Copyright
©The Author(s) 2016.
World J Biol Chem. Feb 26, 2016; 7(1): 14-43
Published online Feb 26, 2016. doi: 10.4331/wjbc.v7.i1.14
Published online Feb 26, 2016. doi: 10.4331/wjbc.v7.i1.14
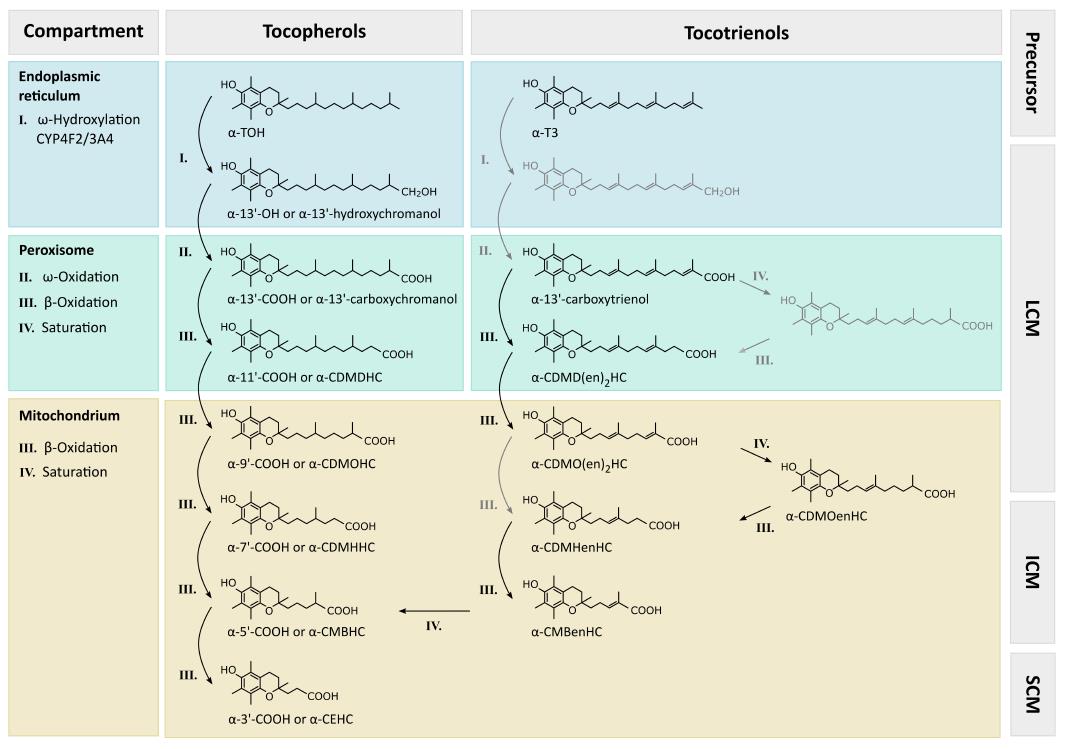
Figure 3 Metabolism of vitamin E.
The metabolism of vitamin E starts with one cycle of ω-hydroxylation followed by five cycles of β-oxidation. The principal catabolic pathway is independent of the saturation of the side-chain[136,137] or the substitution of the chromanol ring system[136], whereas the rate of metabolic degradation is modulated mostly by these two factors[136] (for further details, see the sections on α-TTP in chapter “Intracellular binding proteins” as well as CYP4F2 and kinetics in chapter “Metabolism of vitamin E”). While the intracellular compartmentation of the different reaction steps is known for TOHs[138], it remains unresolved for T3s. The same applies to the structures and arrows marked in grey; they reflect molecules or principle steps which fit into the general concept so far but need further experimental confirmation and molecular characterization. α-TOH: α-tocopherols; α-T3: α-tocotrienols; 13’-OH: 13’-hydroxychromanol; 13’-COOH: 13’-carboxychromanol; LCM: Long-chain metabolites; ICM: Intermediate-chain metabolites; SCM: Short-chain metabolites; CDMD(en)2HC: Carboxydimethyldecadienylhydroxychromanol; CDMOenHC: Carbodimethyloctenylhydroxychromanol; CDMHenHC: Carboxymethylhexenylhydroxychromanol; CMBenHC: Carboxymethylbutadienylhydroxychromanol; CDMOHC: Carboxymethyloctylhydroxychromanol; CDMHHC: Carboxymethylhexylhydroxychromanol; CMBHC: Carboxymethylbutylhydroxychromanol; CEHC: Carboxyethylhydroxychromanols.
- Citation: Schmölz L, Birringer M, Lorkowski S, Wallert M. Complexity of vitamin E metabolism. World J Biol Chem 2016; 7(1): 14-43
- URL: https://www.wjgnet.com/1949-8454/full/v7/i1/14.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v7.i1.14