Copyright
©2014 Baishideng Publishing Group Inc.
World J Biol Chem. May 26, 2014; 5(2): 204-215
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.204
Published online May 26, 2014. doi: 10.4331/wjbc.v5.i2.204
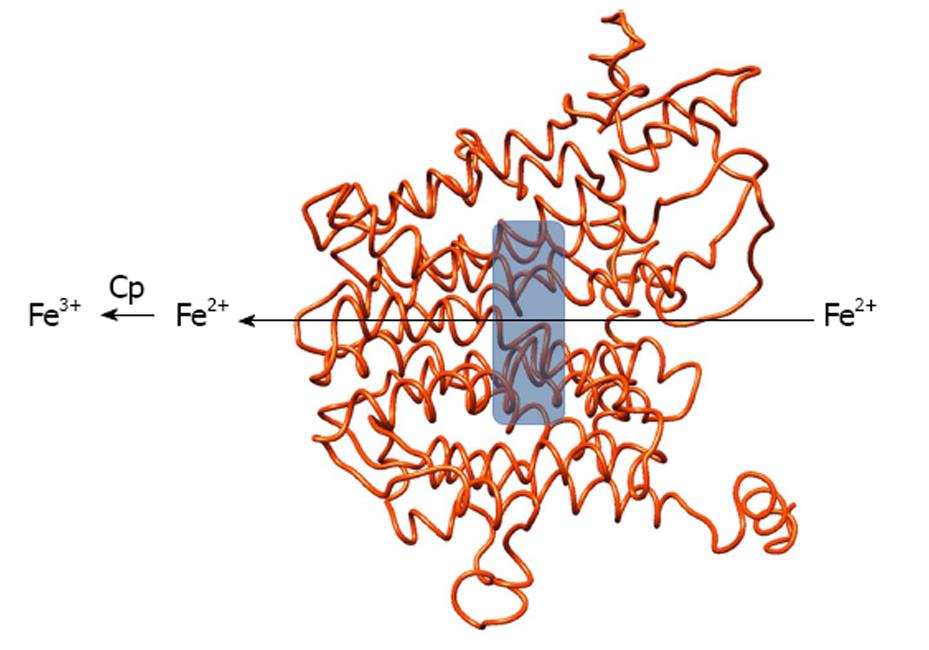
Figure 1 Structural model of human ferroportin viewed along the membrane plane.
The gray box indicates the location of a putative iron-binding site, ferrous iron flows through the protein from the cell interior and is then oxidized by ceruloplasmin at the extracellular side. The figure was produced with Chimera[96].
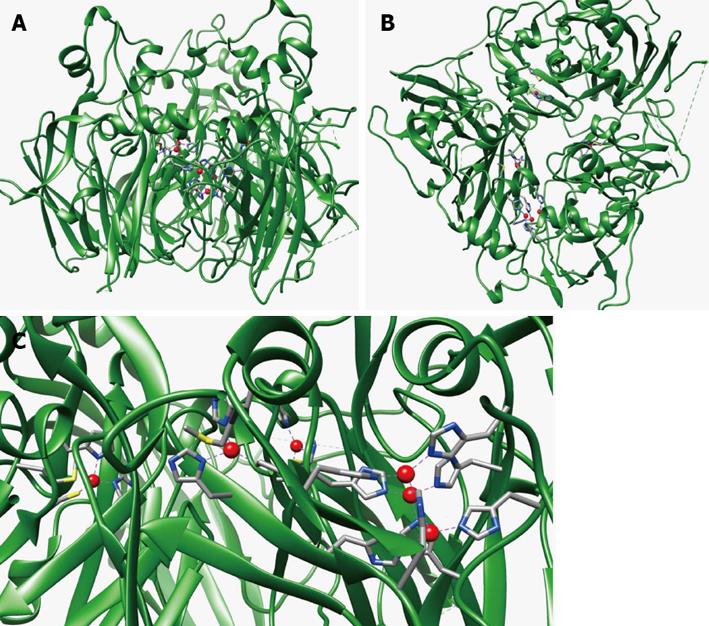
Figure 2 Structure of human ceruloplasmin.
Overall structure of the protein (PDB 1KCW) in two orientations (A: Side view and B: Bottom view), the copper atoms are shown as red spheres, the side chains of the copper ligands are represented as sticks; C: Close-up view of the type 1 and trinuclear cluster catalytic copper binding sites. The figure was produced with Chimera[96].
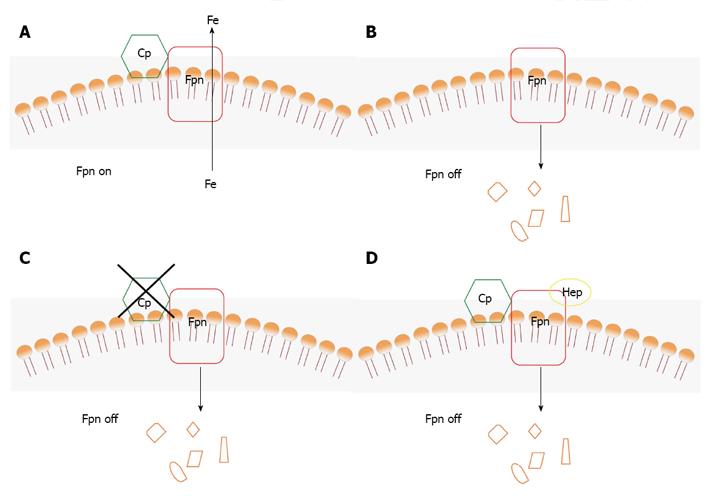
Figure 3 Scheme of the role of ceruloplasmin and hepcidin in the degradation of ferroportin.
A: In the presence of Cp, Fpn is stable and exports iron; B: In the absence of Cp, Fpn is degraded; C: In the presence of inactive Cp, Fpn is degraded; D: In the presence of Cp and hepcidin, Fpn is degraded. Cp: Ceruloplasmin; Fpn: Ferroportin.
- Citation: Musci G, Polticelli F, Bonaccorsi di Patti MC. Ceruloplasmin-ferroportin system of iron traffic in vertebrates. World J Biol Chem 2014; 5(2): 204-215
- URL: https://www.wjgnet.com/1949-8454/full/v5/i2/204.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v5.i2.204