Published online Jun 15, 2017. doi: 10.4239/wjd.v8.i6.304
Peer-review started: March 2, 2017
First decision: March 28, 2017
Revised: April 3, 2017
Accepted: April 23, 2017
Article in press: April 24, 2017
Published online: June 15, 2017
Processing time: 106 Days and 8.9 Hours
To assess circulatory levels of interleukin-18 (IL-18) and determine whether the presence of IL-18 promoter polymorphism influences metabolic syndrome phenotypes.
This study recruited one hundred and eighty individuals divided into three groups with sixty subjects each as: Normal weight (18.0-22.9 kg/m2), overweight (23.0-25.9 kg/m2) and obese (> 26.0 kg/m2) according to South Asian criteria of BMI. Fasting blood glucose (FBG), Lipid profile, insulin, IL-18 and tumor necrosis factor (TNF)α were measured using ELISA kits, whereas low density lipoprotein (LDL)-cholesterol, insulin resistance (HOMA-IR) and insulin sensitivity (QUICKI) were calculated. The body fat percentage (BF) was measured through bioelectrical impedance analysis; waist and hip circumference were measured. Genotyping of IL-18 -607 C/A polymorphism was performed by using tetra-primer amplification refractory mutation system. Student t test, One-way analysis of variance, Hardy-Weinberg equilibrium, Pearson’s χ2 test and Pearson’s correlation were used, where a P value < 0.05 was considered significant.
In an aged matched study, obese subjects showed higher levels of FBG, cholesterol, triglycerides and LDL levels as compared to normal weight (P < 0.001). Highest levels of IL-18 and TNF levels were also seen in obese subjects (IL-18: 58.87 ± 8.59 ng/L) (TNF: 4581.93 ± 2132.05 pg/mL). The percentage of IL-18 -607 A/A polymorphism was higher in overweight and obese subjects vs normal weight subjects (P < 0.001). Moreover, subjects with AA genotype had a higher BF, insulin resistance, TNFα and IL-18 levels when compared with subjects with AC (heterozygous) or CC (wild type) genotypes. However, we did not find any difference in the lipid profile between three subgroups.
This preliminary data suggests that IL-18 polymorphism affects IL-18 levels that might cause low grade inflammation, further exacerbated by increased TNFα. All these increase the susceptibility to develop MetS. Further studies are required to validate our findings.
Core tip: Interleukin-18 (IL-18) gene polymorphisms may influence the expression of its levels. This in turn increases the risk of metabolic syndrome (MetS). Therefore, we aimed to assess the circulatory levels of IL-18 and determine whether the presence of IL-18 promoter polymorphism influences MetS phenotypes. Subjects with AA genotype had a higher body fat, insulin resistance, tumor necrosis factor α and IL-18 levels when compared with subjects with AC (heterozygous) or CC (wild type) genotypes. This preliminary data suggests that IL-18 polymorphism affects IL-18 levels that might cause low grade inflammation. All these increase the susceptibility to develop MetS. Further studies are required to validate our findings.
- Citation: Fatima SS, Jamil Z, Abidi SH, Nadeem D, Bashir Z, Ansari A. Interleukin-18 polymorphism as an inflammatory index in metabolic syndrome: A preliminary study. World J Diabetes 2017; 8(6): 304-310
- URL: https://www.wjgnet.com/1948-9358/full/v8/i6/304.htm
- DOI: https://dx.doi.org/10.4239/wjd.v8.i6.304
Interleukin-18 (IL-18), also known as interferon-gamma inducing factor, is a pro-inflammatory cytokine that belongs to the IL-1 superfamily. It is not only produced by immune cells like macrophages but is also express by keratinocytes, osteoblasts cells, pituitary gland and adrenal cortical cells[1]. IL-18 serves as a mediator of immune response by stimulating T-helper cells (Th-1) against infections[2]. In healthy individuals, its production by the host is in line to utilization as a defense and healing mechanism, maintained in a fine balance. However, it has been established that its over-production results in autoimmune inflammatory disorders[3]. Apart from its role in inflammation, IL-18 has also been associated with increased visceral adiposity and obesity. Studies report abnormally elevated circulating IL-18 levels in obese individuals while reduction in body weight is found to results in a concomitant reduction in IL-18 levels, supporting the fact that reduction in adipose tissue leads to a decline in secretion of pro-inflammatory cytokines[4]. Furthermore, IL-18 mediated inflammation is associated with cardiovascular disorders suggesting its role in atherosclerotic diseases[5]. In the context of hyperglycemia, there are certain oxidative mechanisms that increase circulating cytokines including IL-18, thereby linking high blood glucose to the pro-inflammatory cytokines[6]. Various studies have reported elevated serum IL-18 in patients with type 2 diabetes[7].
As the presence of risk factors for cardiovascular disease and type 2 diabetes mellitus increases the threat of developing metabolic syndrome (MetS), IL-18 has been implicated to play a critical role in such conditions[8]. In terms of polymorphism in the IL-18 gene, various SNP are reported in association with diseases like type 1 diabetes[9], chronic hepatitis B virus infection[10], asthma[11].
The IL-18 gene is located on chromosome 11 (11q22.2-22.3), and contains many polymorphism, especially in the promoter region[12]. One such polymorphism is the -607 A/C that seems to affect the expression level of IL-18 at transcription level[13]. In addition, it has been associated with the development of cardiovascular disease such as vascular endothelial damage and formation of atherosclerosis[14]. The relationship between the promoter region polymorphism of IL-18 and MetS phenotypes is scarce. Therefore, we aimed to assess the circulatory levels of IL-18 and determine whether the presence of IL-18 promoter polymorphism influences MetS phenotypes.
This cross-sectional study recruited 180 healthy male individuals from the waiting areas of outpatient department of Aga Khan University. The subjects were divided into three groups. Group A: normal weight (18.0-22.9 kg/m2), Group B: overweight (23.0-25.9 kg/m2), Group C: obese (> 26.0 kg/m2) according to South Asian criteria of BMI[15]. Subjects with diabetes mellitus, hypertension [resting blood pressure (BP) 170/100 mmHg], dyslipidemia, body weight fluctuation of 5 kg in the recent 6 mo, smokers, alcoholics, any acute illness during last one month, as well as those taking anti-inflammatory medications were excluded. This study was approved by the institutional ethical committee and all participants gave a written and informed consent to participate in this study. The sample size was calculated in order to achieve 80% power to detect an odds ratio of at least 2 among obese, with a two sided alpha value of 95% (NCSS/PASS version 11 software for power analysis and sample size).
The weight and height of all the subjects were measured in kilograms and meters respectively, using a weight scale with a built-in Stadiometer (ZT-120 Health Scale, Nanjing Everich China). Waist circumference and hip circumference was measured using the WHO protocol[16]. Subjects were asked to stand in an erect posture wearing light clothing. BMI was calculated by dividing weight by height squared (kg/m2)[17]. While body fat percentage was measured using Diagnostic Scale BG55 (Beurer Germany) through bioelectrical impedance matching/analysis.
Six milliliter of blood was collected from the study participants after an overnight fast of 12 h. Fasting plasma glucose and Lipid profile were measured using commercially available kits as per the vendor’s instruction (Merck, France). Low density lipoprotein (LDL)-cholesterol levels were calculated using the Friedewald equation[18]. Fasting insulin, IL-18 and TNFα levels were measured using an ELISA kit (DIA source Immuno Assay S.A., Belgium). Insulin resistance was calculated using the homeostasis model assessment of insulin resistance (HOMA-IR) index [fasting insulin (units per milliliter) × fasting glucose milligram/deciliter)/405][19], and insulin sensitivity was calculated by (QUICKI) {1/[log(fasting insulin) + log(fasting glucose)]}[20].
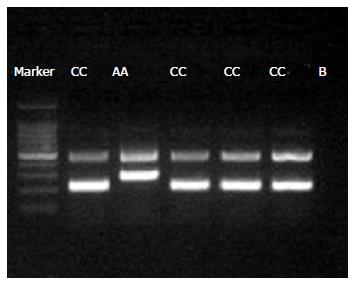
DNA extraction was performed using commercially available Qiagen DNA extraction kit (Cat. #51185, Valencia, CA United States). Genotyping of IL-18 -607 C/A polymorphism was performed by using tetra-primer amplification refractory mutation system (TARMS-PCR) using the GoTaq® Hot Start Green Master Mix (Cat. # M5122, Promega Corporation, United States) as per the manufacturer’s instructions with the following cycling conditions for PCR: 1 cycle for 5 min at 95 °C for initial denaturation followed by 35 cycles at 95 °C for 30 s, 55 °C for 30 s, 72 °C for 30 s followed by a final extension of 10 min at 72 °C. PCR products were electrophoresed in 2% agarose gel. Genotyping quality control was performed in 10% of the samples by duplicate checking (rate of concordance in duplicates was > 99 %). Tetra arms primers used for amplifying IL-18 -607 (C/A) were as follows: Control Band (Outer forward: CCTACAATGTTACAACACTTAAAAT; Outer reverse: ATAAGCCCTAAATATATGTATCCTTA) (product size 440 bp); A allele [Inner forward: GATACCATCATTAGAATTTTGTG (product size 278 bp)] and C allele [Reverse inner GCAGAAAGTGTAAAAATTATCAA (product Size 208 bp)] (Figure 1). The study was approved by the institutional ethical review board (3597-BBS-ERC-15), and all subjects gave a written and informed consent.
A descriptive statistical analysis of continuous variables was performed using SPSS (version 21; SPSS Inc., Chicago, IL, United States). Data on continuous variables were calculated as mean ± SD, whereas data on categorical variables was presented as frequencies and percentages. Statistical comparisons were computed using a student t test, one-way analysis of variance (ANOVA) and Pearson’s χ2 test of independence. Pearson’s correlation (r) were used to determine the correlation between serum IL-18 levels and lipid profile, fasting blood glucose, insulin and body fat parameters. Hardy-Weinberg equilibrium (HWE) was calculated for IL-18 SNP. Significance and effect size of minor allele with study parameters were determined under an additive model of inheritance. In all statistical analysis performed P values < 0.05 were considered significant.
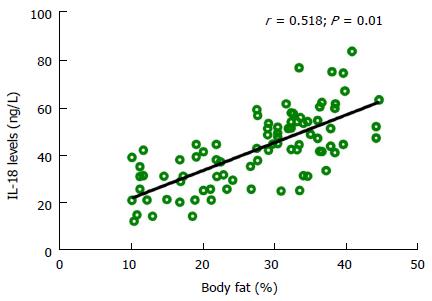
Table 1 shows the biophysical and biochemical data of the study subjects. All three groups were aged matched, therefore no significant difference was observed amongst the groups. Considerable difference was observed in terms of raised BMI and BF in obese group as compared to controls (P < 0.001). Similarly, obese group had a higher FBG, insulin, cholesterol, triglycerides and LDL levels as compared to controls (P < 0.001). Interestingly no differences were seen in the high density lipoprotein (HDL) levels of our study groups. IL-18 and TNF levels showed an increasing trend from normal weight to obese, with the highest levels seen in obese group (IL-18: 58.87 ± 8.59) (TNF: 4581.93 ± 2132.05). We next performed the correlation of IL-18 levels with the study parameters and report a strong positive correlation of IL-18 with BMI, Waist circumference, FBG, insulin, HOMA-IR, QUICKI, Cholesterol, and TNFα while a moderate correlation was seen for LDL and BF. All these associations remained significant after multiple adjustments for confounding factors like age, BMI and BF, except insulin (Table 2 and Figure 2).
| Variables | Normal weight (18-22.9 kg/m2) (n = 60) | Overweight (23-25.9 kg/m2) (n = 60) | Obese (>26 kg/m2) (n = 60) | P value |
| Age (yr) | 26.21 ± 3.876 | 25.76 ± 4.059 | 27.30 ± 5.389 | NS |
| BMI (kg/m2) | 20.48 ± 1.30 | 24.20 ± 0.911 | 28.59 ± 3.341,2 | < 0.001 |
| Body fat (%) | 19.26 ± 7.17 | 28.70 ± 9.101 | 34.89 ± 4.471,2 | < 0.001 |
| Waist circumference (cm) | 88.03 ± 10.53 | 88.46 ± 7.69 | 1.9.26 ± 11.47 | < 0.001 |
| Hip circumference (cm) | 77.69 ± 11.78 | 84.43 ± 0.69 | 100.41 ± 12.89 | < 0.001 |
| WHR (cm) | 0.87 ± 0.05 | 0.871 ± 0.054 | 0.91 ± 0.073 | < 0.001 |
| Fasting blood glucose (mg/dL) | 89.31 ± 15.23 | 105.59 ± 12.501 | 119.00 ± 25.711 | < 0.001 |
| Insulin (uIU/mL) | 20.48 ± 7.95 | 34.96 ± 4.471 | 41.13 ± 7.811,2 | < 0.001 |
| HOMA-IR | 4.70 ± 2.66 | 9.05 ± 1.341 | 11.88 ± 2.811,2 | < 0.001 |
| QUICKI | 0.31 ± 0.02 | 0.27 ± 0.001 | 0.27 ± 0.011 | < 0.001 |
| Cholesterol (mg/dL) | 145.72 ± 30.08 | 147.44 ± 37.91 | 209.06 ± 55.511,2 | < 0.001 |
| Triglyceride (mg/dL) | 124.82 ± 43.92 | 137.58 ± 65.60 | 167.03 ± 55.991,2 | < 0.001 |
| HDL(mg/dL) | 39.96 ± 9.38 | 38.37 ± 8.00 | 36.40 ± 6.40 | NS |
| LDL(mg/dL) | 75.43 ± 30.03 | 79.57 ± 40.01 | 134.78 ± 58.991,2 | < 0.001 |
| TNFα (pg/mL) | 810 ± 1233 | 4455 ± 23901 | 4581 ± 21321 | < 0.001 |
| IL-18 (ng/L) | 25.34 ± 6.57 | 41.96 ± 4.501 | 58.87 ± 8.591,2 | < 0.001 |
| Variable | Unadjusted r | Adjusted r (age, BMI and body fat %) |
| Age (yr) | 0.302 | -- |
| BMI (kg/m2) | 0.751 | -- |
| Body fat (%) | 0.518 | -- |
| Waist circumference (cm) | 0.333 | 0.413 |
| Hip circumference (cm) | 0.695 | -0.110NS |
| Fasting blood glucose (mg/dL) | 0.559 | 0.376 |
| Insulin (uIU/mL) | 0.655 | 0.205NS |
| HOMA-IR | 0.699 | 0.344 |
| QUICKI | -0.600 | -0.496 |
| Cholesterol (mg/dL) | 0.514 | 0.265 |
| LDL(mg/dL) | 0.464 | 0.245 |
| TNFα | 0.577 | 0.491 |
The genotype distributions was in accordance with HWE in total study subjects (P = 0.222) and in subgroups (Normal Weight: P = 0.281; Overweight: P = 0.663; Obese: P = 0.196). The percentage of IL-18 -607 A/A genotype was higher in overweight and obese subjects vs normal weight subjects (P < 0.001 Table 3). Moreover, subjects with AA genotype had a higher BF, insulin resistance, TNFα and IL-18 levels when compared with subjects with AC or CC genotypes. However, we did not find any difference in the lipids profile between three subgroups (Table 4).
| Variables | CC (n = 57) | AC (n = 72) | AA (n = 51) | P value |
| BMI (kg/m2) | 23.36 ± 4.05 | 24.60 ± 4.137 | 25.25 ± 3.351 | 0.05 |
| Body fat (%) | 24.70 ±10.30 | 28.70 ± 8.592 | 29.31 ± 9.461 | < 0.001 |
| Waist circumference(cm) | 92.78 ± 16.61 | 96.48 ± 13.21 | 97.32 ± 13.82 | 0.085 |
| Hip circumference (cm) | 74.18 ± 4.23 | 67.40 ±3.99 | 64.51 ± 4.84 | 0.694 |
| WHR (cm) | 0.88 ± 0.069 | 0.88 ± 0.061 | 0.89 ± 0.06 | 0.490 |
| Fasting blood glucose (mg/dL) | 103.41 ± 23.43 | 101.79 ± 21.01 | 110.32 ± 21.97 | > 0.05 |
| Insulin (uIU/mL) | 28.83 ± 11.91 | 32.76 ± 11.392 | 34.67 ± 9.611 | 0.002 |
| HOMA-IR | 7.75 ± 4.34 | 8.44 ± 3.71 | 9.47 ± 3.001 | 0.007 |
| QUICKI | 0.29 ± 0.02 | 0.28 ± 0.02 | 0.28 ± 0.011 | 0.046 |
| Cholesterol (mg/dL) | 161.01 ± 39.01 | 165.12 ± 55.03 | 177.42 ± 60.72 | > 0.05 |
| Triglyceride (mg/dL) | 135.66 ± 42.29 | 145.61 ± 61.45 | 146.78 ± 64.08 | > 0.05 |
| HDL(mg/dL) | 38.29 ± 8.84 | 38.48 ± 56.32 | 37.17 ± 6.75 | > 0.05 |
| LDL(mg/dL) | 88.99 ± 5.38 (SEM) | 94.73 ± 6.63 (SEM) | 107.77 ± 7.89 (SEM) | > 0.05 |
| TNFα (pg/mL) | 2521 ± 353.9 (SEM) | 3403.08 ± 313.25 (SEM) | 3760.35 ± 336.551 (SEM) | 0.008 |
| IL-18 (ng/L) | 37.16 ± 19.35 | 43.00 ± 13.192 | 46.311 ± 13.071 | 0.001 |
IL-18 is pleiotropic cytokine acting in both acquired and innate immunity. Additionally it also acts to stimulate the production of TNFα[1] which is also a key player associated with higher BMI[21,22]. These cytokines in turn predispose an individual to develop MetS phenotypes.
In an age matched study group, we observed a positive association of serum IL-18 concentration with BMI, FBG, and serum TG, where body fat percentage contributed most to the variation of serum IL-18 concentration. Furthermore, our study shows circulating IL-18 levels were associated with measures of insulin resistance (HOMA-IR) and decreased insulin sensitivity (QUICKI) in apparently healthy obese subjects. Among other factors causing a rise in IL-18 levels, nutritional states, such as hyperglycemia, and fat mass increase[23] have been validated the most. In particular, adipocytes from obese individuals were found to secrete a threefold higher IL-18 vs lean ones[24], identifying an essential role of IL-18 in regulating fat distribution[25]. Though, an animal study demonstrated that deficiency of IL-18 resulted in obesity and insulin resistance in mice and the phenotype could be rescued by exogenous administration of IL-18[26].
We further evaluated the presence of a promote gene polymorphism in these subjects. Interestingly, we report that subjects with AA genotype had a higher BMI, BF, insulin resistance and IL-18 levels. Moreover, raised IL-18 increased the chances of developing of MetS in our study subjects (OR = 2.72, 95%CI: 1.28-5.74, P = 0.008). Results regarding the association of -607 SNP and MetS phenotypes are not consistent. One study reported a decreased proportion of A/A genotype in type 1 diabetic patients relative to control subjects[9]. However, another[27] found higher proportion of A/A genotype in type 1 diabetic patients but no risk association could be identified. Another study, conducted in Chinese population reported a higher proportion of A/A genotype in patients with type 2 diabetes, which is somewhat similar to our report. Therefore, it is empirical to identify the different genetic influences among different races when considering genotype data and risk association. For instance, A allele at position of -607 was a protective allele from type 1 diabetes in a Polish population[9], while in a population from United Kingdom, the significant association was not found[27].
These seemingly conflicting results suggested that IL-18 probably acts as a feedback signal for obesity, hyperglycemia, and positive energy balance. Alternatively, it might be a consequence of sensitivity in those subjects to the effect of IL-18. This may prove that inflammatory marker (IL-18) may just not be an indicator for chronic inflammation and obesity but has a role in pathway leading to MetS. Another outcome of our study was the relation of IL-18 with increased levels of cholesterol and LDL. This opens up another avenue about the role of IL-18 in atherosclerosis and presents a great opportunity to work further in this field. Some of previous studies have shown association of circulating IL-18 levels with cardiovascular mortality among patients with coronary artery disease[5]. However, contrasting data also exists which states no or weak association of IL-18 levels with BMI and lipid profile in European men[28], this may suggest that association may vary according to the population. In addition to Interleukin 18, recent repost suggested that other variants such as Interleukin-23/IL-17 axis has also been independently affiliated with obesity in women especially related to increase visceral fat, insulin resistance, and leptin levels[29] as well as in causing hypertension and increased cardiovascular risk[30].
One of the limitations of this study rests in the study-design. As this was a cross sectional study the association of IL-18 with all of these metabolic traits could not be established, further more we were unable to record the nonalcoholic fatty liver disease through ultra sonographic analysis. Nevertheless our results clearly show that IL-18 can be used a marker for obesity and supports the hypothesis that IL-18 may be involved in pathway of MetS and form a link between metabolic risk factors, diabetes, and cardiovascular diseases specially in south Asian population.
Cytokines are implicated for causing lipid derangement and insulin resistance. Furthermore, polymorphisms in the interleukin-18 (IL-18) genes influences expression levels and may increase the risk of metabolic syndrome (MetS).
The authors’ results clearly show that IL-18 can be used a marker for obesity and supports the hypothesis that IL-18 polymorphism may be involved in pathway of MetS and form a link between metabolic risk factors, diabetes, and cardiovascular diseases specially in south Asian population. This can lead to precision treatments to reduce the burden of obesity.
The literature suggests a mixed role of IL-18 gene polymorphisms in MetS or diabetes. However, the present study suggests a new role promter gene polymorphisms in modulating MetS phenotypes.
The authors’ study provides a preliminary report of association of IL-18 levels and its polymorphism though at this stage no therapeutic role can be elucidated.
MetS: It is a cluster of conditions such as diabetes, hypertension, increased waist circumference and lipid levels in in individual; Polymorphism: The presence of genetic variation within a population.
Preliminary results of this work are very interesting.
Manuscript source: Invited manuscript
Specialty type: Endocrinology and metabolism
Country of origin: Pakistan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Dinc M, Fiori E, Saisho Y, Schoenhagen P, Tarantino G S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Okamura H, Kashiwamura S, Tsutsui H, Yoshimoto T, Nakanishi K. Regulation of interferon-gamma production by IL-12 and IL-18. Curr Opin Immunol. 1998;10:259-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 401] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 2. | Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 429] [Cited by in RCA: 650] [Article Influence: 54.2] [Reference Citation Analysis (0)] |
| 3. | Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 958] [Cited by in RCA: 1021] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 4. | Esposito K, Nappo F, Giugliano F, Di Palo C, Ciotola M, Barbieri M, Paolisso G, Giugliano D. Cytokine milieu tends toward inflammation in type 2 diabetes. Diabetes Care. 2003;26:1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Blankenberg S, Tiret L, Bickel C, Peetz D, Cambien F, Meyer J, Rupprecht HJ. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation. 2002;106:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 405] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 6. | Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1329] [Cited by in RCA: 1426] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 7. | Moriwaki Y, Yamamoto T, Shibutani Y, Aoki E, Tsutsumi Z, Takahashi S, Okamura H, Koga M, Fukuchi M, Hada T. Elevated levels of interleukin-18 and tumor necrosis factor-alpha in serum of patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Metabolism. 2003;52:605-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Hung J, McQuillan BM, Chapman CM, Thompson PL, Beilby JP. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:1268-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 9. | Kretowski A, Mironczuk K, Karpinska A, Bojaryn U, Kinalski M, Puchalski Z, Kinalska I. Interleukin-18 promoter polymorphisms in type 1 diabetes. Diabetes. 2002;51:3347-3349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Hirankarn N, Manonom C, Tangkijvanich P, Poovorawan Y. Association of interleukin-18 gene polymorphism (-607A/A genotype) with susceptibility to chronic hepatitis B virus infection. Tissue Antigens. 2007;70:160-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Lachheb J, Chelbi H, Ammar J, Hamzaoui K, Hamzaoui A. Promoter polymorphism of the IL-18 gene is associated with atopic asthma in Tunisian children. Int J Immunogenet. 2008;35:63-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Smith AJ, Humphries SE. Cytokine and cytokine receptor gene polymorphisms and their functionality. Cytokine Growth Factor Rev. 2009;20:43-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 279] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 13. | Giedraitis V, He B, Huang WX, Hillert J. Cloning and mutation analysis of the human IL-18 promoter: a possible role of polymorphisms in expression regulation. J Neuroimmunol. 2001;112:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 353] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 14. | Liu W, Tang Q, Jiang H, Ding X, Liu Y, Zhu R, Tang Y, Li B, Wei M. Promoter polymorphism of interleukin-18 in angiographically proven coronary artery disease. Angiology. 2009;60:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Snehalatha C, Viswanathan V, Ramachandran A. Cutoff values for normal anthropometric variables in asian Indian adults. Diabetes Care. 2003;26:1380-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 289] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 16. | Consultation WE. Waist circumference and waist-hip ratio. Report of a WHO Expert Consultation Geneva. World Health Organization. 2008;8-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Garrow JS, Webster J. Quetelet’s index (W/H2) as a measure of fatness. Int J Obes. 1985;9:147-153. [PubMed] |
| 18. | Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499-502. [PubMed] |
| 19. | Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22373] [Cited by in RCA: 24471] [Article Influence: 611.8] [Reference Citation Analysis (0)] |
| 20. | Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402-2410. [PubMed] |
| 21. | Tracy RP. Is visceral adiposity the “enemy within”? Arterioscler Thromb Vasc Biol. 2001;21:881-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Adabimohazab R, Garfinkel A, Milam EC, Frosch O, Mangone A, Convit A. Does Inflammation Mediate the Association Between Obesity and Insulin Resistance? Inflammation. 2016;39:994-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 23. | Escobar-Morreale HF, Botella-Carretero JI, Villuendas G, Sancho J, San Millán JL. Serum interleukin-18 concentrations are increased in the polycystic ovary syndrome: relationship to insulin resistance and to obesity. J Clin Endocrinol Metab. 2004;89:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 134] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Skurk T, Kolb H, Müller-Scholze S, Röhrig K, Hauner H, Herder C. The proatherogenic cytokine interleukin-18 is secreted by human adipocytes. Eur J Endocrinol. 2005;152:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Zorrilla EP, Sanchez-Alavez M, Sugama S, Brennan M, Fernandez R, Bartfai T, Conti B. Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci USA. 2007;104:11097-11102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 26. | Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, Tack CJ, van Krieken H, Kim SH, Stalenhoef AF. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 330] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 27. | Szeszko JS, Howson JM, Cooper JD, Walker NM, Twells RC, Stevens HE, Nutland SL, Todd JA. Analysis of polymorphisms of the interleukin-18 gene in type 1 diabetes and Hardy-Weinberg equilibrium testing. Diabetes. 2006;55:559-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Blankenberg S, Luc G, Ducimetière P, Arveiler D, Ferrières J, Amouyel P, Evans A, Cambien F, Tiret L. Interleukin-18 and the risk of coronary heart disease in European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME). Circulation. 2003;108:2453-2459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 240] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 29. | Sumarac-Dumanovic M, Stevanovic D, Ljubic A, Jorga J, Simic M, Stamenkovic-Pejkovic D, Starcevic V, Trajkovic V, Micic D. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond). 2009;33:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 30. | Nosalski R, McGinnigle E, Siedlinski M, Guzik TJ. Novel Immune Mechanisms in Hypertension and Cardiovascular Risk. Curr Cardiovasc Risk Rep. 2017;11:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |