Copyright
©2010 Baishideng Publishing Group Co.
World J Biol Chem. Sep 26, 2010; 1(9): 286-290
Published online Sep 26, 2010. doi: 10.4331/wjbc.v1.i9.286
Published online Sep 26, 2010. doi: 10.4331/wjbc.v1.i9.286
Figure 1 Benfang Lei, PhD, Department of Veterinary Molecular Biology, Montana State University, 960 Technology Blvd, PO Box 173610, Bozeman, MT 59717, United States.
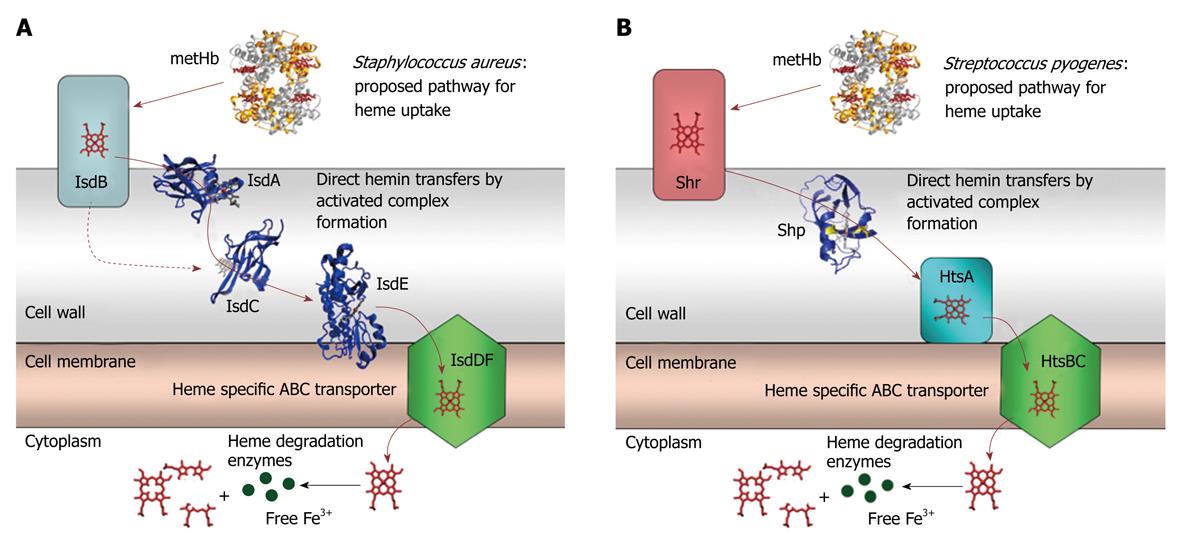
Figure 2 Cartoons for the proposed pathway of heme acquisition from metHb by the Staphylococcus aureus Isd (A) and Group A Streptococcus Shr/Shp/HtsABC (B) systems.
The arrows indicate the direction of direct heme transfer. Heme transfer from IsdB to IsdC represented by the dotted arrow may be prevented in vivo by their physical locations in the cell wall. The structure models of the proteins were from the coordinates 2Q8Q, 2ITF, 2O6P, 2Q7A, and 1HHO. This figure was originally published in J Biol Chem. Zhu H, Xie G, Liu M, Olson JS, Fabian M, Dooley DM, Lei B. Pathway for heme uptake from human methemoglobin by the iron-regulated surface determinants system of Staphylococcus aureus. J Biol Chem 2008; 283: 18450-18460[23].
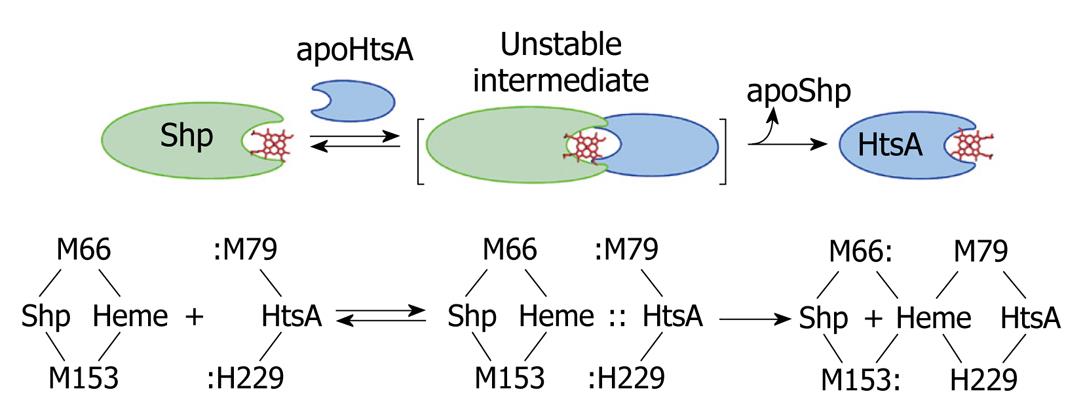
Figure 3 A direct heme axial ligand displacement model for the holoShp-holoHtsA reactions.
The side chains of the heme axial ligands, M79 and H229, in apoHtsA are proposed to be inserted into the axial positions of heme in Shp, simultaneously displace M66 and M153 of Shp, and extract heme from the donor. M66/M153 and M79/H229 are the axial ligand residues of the heme iron in Shp and HtsA, respectively.
- Citation: Lei B. Benfang Lei’s research on heme acquisition in Gram-positive pathogens and bacterial pathogenesis. World J Biol Chem 2010; 1(9): 286-290
- URL: https://www.wjgnet.com/1949-8454/full/v1/i9/286.htm
- DOI: https://dx.doi.org/10.4331/wjbc.v1.i9.286