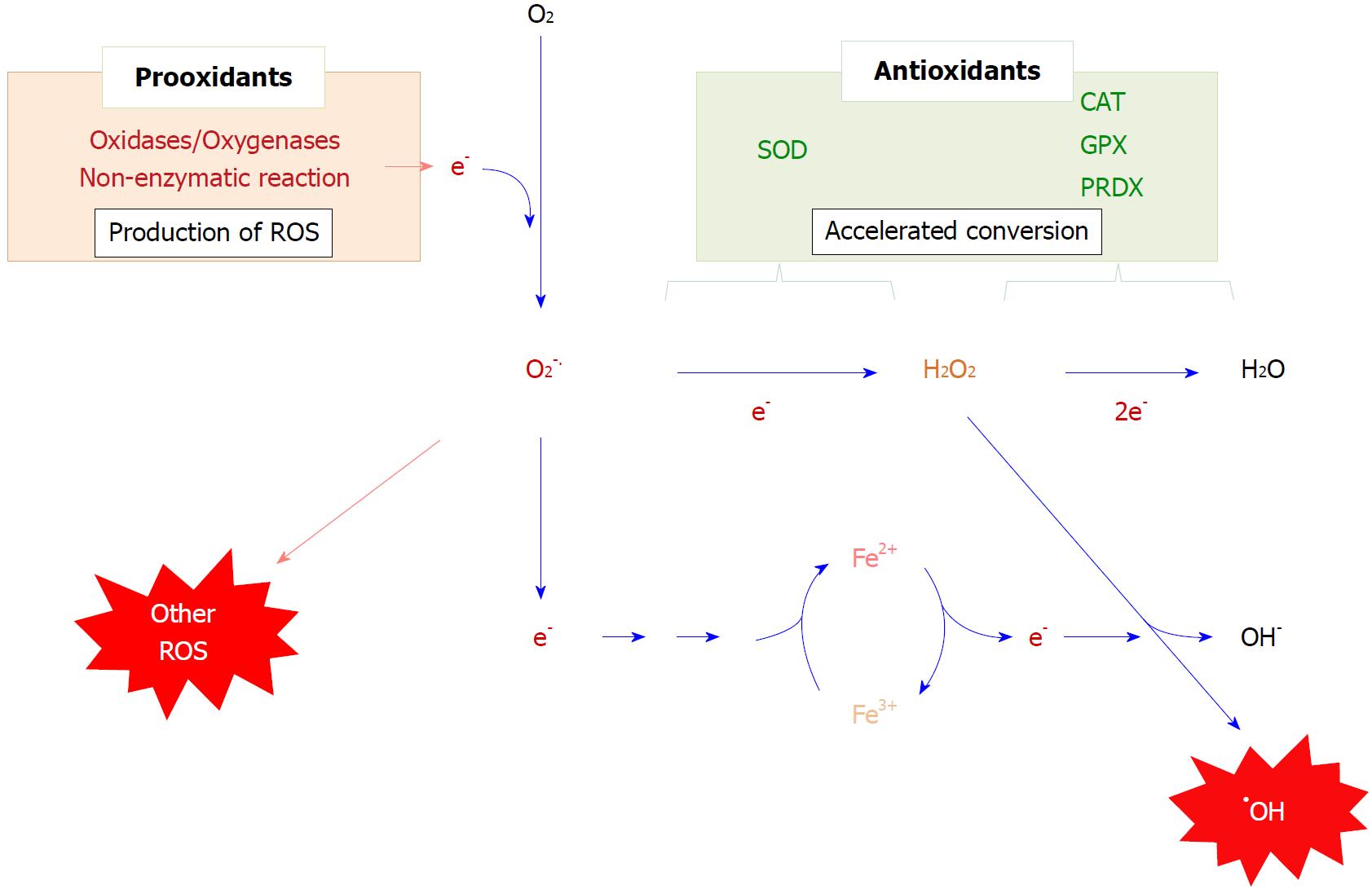
Figure 2 Production, conversion, and suppression of reactive oxygen species.
The production of reactive oxygen species is largely initiated by a one-electron donation to an oxygen molecule, resulting in the production of superoxide (O2.-). Superoxide undergoes spontaneous dismutation to hydrogen peroxide, a process that is markedly accelerated by superoxide dismutase. The resulting hydrogen peroxide can be rapidly eliminated by peroxidases, such as catalase, glutathione peroxidase and peroxiredoxin In the meantime, a one-electron reduction of a transition metal, notably ferric to ferrous ion, results in the conversion of hydrogen peroxide to hydroxyl radicals (•OH). ROS: Reactive oxygen species; SOD: Superoxide dismutase; CAT: Catalase; GPX: Glutathione peroxidase; PRDX: Peroxiredoxin.
- Citation: Fujii J, Homma T, Kobayashi S, Seo HG. Mutual interaction between oxidative stress and endoplasmic reticulum stress in the pathogenesis of diseases specifically focusing on non-alcoholic fatty liver disease. World J Biol Chem 2018; 9(1): 1-15
- URL: https://www.wjgnet.com/1949-8454/full/v9/i1/1.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v9.i1.1