Published online Jul 10, 2015. doi: 10.4239/wjd.v6.i7.912
Peer-review started: August 2, 2014
First decision: December 17, 2014
Revised: December 30, 2014
Accepted: March 30, 2015
Article in press: April 2, 2015
Published online: July 10, 2015
Hypoglycemia unawareness (HU) is defined at the onset of neuroglycopenia before the appearance of autonomic warning symptoms. It is a major limitation to achieving tight diabetes and reduced quality of life. HU occurs in approximately 40% of people with type 1 diabetes mellitus (T1DM) and with less frequency in T2DM. Though the aetiology of HU is multifactorial, possible mechanisms include chronic exposure to low blood glucose, antecedent hypoglycaemia, recurrent severe hypoglycaemia and the failure of counter-regulatory hormones. Clinically it manifests as the inability to recognise impeding hypoglycaemia by symptoms, but the mechanisms and mediators remain largely unknown. Prevention and management of HU is complex, and can only be achieved by a multifactorial intervention of clinical care and structured patient education by the diabetes team. Less know regarding the impact of medications on the development or recognition of this condition in patients with diabetes. Several medications are thought to worsen or promote HU, whereas others may have an attenuating effect on the problem. This article reviews recent advances in how the brain senses and responds to hypoglycaemia, novel mechanisms by which people with insulin-treated diabetes develop HU and impaired counter-regulatory responses. The consequences that HU has on the person with diabetes and their family are also described. Finally, it examines the evidence for prevention and treatment of HU, and summarizes the effects of medications that may influence it.
Core tip: This review describes novel mechanisms by which people with insulin-treated diabetes develop hypoglycemia unawareness (HU), the consequences that HU has on the person with diabetes and their family, the evidence for prevention and treatment of HU, and the effects of medications that may influence it.
- Citation: Martín-Timón I, del Cañizo-Gómez FJ. Mechanisms of hypoglycemia unawareness and implications in diabetic patients. World J Diabetes 2015; 6(7): 912-926
- URL: https://www.wjgnet.com/1948-9358/full/v6/i7/912.htm
- DOI: https://dx.doi.org/10.4239/wjd.v6.i7.912
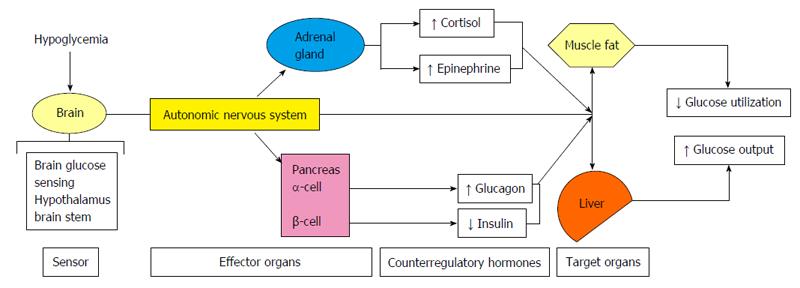
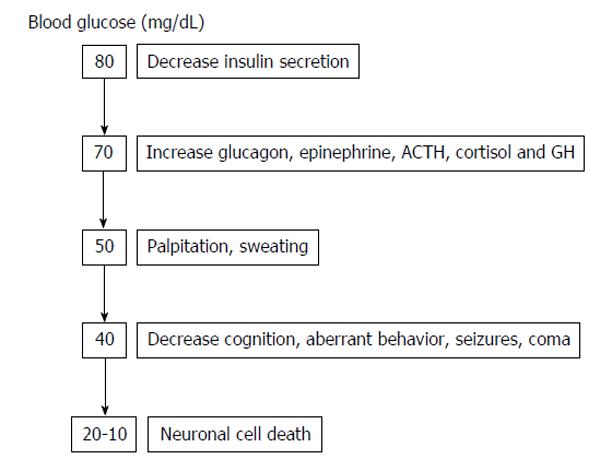
Hypoglycemia is usually defined as a plasma glucose level < 70 mg/dL (3.9 mmol/L)[1]. Since the brain is permanently dependent on glucose, strong counter-regulatory mechanisms exists to quickly increase glucose levels to protect the human body from the negative consequences of hypoglycemia. Counter-regulatory response to hypoglycemia (Figure 1) includes inhibition of the endogenous insulin secretion and stimulation of glucagon, catecholamines (norepinephrine, epinephrine), cortisol and growth hormone secretion, which all together stimulate hepatic glucose production and cut down glucose utilization in peripheral tissues, increasing in this way plasma glucose levels. As glycaemia comes down, the activation of the autonomic nervous system leads to neurogenic symptoms (palpitations, sweating, hunger, anxiety, tremors, etc.), which allows the perception of hypoglycaemia (hypoglycaemia awareness) (Figure 2).
Hypoglycemia unawareness (HU) is defined as the onset of neuroglycopenia before the appearance of autonomic warning symptoms[2] or as the failure to sense a significant fall in blood glucose below normal levels[3]. In patients with type 1 (T1DM) or type 2 diabetes mellitus (T2DM), recurrent hypoglycemia has been shown to reduce the glucose level that precipitates the counter-regulatory response necessary to restore euglycemia during a subsequent episode of hypoglycemia[4,5].
HU was observed in 40% T1DM patients[6] and less frequently in T2DM patients with low C-peptide levels. The presence of HU increases the risk of severe hypoglycaemia (six-fold for T1DM[7] and 17-fold for T2DM[8]). HU is more common in individuals with longer duration of diabetes, history of recent and/or recurrent hypoglycaemic events, patients with intensive glycemic therapy and in advanced age[9].
Presently, the major risk factors for the development of HU are duration of the disease and improved metabolic control. The severity of HU was associated with longer diabetes duration and with a history of frequent low glycemic levels[6], whereas aging and the blood glucose decreasing rate using professional continuous glucose monitoring systems (CGMS), which falls from near blood glucose level, were risk of severe HU[10]. Data from Pittsburgh Epidemiology of Diabetes Complications[11] showed that diabetes duration, HbA1c and intensive insulin therapy predicted HU in men, whereas severity and frequency of hypoglycemia, QTc interval and hypertension predicted HU in women. Thus, women are more likely to have HU, which unlike in men, is also marginally related to hypertension, QTc interval and hypoglycemia. On the other hand, in patients with T1DM, HU was 3.4-fold more common among patients homozygous for Gly16 than among patients with other variants of the Arg16Gly polymorphism, so that T1DM patients who carry two alleles of the Gly16 variant of ADRB2 are at increased risk of developing HU[12]. Finally, in both T1 and T2DM patients with impaired HU, hypoglycemia-induced electroencephalogram changes, such increased theta band amplitude, were not affected by antecedent of hypoglycemia[13].
This article reviews recent advances in how the brain senses and responds to hypoglycemia, novel mechanisms by which people with insulin-treated diabetes develop HU and impaired counter-regulatory responses. The consequences that HU had on the person with diabetes and their family is also described. Finally, it examines the evidence for prevention and management of HU, and summarizes the effects of medications that may influence it.
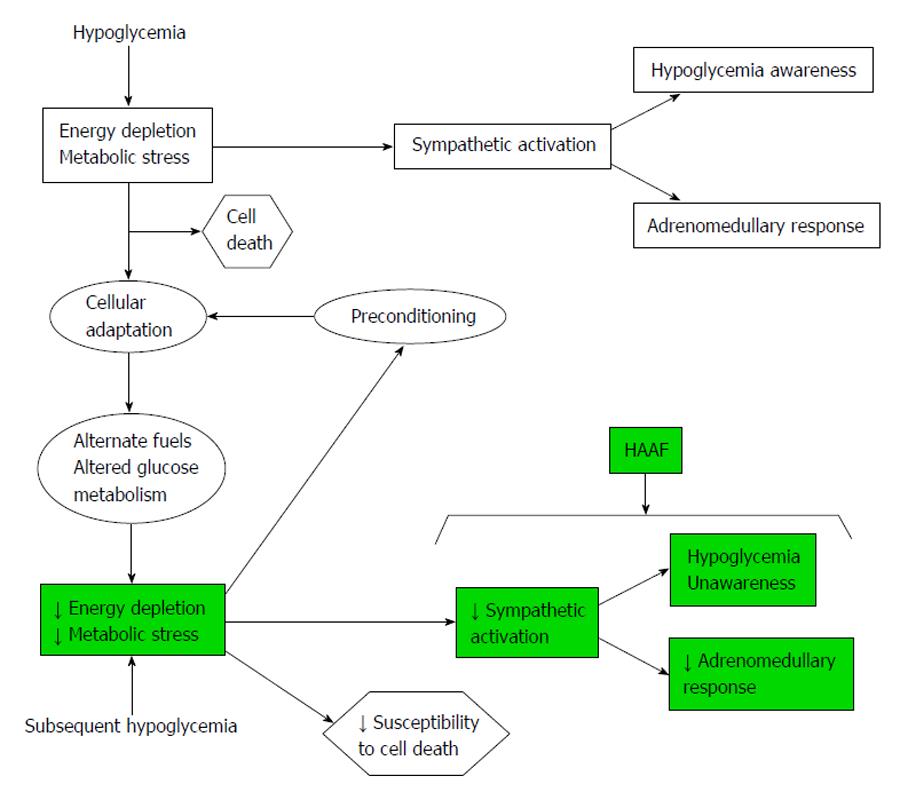
Aberrant glucose counter-regulation (as a result of a failure in the reduction of insulin production and an increase in glucagon release), and HU (as the result of an attenuated increase in sympathoadrenal activity) are the components of hypoglycemia-associated autonomic failure (HAAF) in diabetics patients. HAAF is most often caused by recent/recurrent iatrogenic hypoglycemia, and indeed HAAF is maintained by recurrent hypoglycemia[14,15] (Figure 3).
Catecholamines: Previous hypoglycemia leads to a blunted catecholamine response to a following episode of hypoglycemia. These has been demonstrated in several studies; for example Ramanathan et al[17] showed that intravenous infusion of adrenergic blockers on one day of a hypoglycemia prevent the counter-regulatory failure in the response on the next day of hypoglycemia. This study implicates that HAAF needs a previous hypoglycemia (with its sympathoadrenal responses). If we use this hypothesis to think in a possible pharmacologic treatment, we can concluded that blocking the action of catecholamines we can limit the development of HAAF and protect against subsequent hypoglycemias; but unfortunately, blocking the action of catecholamines in periphery we would tend to an increase in the severity of hypoglycemia. We would need to develop a selective adrenergic receptor modulators that favourably change central nervous system response without modify the beneficial peripheral effects of the sympathoadrenal response.
Sleep: Sleep is a peripheral mediator of HAAF linked with catecholamine response. Patients with T1DM, while they are sleeping, they have a significantly decreased epinephrine response to hypoglycemia[18], and also a reduced awakening from sleep during hypoglycemia[19]. So, because of the HU and the impaired adrenomedullary response, we can explain some of the overnight deaths of healthy young people with T1DM.
Cortisol: Hypoglycemia is associated with an elevation in systemic corticosteroids, and this has been proposed to feedback to the hypothalamus contributing to HAAF[20-22]. However it remains controversial if the endogenous hypercortisolemia is of sufficient magnitude to blunt de counter-regulatory response to hypoglycemia[23,24]. It have been shown that corticotrophin releasing hormone agonist impair the counter-regulatory response to a subsequent hypoglycemia, suggesting a possible role in HAAF[25].
Opioids: Preclinical and clinical studies with opioids demonstrated a rise in endogenous opioids during hypoglycemia, for example naloxone (an opioid receptor blocker), increased the sympathoadrenal response to hypoglycemia, and when is infused during previous hypoglycemia, it prevent HAAF[26,27]. Hence there is a potential therapeutic function for opioid receptor blockade to protect against HAAF.
Exercise: The inability to reduced circulating insulin during exercise, lead T1DM patients, at an increased risk for hypoglycemia during or after exercise. In addition to, during exercise the opioid beta endorphin is released to activate the sympathoadrenal response. In a recent study, healthy individuals who exercised and elevated endorphin levels, they had reduced catecholamine response during hypoglycemia in the next day[28], suggesting that endogenous opioids, again, play a role in HAAF, and that blocking their action may protect against exercise-autonomic failure.
Clinically HAAF can be viewed as both, maladaptative or adaptative response[29]. At one end, patients with T1DM and HU make tests of cognitive function during hypoglycemia better than patients with HU. Additionally, the time necessary for complete cognitive recovery after restoration of normoglycemia is faster in patients who have HU[30]. HAAF in humans may be similar than in rats; rats with recurrent moderate hypoglycemia had less brain cell death[31] and less mortality during or following marked hypoglycemia than those without recurrent hypoglycemia. On the other hand, HAAF is without doubt a maladaptive response if we consider that defective glucose counter-regulation and HU rise the risk of severe hypoglycemia with its morbidity and potential mortality[32].
Although it is well established that recurrent hypoglycemia leads to HU, the mechanism responsible for this are unknown. Several current mechanistic hypotheses are discussed below.
The brain glucose transport or glucose metabolism hypothesis: Several studies have identified specific brain regions that exhibit decrease glucose uptake. In diabetic patients with and without HU, the effects of acute moderate hypoglycemia and the condition of HU on regional brain uptake of the labeled glucose analog [(18)F]fluorodeoxyglucose (FDG) using positron emission tomography were examined[33,34]. In the group with hypoglycemia awareness, there was an increase in the normalized FDG uptake in a subthalamic brain region[33], in left amygdale and in bilateral ventral striatum[34] in response to hypoglycemia; whereas in the group with HU the uptake in these brain regions fell significantly[33,34]. Reduced responses in these brain regions in HU, suggest habituation of higher behavioral responses to hypoglycemia as a basis for unawareness, and demonstrated a change in its metabolic function associated with the failure to trigger a counter-regulatory response. On the other hand, in subjects with T1DM and HU a positive correlation was observed between thalamic response and epinephrine response to hypoglycemia, suggesting that this brain region may be involved in the coordination of the counter-regulatory response to hypoglycemia[35]. During recurrent hypoglycemia, cerebral blood flow reduced significantly in the thalamus and hypothalamus of T1DM subjects, compared to healthy controls[36], suggesting that there is reduced neuronal activation in these brain regions that participate in glucose sensing and/or coordination of counter-regulation response in subjects with T1DM that likely contributes to the development of HU.
It has been hypothesized that recurrent hypoglycemia leads to HU through an alteration in the glucose transport or metabolism. Altered glucose transport or metabolism as a cause of HU is less substantiated in humans. Subjects with T1DM and HU had significantly higher brain glucose concentrations compared to that in controls under the same conditions[37]. These date suggest that changes in brain glucose transport or metabolism may occur as a result of recurrent hypoglycemia.
The brain glycogen supercompensation hypothesis: It has been hypothesized that increased brain glycogen contributes to the development of HU and impaired sympathoadrenal responses by providing energy for the brain during periods of systemic hypoglycemia. Experimental studies and in humans have shown that after one or more episodes of hypoglycemia, increased glycogen content in the brain[38,39]. Subsequent studies indicated lower glycogen content in brain of humans with T1DM, implying that glycogen supercompensation does not contribute to the development of HU[40]. The most important question to resolve is whether changes to brain glucose levels, physiologically or pharmacologically induced, may provide people who suffer from recurrent hypoglycemia a therapeutic benefit to preserve both the sympathoadrenal response and HU.
The brain fuel hypothesis: When there is a decrease in the supply of glucose from the periphery, the brain may be able to keep your metabolic processes by increasing uptake of alternative carbon fuels such as lactate or ketones. Plasma lactate concentrations are approximately tenfold higher than those of acetate, making it a primary candidate as an alternative brain fuel during hypoglycemia. On the other hand, increased of blood-brain barrier monocarboxylic acid (MCA) transport and metabolism among T1DM individuals with HU may be a mechanism to supply the brain with non-glucose fuels during episodes of acute hypoglycemia and may contribute to the maintenance of brain energetic during hypoglycemia and to the syndrome of HU, independent of diabetes[41]. Finally, in T1DM patients with HU, upregulation of the MCA transporter promotes increased brain lactate uptake[42].
The brain neuronal communication hypothesis: Neuronal communication relies on the release of classical neurotransmitters, such as Gamma-Aminobutyric Acid (GABA), a potent inhibitory neurotransmitter. GABA levels in ventromedial hypothalamus (VMH) interstitial fluid are decreased during acute hypoglycemia[43]. Recurrent hypoglycemia leads to a significant increase in VMH GABA concentrations[44], that fail to decrease normally during subsequent hypoglycemia, and which correlates with the reduced glucagon and epinephrine responses[45]. These data suggest that recurrent hypoglycemia results in increased VMH GABA inhibitory tone, and that altered GABA tone may be an important common mediator in the development of HAAF, especially in diabetic patients.
People who have HU have a much greater risk of severe hypoglycemia, up to six fold, with its attendant morbidity[46,47]. HU may result in many serious forms of morbidity including seizure, coma, fractures and joint dislocation and cardiac arrhythmias, and is occasionally fatal.
Severe episodes of hypoglycemia or HU requiring the assistance of another have been shown to be associated with an increased risk of mortality in both the Action to Control Cardiovascular Risk in Diabetes (ACCORD)[48] and the Action in Diabetes and Vascular Disease[49] studies. On the other hand, post hoc analysis of the ACCORD study cohort, to examine the relationship between frequent and unrecognized hypoglycemia and mortality, 10096 ACCORD study participants were included. In this study, recognized and unrecognized hypoglycemia was more common in the intensive group than in the standard group; and in the intensive group, a small but statistically significant inverse relationship was identified between the number of hypoglycemic episodes and the risk of death among participants[50]. This latter finding does not mean that we should change our clinical practice and include frequent episodes of hypoglycemia in the targets of T2DM patients and cardiovascular risk factors. Instead, we must strive to achieve optimal glycemic control in our patients, without episodes of hypoglycemia.
Several prospective studies as the Diabetes Control and Complications Trial[51] and the Stockholm Diabetes Intervention Study[52] suggests that cognitive function does not deteriorate in patients with T1DM who suffer recurrent hypoglycemia, at least less than 10 years of these studies.
Gold et al[53] to compare the degree of cognitive dysfunction experienced by T1DM patients who had normal awareness of the onset of hypoglycemia with patients who had history of impaired awareness of hypoglycemia, found that T1DM patients with HU exhibited more profound cognitive dysfunction during acute hypoglycemia which persisted for longer following blood glucose recovery. Intellectual activity is likely to be affected and cause sub-optimal performance during this recovery period. Recent investigations with advanced imaging techniques have demonstrated that adults with T1DM appear to call upon a greater volume of the brain to perform a working memory task during hypoglycemia[54]. These findings suggest that adults with T1DM must recruit more regions to preserve cognitive function during hypoglycemia than adults without the disease.
Evidence of clinical audit in T1DM patients with intensive insulin therapy with HU showed that these patients had less adhesion to changes in insulin regimens to compare them with patients with hypoglycemia awareness, despite the observed increase in clinical contacts[55]. Neuroimaging studies have shown that patients with HU showed a reduced activation in appetitive motivational networks associated with integrated behavioral responses to hypoglycemia[34]. This may suggest that in some patients with HU behavioral strategies are more important than educational strategies; however treatment of HU will require a combination of both strategies, behavioral and educational, along with the use of technology, such as therapy with continuous insulin pump and online glucose monitoring[56].
A significant proportion of children and adolescents with T1DM have HU. Screening for HU is an important component of routine diabetes care and can identify patients at increased risk of severe hypoglycemic events[57]. The youngest patients are most vulnerable to the adverse consequences of hypoglycemia. Ongoing maturation of the central nervous system puts these children at greater risk for cognitive deficits as a consequence of HU[58]. HU is a significant problem for children and adolescents with T1DM and the major risk factor for development of hypoglycemia[57]. Those children with T1DM diagnosed before age of 6, who suffer repeated and severe episodes of hypoglycemia may have more increased range of cognitive dysfunction, brain abnormalities[59], structural brain changes[60], lower mental abilities latter on in life, and behavior problems than those who do not have HU until latter[61,62].
HU is less common in T2DM patients. Two retrospective surveys of subjects with insulin-treated T2DM showed that only 8% and 9.8% respectively had HU estimated by a validated scoring system[8,46]. However, in the patients with HU the incidence of severe hypoglycemia was nine-fold and 17-fold higher respectively than those with normal hypoglycemia awareness[8,46]. In several studies, using continuous monitoring system, asymptomatic hypoglycemia was detected in 47%[63] and 56%[64] of subjects with T2DM, treated with different treatment regimes. These findings suggest that HU may be more prevalent in T2DM than is appreciated.
Severe hypoglycemia, due to HU, was associated in T2DM patients with cardiovascular and neurological complications[1,48]. In patients with T2DM and coronary artery disease, severe hypoglycemia was associated with ischemic electrocardiogram changes and chest pain, and may account for sudden mortality[65,66]. In a retrospective study in T2DM subjects, the patients who experienced outpatient severe hypoglycemia were also shown to have a 79% higher odds ratio of experiencing acute cardiovascular events than patients without severe hypoglycemia[67]; and a case-control study in patients with T2DM showed a 65% increase in the odds of myocardial infarction with severe hypoglycemia within the previous two weeks; the risk of myocardial infarction persisted elevated for up to six months following a hypoglycemic event[68].
Behavioral changes, cognitive impairment, seizures, coma and a mortality rate estimated at between 4.9% and 9% are well-known neurological complications of severe and prolonged hypoglycemia secondary to HU[69-71]. Severe hypoglycemia secondary to HU can cause neuronal cell death and may damage regions of the brain that oversea memory, especially in older people with T2DM[72].
Finally, a frequently problem in T2DM is nocturnal hypoglycemia. Undetected nocturnal hypoglycemia often contributes to HU. Nocturnal hypoglycemia has been associated with cardiac arrhythmias resulting in sudden death[73].
Patients in the older age-groups are especially vulnerable to HU. Aging modifies the cognitive, symptomatic, and counter-regulatory hormonal responses to hypoglycemia[74]. Older adults with diabetes are at much higher risk for the geriatric syndrome, which includes falls, incontinence, frailty, cognitive impairment and depressive symptoms[75]. In the elderly subjects, episodes of severe hypoglycemia are more likely to be followed by changes in the blood brain circulation which may further increase the risk of neurological damage in this population[76,77]. In older patients with T2DM, Whitmer et al[72] found a significant association between the number of severe hypoglycemic episodes and dementia; with ≥ 3 episodes almost doubling the risk more episodes of severe hypoglycemia secondary to HU had increasing likelihoods of being subsequently diagnosed with dementia. Another authors also found an association between severe hypoglycemia and cognitive impairment in these patients[78]. These reports suggest that severe hypoglycemia and HU in older people with diabetes may be associated with cognitive decline[79].
Pregnancy is associated with a high risk of severe hypoglycemia in diabetic subjects. History of HU has been documented as risk factors of severe hypoglycemia during pregnancy[80-82]. Reduced sympathoadrenal responses during hypoglycemia may contribute to defective glucose counter-regulation and HU[83,84]. In pregnant woman severe hypoglycemia episodes and HU occur three to five times more frequently in first trimester than third trimester when compared with the incidence in the year preceding the pregnancy[80,81,85] and may lead to severe morbidity and even death[86].
Hypoglycemia and HU are associated with significant reductions in quality of life measures in both T1DM and T2DM patients[87-89]. The wellbeing of patients may be affected both from the effects of hypoglycemia and from fear of recurrence[89,90]. A positive association was found between severity and/or frequency of hypoglycemic events and greater fear of hypoglycemic episodes[71]. As a result fear of hypoglycemia makes the patients to promote compensatory behaviors in a way to have less episodes of hypoglycemia such as decreased insulin doses resulting in negative glycemic control, and an increased risk of serious health consequences[91]. Patients with recurrent hypoglycemia and HU were more likely to have a lower quality of life in several parameters including depression and anxiety[89,92,93], increased pain and limitations in mobility and usual activities[89], and decline in the quantity and quality of sleep[94]. On the other hand, young adults with T1DM reported the presence of interpersonal conflict, and difficulty talking about issues related to hypoglycemia with significant others[95], that may carry over to their work life, where hypoglycemia has been linked to reduced productivity[88].
Despite that many countries require documentation that severe hypoglycemia and HU is not occurring before persons with diabetes are permitted to have a license to operate a motor vehicle; HU has not consistently been associated with an increased risk of car collisions[96-98].
In the subjects with diabetes, HU can have a profound impact on the lives of their family members, and are often reliant on immediate relatives or partners to detect and treat hypoglycemia episodes. A recent study based in-depth interviews with 24 adult family members of persons with T1DM and HU, showed that family members restricted their own lives in order to help the person with HU to detect and treat hypoglycemia[99]. In this study, some family members of people with HU, report that they are afraid of their partners, during episodes of hypoglycemia because of their aggressive behavior and their personality changes, making it difficult managing their treatment. The study showed that family members of patients with HU restricted their own lives in order to help the person with HU to detect and treat hypoglycemia, and felt anxious about the safety of the person with HU; which sometimes leads family members to neglect their own health, leading to resentment over time[100]. On the other hand, personality changes during hypoglycemia events of the person with diabetes, such as aggression, also caused, in some family members, physical fear of your partner or relative, and made treatment difficult. Family members emphasized that there is an unmet need for information and emotional support for caregivers, and the researchers suggest that proactive support for the families of patients with diabetes and HU should be considered and provided by healthcare professionals[99].
The psychological consequences of HU include subsequent fear to hypoglycemia, and secondary poor treatment compliance, increased anxiety and decreased levels of satisfaction and happiness. Fear of hypoglycemia will be a barrier to achieving good glycemic control. The hypoglycemia fear survey (HFS) used to measure behaviors (HFS-B) and worries (HFS-W) related to hypoglycemia in adults with T1DM, such as maintaining higher blood glucose levels than recommended, and limiting exercise or physical activity, or concerns may have about hypoglycemic episodes, such as nocturnal episodes; have been shown to be significantly higher in women than in men and among patients who have experienced severe hypoglycemia in the past compared with those that have not[100]. If patients experience repeated severe hypoglycemic events, both the patient’s and the physician’s subsequent treatment policy are affected. In one study that reviewed hospital records and examined daily insulin doses and HbA1c levels before and after and episode of severe hypoglycemia in patients with insulin-dependent diabetes, it was found that, in 69% of these cases, either the physician or patient or both decreased the daily insulin dose. Furthermore, physicians decreased the insulin dose in a third of patients in whom the cause of hypoglycemia was preventable and due to a cause other than erroneous administration of excess insulin[101].
The economic consequences of severe hypoglycemia events and HU in patients with diabetes are higher than that of a mild episode and have been examined in a number of studies in Europe and United States[102-105]. Reported costs of a severe hypoglycemic event varied from approximately $80 to $5000, depending on the requirement for resources including hospitalization, emergency services, healthcare professionals and diagnostic test.
A United Kingdom study estimated the total cost of emergency treatments of 244 episodes of severe hypoglycemia in 160 patients with T1DM and T2DM over the course of one year. The total cost was approximately £92078 (£400 per episode)[102]. On the other hand, in a Swedish study the total cost (direct and indirect) of severe hypoglycemia in T2DM patients was between $12.90 and $14.10 for one month period[90].
An analysis of several United States studies, the estimated annual total cost attributable to severe hypoglycemia was between $1400 and $1500[106]. In this analysis the estimated work days lost per hypoglycemic event was between 0.22 and 6.60 d[103]. A recent study estimated that in patients with diabetes who experienced severe hypoglycemia, the lost of productivity ranged from $15.26 to $93.47 per severe hypoglycemic event, representing 8.3-15.9 h of lost work time per month[106]. Among the patients who experimented a severe hypoglycemic event at work, 18.3% missed work for a mean duration of 9.9 h, whereas the patients who had severe hypoglycemic event outside working hours, 22.7% arrived late for work or missed a full day[104]. If the hypoglycemia has occurred during the night, the number of working hours lost increased to 14.7 h[104].
Prevention of HU is an important part of modern day intensive diabetes therapy. To prevent HU, the goal is the complete avoidance of hypoglycemia, which is very difficult to achieve[105]. Blood glucose monitoring, individualized targets and educational programs are important in the bid to prevent and manage HU.
Blood glucose monitoring: CGMS, that can detect hypoglycemia, represents an important technological advance on the methods used for self-monitoring of blood glucose, and they are welcome to both patients and clinicians[106]. The ability of CGMS systems is to advise patients when glucose levels fall too low or rise too high, and has the potential to reduce de duration of hypoglycemia and hyperglycemia events[107,108]. Also, CGMS can be used for objective detection of patients with HU[109]. In adult patients with long-standing T1DM, a fasting level of C peptide of ≤ 0.6 ng/mL, and a HbA1c ≤ 9%, hypoglycemic episodes with a duration more than 90 minutes detected by CGMS, identified patients who had HU with an 88% specificity and 75% sensitivity[109]. On the other hand, the epinephrine response to hypoglycemia in adolescents patients with T1DM with HU was greater after the use of real-time CGMS with low glucose alarms than with standard medical therapy alone[110]. This suggests that real-time CGMS is a useful clinical tool to improve HU in adolescents with T1DM[110]. Choudhary et al[111] assessed the effect of CGMS on the frequency of severe hypoglycemia episodes, using the Gold scoring method[46] in 35 people with T1DM who have HU, via retrospective audit. A significant decline was observed in the mean rate of severe hypoglycemia (8.1 to 0.6 events per year) and also in HbA1c level (8.1% to 7.6%), between its initiation and the end of the 1-year follow-up period; while the mean Gold score did not change significantly[111]. These results support previous reports that CGMS can lower the incidence of severe hypoglycemia in patients with T1DM and HU, with no impact on the severity of HU over a 1-year period. A randomized cross-over study to assess the effects of CGMS use on glycemic levels and quality of life in patients with T1DM and HU, using the change in the Gold scoring as one of the secondary endpoints, is currently in progress and the results will not be available until 2015[112].
The impact of closed-loop CGMS, which link CGM technology with insulin pumps, whereby insulin infusion is programmed to stop automatically when glucose levels drop below a pre-determined glycemic threshold, on reducing the incidence of hypoglycemia events appears to be limited and so their usefulness in improving HU is debatable[16].
Individualized targets: In diabetic patients with HU blood glucose targets should be relaxed but not abandoned. Appropriate targeting of plasma glucose may help patients and practitioners achieve HbA1c goals, reduce excessive self-testing and minimize the occurrence of severe hypoglycemic events[113]. Glycemic goals should be individualized with some degree of safety particularly for patients with long duration of diabetes, patients who have a high risk of HU and severe hypoglycemia development, and/or subjects with multiple co-morbidities[114,115]. Basically, an HbA1c goal of less than 7% remains recommended, but is there a safe range for HbA1c? In patients with T1DM undertaking insulin therapy, the rates of severe hypoglycemia were increased among those with HbA1c < 6% and therefore it was suggested that using current therapy, an HbA1c of between 6%-7% represents the best compromise between the risk of severe hypoglycemia and that of developing microvascular complications[116].
Educational programs: The central objective of a hypoglycemia-reversal program is to prevent any period of hypoglycemia for at least four weeks. In diabetic patients with HU an appropriate educational program includes an emphasis on regular snacks at right times, warnings to take special care at periods of greater risk such as before lunch, moderation in alcohol intake and about the danger of delayed hypoglycemia after heavy alcohol intake or prolonged exercise. Diabetes self-management education can have physical and psychosocial benefits, and results in behavior changes with positive influence in outcome. A self-awareness intervention of 8 sessions, each lasting 3 h, was designed to determine whether there are psychosocial and physical benefits of self-awareness intervention in 29 adults with T1DM and HU. Post-intervention the participants detected more cues of euglycemia and hypoglycemia and experienced significant increases in integration and metabolic control[117].
In a randomized, prospective multi-centre trial, the effect of a specific training program for patients with hypoglycemia problem was compared with a control group receiving a standardized education program aiming of at avoidance of hypoglycemia by optimization of insulin therapy[118]. Compared to control group, the specific training program demonstrates additional benefits in terms of improving HU, reducing mild hypoglycemia, and detecting ant treating low blood glucose[118]. In the Dose Adjustment for Normal Eating-Hypoglycemia Awareness Restoration study, a 6-wk pilot intervention using motivational interviews and cognitive behavioral techniques around hypoglycemia, in 23 people with HU; support the importance of educational programs to improve HU. One year after the intervention HU had improved, mean rates of severe hypoglycemia fell from 3 to 0 per person per year, and worry and behavior around hypoglycemia improved[119]. In a sub-study of HypoCOMPaSS trial aimed to assess the restoration of impaired hypoglycemia awareness and defective hypoglycemia counter-regulation by an educational strategy targeted at hypoglycemia avoidance, in 18 adults patients with T1DM; following the 6-mo intervention the mean glucose concentration at which participants first experienced symptoms of hypoglycemia significantly increased from baseline (from 2.6 to 3.1 mg/dL), and counter-regulatory responses to hypoglycemia were also enhanced[120].
Jointly, the results of these three studies suggest that interventions that include education around hypoglycemia avoidance may help to decrease HU.
The treatment options for the management of HU are listed in Table 1.
| Treatments options | Mechanism of action |
| Optimizing insulin treatment | Avoidance of hypoglycemia |
| Pharmacological therapy | |
| β2-adrenergic agents | Enhancement of adrenaline effect |
| Methylxanthine derivates (caffeine, theophylline) | Central nervous system stimulation |
| Serotonin reuptake inhibitors (fluoxetine, sertraline, paroxetine) | Unknown. It has been hypothesized that the effect could be mediated by an atypical presentation of serotonin syndrome that will lead to autonomic dysfunction |
| KATP channel modulators | Modulation of hypoglycemia sensing |
| Other treatments | |
| Islet cell transplantation | Improving metabolic control |
| Fructose | Modulation of hypoglycemia sensing |
Optimizing insulin treatment: It is important that in patients with a history of recurrent hypoglycemia and HU, the time of episodes be identified and the treatment regimen be adjusted accordingly[121]. Compared with regular insulin, rapid-acting insulin analogs have a more rapid onset of action, higher peak action, and shorter duration of action, which more closely approximates endogenous mealtime insulin response, allowing more flexibility in the time of meals and exercise, and, consequently, a lower risk of severe hypoglycemic events[122]. Similarly, long-acting insulin analogs exhibit a more consistent, longer, and flatter action profile than NPH insulin, and demonstrate a lower risk of hypoglycemia, particularly nocturnal[123,124]. In diabetic patients with HU substitution of regular insulin with rapid-acting insulin analogs (aspart, lispro or glulisine) reduces frequency of daytime hypoglycemia; and substitution of long-acting insulin analogues (detemir or glargine) for intermediate-acting insulin (NPH or premix) reduces the frequency of nocturnal and day time hypoglycemia[121,125]. Compared with insulin glargine, the newest basal analog insulin degludec offers a more constant time-action profile, a long duration of action, and a lower risk of hypoglycemia[126,127]. While clinical experience with insulin degludec is limited, a meta-analysis evaluating 5 clinical trials of 3372 subjects with T2DM demonstrated a 17% lower rate of overall hypoglycemia and a 32% lower rate of nocturnal hypoglycemia with insulin degludec, compared with insulin glargine[128]. These characteristics may facilitate the achievement of glycemic control with insulin degludec with fewer hypoglycemic events in patients with HU.
An alternative approach is to use continuous subcutaneous insulin infusion (CSII). A study was designed by Giménez et al[129] to evaluate the effect of CSII on hypoglycemia awareness and on glucose profile in a cohort of T1DM subjects in which 95% had established HU and had experienced two or more episodes of severe hypoglycemia in the preceding two years, for a 24-mo period. Severe hypoglycemic episodes fell from 1.25 per subject-year to 0.05 after 24 mo, an improvement in all the aspects of quality of life, and an improved symptomatic response to experimentally-induced hypoglycemia was observed[130]. Previous studies[130-132] have also shown a reduction in hypoglycemia with CSII, particularly when a short-acting insulin analogue is used[2,133]. The decrease is partly due to better pharmacokinetic delivery of insulin and a 15%-20% reduction in insulin requirements compared with multiple doses of insulin[134]. Substitution of CSII for NPH insulin in patients with T1DM, especially at bedtime, resulted in a lower frequency of hypoglycemic episodes, and improved counter-regulatory and symptomatic responses during subsequent acute hypoglycemia[135]. On the other hand, administration of bolus doses of glucagon at times of impeding hypoglycemia during CSII lowered the frequency of hypoglycemia[136].
Pharmacological therapy:β-adrenergic antagonists or β-blockers alter the effects of epinephrine and could have potential effects on glucose homeostasis and the hypoglycemic counter-regulatory system. The more troubling concern regarding β-blockers is their potential effect on HU and blunting of the return to euglycemic levels after hypoglycemia has occurred, through the suppression of all adrenergically mediated symptoms of hypoglycemia. In patients with T1DM without HU, adrenergic symptoms did occur at lower glucose levels when subjects were treated with β-blockers[137]. Cardioselective β-blockers cause less alteration in the perception of hypoglycemia and may have an effect on correction of hypoglycemia than do their noncardioselective counterparts[138]. These agents should not be avoided in patients with diabetes but should be used with the same caution as when any new medication is added to a patient’s therapeutic regime.
It has been suggested that people with HU may have reduced β-adrenergic sensitivity, and this can be reversed by strict avoidance of hypoglycemia[139]. In T1DM patients, the use of β-adrenergic agonist terbutaline was associated with statistically significant higher glucose levels compared to control subjects during the first half and second half of the night, and with reduction of nocturnal hypoglycemic episodes (22 in the control group vs 1 in the group of terbutaline). β-adrenergic agonist had therefore been suggested as possible therapeutic options for HU, at the cost of inducing morning hyperglycemia. One of the concerns about using β-adrenergic agonist for the treatment of HU was associated with reduced β2 sensitivity observed in vitro. A recent study from De Galan et al[140] showed that sensitivity to β2-adrenergic receptor agonist stimulation is preserved in T1DM patients with HU. No long-term clinical trials to evaluate the usefulness of β-adrenergic agonist in the prevention of HU have been reported.
Several studies have evaluated the effects of the methylxantines derivatives caffeine and theophylline on HU and the counter-regulatory response to hypoglycemia. Both have been shown to augment symptom intensity and improve counter-regulatory responses in patients with T1DM with and without HU[2,141]. Using functional magnetic imaging, caffeine can restore regional brain activation normally lost during acute hypoglycemia[142]. In another trial designed to assess the impact of caffeine on the frequency and perception of hypoglycemia over a 3-mo period; patients receiving caffeine (200 mg/twice-daily) had statistically significant more symptomatic hypoglycemia episodes and more intense warning symptoms than patients receiving placebo[143]. These results suggest that modest amounts of caffeine enhance the sensitivity of hypoglycemia warning symptoms in patients with T1DM without increasing the incidence of severe hypoglycemia. de Galan et al[144] planned one study to evaluate the impact of theophylline on the response to hypoglycemia in 15 patients with T1DM who had a history of HU and 15 matched healthy control subjects. When compared with placebo, theophylline (2.8 mg/kg) improves de counter-regulatory response to a perception of hypoglycemia in the group with T1DM with HU[144]. Although modest doses of caffeine and theophylline may be effective at reducing HU in patients with T1DM at a low cost and without significant toxicity, larger doses may carry risk, and large trials are needed to determine efficacy, toxicity and dose-response curves.
The development of HU was associated with the use of selective serotonin reuptake inhibitors (SSRIs) in three patients with T1DM treated with different SSRIs (fluoxetine, sertraline and paroxetine) for depression and who were previously able to recognize and treat hypoglycemia symptoms[145]. HU occurred in all three patients within weeks of starting SSRI therapy. HU reversed after discontinuation of SSRI therapy[145]. The mechanism by which SSRIs might be associated with HU is unknown, but it has been hypothesized that the effect could be mediated by an atypical presentation of serotonin syndrome that will lead to autonomic dysfunction[146]. These observations suggest that in some patients, treatment with SSRIs may alter the perception of hypoglycemia, and should be used with caution in diabetic subjects with HU.
Infusion of the opioid-receptor antagonist naloxone increases the plasma epinephrine response to hypoglycemia and, when administered during hypoglycemia prevents attenuation of the plasma epinephrine response to subsequent hypoglycemia in humans[26,27].
Administration of a selective Kir6.2/SUR-1 KATP-channel agonist increases the epinephrine response to hypoglycemia in rats[147]. However, systemic administration of the nonselective KATP-channel agonist diazoxide suppresses the glucagon response and has no effect on the epinephrine response to hypoglycemia in nondiabetic humans[148]. These results suggest that KATP-channel modulators are not effective in humans, possibly due to inability to cross blood-brain barrier.
Other treatments: Islet cell transplantation (ICTx) prevents severe hypoglycemia[149], and restores some counter-regulatory hormone secretion[150]. In a retrospective study conducted in 31 T1DM recipients of ICTx, HU was assessed using the Clark hypoglycemic score (minimum = 0; maximum = 7; no hypoglycemia = 0; HU ≥ 4)[151] twice. A reduction in the proportion of patients with HU was observed post-ICTx (pre vs post-ICTx: 87% vs 13%) and a significant increase in glucose threshold that resulted in symptoms (pre vs post-ICTx: 41.4 mg/dL vs 58.4 mg/dL)[152]. These results were sustained even after the patient’s stratification based in islet function, graft dysfunction and graft failure[152]. These results suggests that improved metabolic control achieved with ICTx can restore hypoglycemia awareness in patients with T1DM, persisting even after islet graft failure.
Fructose infusion amplifies epinephrine and glucagon responses and increases glucose production during hypoglycemia in humans[153]. Fructose is a promising treatment but has not been tested in clinical trials.
HU is a complex, difficult-to-study phenomenon that carries with it great risk to patients. HU is common in people with T1DM and is observed with less frequency in insulin-treated T2DM. Exposure to antecedent hypoglycemia, especially repeated episodes, is an important factor in the pathogenesis of HU. Although enormous advances have been made in our knowledge of the mechanisms of HU, further research is needed to elucidate the pathophysiology of counter-regulatory impairment and HU, and enable the development of more targeted strategies that support glucose counter-regulation and consequently reduce hypoglycemia. Numerous research studies have begun to uncover the mechanisms by which the central nervous system responds and adapts to hypoglycemia. Understanding these mechanisms will lead to better management and therapies that reduce the risk for hypoglycemia. Studies aiming to improve or even reverse HU have met with variable success and a number of research groups are considering new candidate pathways to develop a therapy. Therefore, until effective measures are developed to reverse HU, part of the role of the healthcare professional should be to educate people with diabetes on the risks associated with HU and should discuss hypoglycemia prevention strategies with their patients, so that they can have a better chance of achieving their glucose controls goals while avoiding the morbidity and mortality associated with hypoglycemia.
P- Reviewer: Das UN, Osian G, Skok P S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
| 1. | Desouza CV, Bolli GB, Fonseca V. Hypoglycemia, diabetes, and cardiovascular events. Diabetes Care. 2010;33:1389-1394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 285] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 2. | de Galan BE, Schouwenberg BJ, Tack CJ, Smits P. Pathophysiology and management of recurrent hypoglycaemia and hypoglycaemia unawareness in diabetes. Neth J Med. 2006;64:269-279. [PubMed] [Cited in This Article: ] |
| 3. | Moghissi E, Ismail-Beigi F, Devine RC. Hypoglycemia: minimizing its impact in type 2 diabetes. Endocr Pract. 2013;19:526-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Briscoe VJ, Davis SN. Hypoglycemia in type 1 and type 2 diabetes: physiology, pathophysiology, and management. Clinical Diabetes. 2006;24:115-121. [DOI] [Cited in This Article: ] |
| 5. | Vignesh JP, Mohan V. Hypoglycaemia unawareness. J Assoc Physicians India. 2004;52:727-732. [PubMed] [Cited in This Article: ] |
| 6. | Czyzewska K, Czerniawska E, Szadkowska A. Prevalence of hypoglycemia unawareness in patients with type 1 diabetes. Pediatr Diabet. 2012;13 Suppl 17:77. [DOI] [Cited in This Article: ] |
| 7. | Geddes J, Schopman JE, Zammitt NN, Frier BM. Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. Diabet Med. 2008;25:501-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 259] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 8. | Schopman JE, Geddes J, Frier BM. Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res Clin Pract. 2010;87:64-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes. 2008;57:3169-3176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 528] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 10. | Miura J, Kajiura M, Hoshina S, Kobayashi H, Uchigata Y. The investigation of risk factor for the hypoglycemia unawareness in patients with type 1 diabetes using CGMS. Diabetes. 2012;61:A554. [Cited in This Article: ] |
| 11. | Pambianco GL, Costacou T, Orchard TJ. Does hypoglycemia unawareness (HU) differ by gender in type 1 diabetes (T1D)? Diabetes. 2009;58 Suppl 1:A544. [Cited in This Article: ] |
| 12. | Schouwenberg BJ, Veldman BA, Spiering W, Coenen MJ, Franke B, Tack CJ, de Galan BE, Smits P. The Arg16Gly variant of the beta2-adrenergic receptor predisposes to hypoglycemia unawareness in type 1 diabetes mellitus. Pharmacogenet Genomics. 2008;18:369-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 13. | Sejling AS, Kjaer TW, Pedersen-Bjergaard U, Remvig LS, Larsen A, Nielsen MN, Tarnow L, Thorsteinsson B, Juhl CB. The effect of recurrent hypoglycaemia on cerebral electrical activity in patients with type 1 diabetes and hypoglycaemia unawareness. Diabetes. 2013;62:A104. [Cited in This Article: ] |
| 14. | Dagogo-Jack S, Rattarasarn C, Cryer PE. Reversal of hypoglycemia unawareness, but not defective glucose counterregulation, in IDDM. Diabetes. 1994;43:1426-1434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 192] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Fanelli C, Pampanelli S, Epifano L, Rambotti AM, Di Vincenzo A, Modarelli F, Ciofetta M, Lepore M, Annibale B, Torlone E. Long-term recovery from unawareness, deficient counterregulation and lack of cognitive dysfunction during hypoglycaemia, following institution of rational, intensive insulin therapy in IDDM. Diabetologia. 1994;37:1265-1276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 115] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Reno CM, Litvin M, Clark AL, Fisher SJ. Defective counterregulation and hypoglycemia unawareness in diabetes: mechanisms and emerging treatments. Endocrinol Metab Clin North Am. 2013;42:15-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Ramanathan R, Cryer PE. Adrenergic mediation of hypoglycemia-associated autonomic failure. Diabetes. 2011;60:602-606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Jones TW, Porter P, Sherwin RS, Davis EA, O’Leary P, Frazer F, Byrne G, Stick S, Tamborlane WV. Decreased epinephrine responses to hypoglycemia during sleep. N Engl J Med. 1998;338:1657-1662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 219] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 19. | Banarer S, Cryer PE. Sleep-related hypoglycemia-associated autonomic failure in type 1 diabetes: reduced awakening from sleep during hypoglycemia. Diabetes. 2003;52:1195-1203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 117] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | McGregor VP, Banarer S, Cryer PE. Elevated endogenous cortisol reduces autonomic neuroendocrine and symptom responses to subsequent hypoglycemia. Am J Physiol Endocrinol Metab. 2002;282:E770-E777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 21. | Davis SN, Shavers C, Davis B, Costa F. Prevention of an increase in plasma cortisol during hypoglycemia preserves subsequent counterregulatory responses. J Clin Invest. 1997;100:429-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Davis SN, Shavers C, Costa F, Mosqueda-Garcia R. Role of cortisol in the pathogenesis of deficient counterregulation after antecedent hypoglycemia in normal humans. J Clin Invest. 1996;98:680-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 146] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 23. | Raju B, McGregor VP, Cryer PE. Cortisol elevations comparable to those that occur during hypoglycemia do not cause hypoglycemia-associated autonomic failure. Diabetes. 2003;52:2083-2089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Goldberg PA, Weiss R, McCrimmon RJ, Hintz EV, Dziura JD, Sherwin RS. Antecedent hypercortisolemia is not primarily responsible for generating hypoglycemia-associated autonomic failure. Diabetes. 2006;55:1121-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | McCrimmon RJ, Song Z, Cheng H, McNay EC, Weikart-Yeckel C, Fan X, Routh VH, Sherwin RS. Corticotrophin-releasing factor receptors within the ventromedial hypothalamus regulate hypoglycemia-induced hormonal counterregulation. J Clin Invest. 2006;116:1723-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 26. | Caprio S, Gerety G, Tamborlane WV, Jones T, Diamond M, Jacob R, Sherwin RS. Opiate blockade enhances hypoglycemic counterregulation in normal and insulin-dependent diabetic subjects. Am J Physiol. 1991;260:E852-E858. [PubMed] [Cited in This Article: ] |
| 27. | Vele S, Milman S, Shamoon H, Gabriely I. Opioid receptor blockade improves hypoglycemia-associated autonomic failure in type 1 diabetes mellitus. J Clin Endocrinol Metab. 2011;96:3424-3431. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Milman S, Leu J, Shamoon H, Vele S, Gabriely I. Magnitude of exercise-induced β-endorphin response is associated with subsequent development of altered hypoglycemia counterregulation. J Clin Endocrinol Metab. 2012;97:623-631. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Seaquist ER, Anderson J, Childs B, Cryer P, Dagogo-Jack S, Fish L, Heller SR, Rodriguez H, Rosenzweig J, Vigersky R. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384-1395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 955] [Cited by in F6Publishing: 915] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 30. | Zammitt NN, Warren RE, Deary IJ, Frier BM. Delayed recovery of cognitive function following hypoglycemia in adults with type 1 diabetes: effect of impaired awareness of hypoglycemia. Diabetes. 2008;57:732-736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Puente EC, Silverstein J, Bree AJ, Musikantow DR, Wozniak DF, Maloney S, Daphna-Iken D, Fisher SJ. Recurrent moderate hypoglycemia ameliorates brain damage and cognitive dysfunction induced by severe hypoglycemia. Diabetes. 2010;59:1055-1062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Cryer PE. Death during intensive glycemic therapy of diabetes: mechanisms and implications. Am J Med. 2011;124:993-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 33. | Cranston I, Reed LJ, Marsden PK, Amiel SA. Changes in regional brain (18)F-fluorodeoxyglucose uptake at hypoglycemia in type 1 diabetic men associated with hypoglycemia unawareness and counter-regulatory failure. Diabetes. 2001;50:2329-2336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Dunn JT, Cranston I, Marsden PK, Amiel SA, Reed LJ. Attenuation of amydgala and frontal cortical responses to low blood glucose concentration in asymptomatic hypoglycemia in type 1 diabetes: a new player in hypoglycemia unawareness? Diabetes. 2007;56:2766-2773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Mangia S, Tesfaye N, De Martino F, Kumar AF, Kollasch P, Moheet AA, Eberly LE, Seaquist ER. Hypoglycemia-induced increases in thalamic cerebral blood flow are blunted in subjects with type 1 diabetes and hypoglycemia unawareness. J Cereb Blood Flow Metab. 2012;32:2084-2090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Tesfaye N, Nangia S, De Martino F, Kumar A, Moheet A, Iverson E, Eberly LE, Seaquist ER. Hypoglycemia-induced increases in cerebral blood flow (CBF) are blunted in subjects with type 1 diabetes (TID) and hypoglycemia unawareness (HU). Diabetes. 2011;60:A79-A80. [Cited in This Article: ] |
| 37. | Criego AB, Tkac I, Kumar A, Thomas W, Gruetter R, Seaquist ER. Brain glucose concentrations in patients with type 1 diabetes and hypoglycemia unawareness. J Neurosci Res. 2005;79:42-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Oz G, Kumar A, Rao JP, Kodl CT, Chow L, Eberly LE, Seaquist ER. Human brain glycogen metabolism during and after hypoglycemia. Diabetes. 2009;58:1978-1985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 39. | Canada SE, Weaver SA, Sharpe SN, Pederson BA. Brain glycogen supercompensation in the mouse after recovery from insulin-induced hypoglycemia. J Neurosci Res. 2011;89:585-591. [PubMed] [Cited in This Article: ] |
| 40. | Öz G, Tesfaye N, Kumar A, Deelchand DK, Eberly LE, Seaquist ER. Brain glycogen content and metabolism in subjects with type 1 diabetes and hypoglycemia unawareness. J Cereb Blood Flow Metab. 2012;32:256-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Gulanski BI, De Feyter HM, Page KA, Belfort-DeAguiar R, Mason GF, Rothman DL, Sherwin RS. Increased brain transport and metabolism of acetate in hypoglycemia unawareness. J Clin Endocrinol Metab. 2013;98:3811-3820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 42. | De Feyter HM, Mason GF, Shulman GI, Rothman DL, Petersen KF. Increased brain lactate concentrations without increased lactate oxidation during hypoglycemia in type 1 diabetic individuals. Diabetes. 2013;62:3075-3080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 43. | Moheet A, Emir UE, Terpstra M, Kumar A, Eberly LE, Seaquist ER, Öz G. Initial experience with seven tesla magnetic resonance spectroscopy of hypothalamic GABA during hyperinsulinemic euglycemia and hypoglycemia in healthy humans. Magn Reson Med. 2014;71:12-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Chan O, Cheng H, Herzog R, Czyzyk D, Zhu W, Wang A, McCrimmon RJ, Seashore MR, Sherwin RS. Increased GABAergic tone in the ventromedial hypothalamus contributes to suppression of counterregulatory responses after antecedent hypoglycemia. Diabetes. 2008;57:1363-1370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 45. | Chan O, Paranjape S, Czyzyk D, Horblitt A, Zhu W, Ding Y, Fan X, Seashore M, Sherwin R. Increased GABAergic output in the ventromedial hypothalamus contributes to impaired hypoglycemic counterregulation in diabetic rats. Diabetes. 2011;60:1582-1589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care. 1994;17:697-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 522] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 47. | Choudhary P, Geddes J, Freeman JV, Emery CJ, Heller SR, Frier BM. Frequency of biochemical hypoglycaemia in adults with Type 1 diabetes with and without impaired awareness of hypoglycaemia: no identifiable differences using continuous glucose monitoring. Diabet Med. 2010;27:666-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 48. | Gerstein HC, Miller ME, Byington RP, Goff DC, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545-2559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5985] [Cited by in F6Publishing: 5391] [Article Influence: 336.9] [Reference Citation Analysis (0)] |
| 49. | Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S. Severe hypoglycemia and risks of vascular events and death. N Engl J Med. 2010;363:1410-1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1067] [Cited by in F6Publishing: 1048] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 50. | Seaquist ER, Miller ME, Bonds DE, Feinglos M, Goff DC, Peterson K, Senior P. The impact of frequent and unrecognized hypoglycemia on mortality in the ACCORD study. Diabetes Care. 2012;35:409-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 51. | Jacobson AM, Musen G, Ryan CM, Silvers N, Cleary P, Waberski B, Burwood A, Weinger K, Bayless M, Dahms W. Long-term effect of diabetes and its treatment on cognitive function. N Engl J Med. 2007;356:1842-1852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 396] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 52. | Reichard P, Pihl M. Mortality and treatment side-effects during long-term intensified conventional insulin treatment in the Stockholm Diabetes Intervention Study. Diabetes. 1994;43:313-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 78] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Gold AE, MacLeod KM, Deary IJ, Frier BM. Hypoglycemia-induced cognitive dysfunction in diabetes mellitus: effect of hypoglycemia unawareness. Physiol Behav. 1995;58:501-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Bolo NR, Musen G, Jacobson AM, Weinger K, McCartney RL, Flores V, Renshaw PF, Simonson DC. Brain activation during working memory is altered in patients with type 1 diabetes during hypoglycemia. Diabetes. 2011;60:3256-3264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Smith CB, Choudhary P, Pernet A, Hopkins D, Amiel SA. Hypoglycemia unawareness is associated with reduced adherence to therapeutic decisions in patients with type 1 diabetes: evidence from a clinical audit. Diabetes Care. 2009;32:1196-1198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Graveling AJ, Frier BM. Hypoglycemia unawareness is associated with reduced adherence to therapeutic decisions in patients with type 1 diabetes: evidence from a clinical audit: response to Smith et al. Diabetes Care. 2010;33:e15; author reply e16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 57. | Ly TT, Gallego PH, Davis EA, Jones TW. Impaired awareness of hypoglycemia in a population-based sample of children and adolescents with type 1 diabetes. Diabetes Care. 2009;32:1802-1806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Hannonen R, Tupola S, Ahonen T, Riikonen R. Neurocognitive functioning in children with type-1 diabetes with and without episodes of severe hypoglycaemia. Dev Med Child Neurol. 2003;45:262-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 59. | Northam EA, Anderson PJ, Jacobs R, Hughes M, Warne GL, Werther GA. Neuropsychological profiles of children with type 1 diabetes 6 years after disease onset. Diabetes Care. 2001;24:1541-1546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 246] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 60. | Ho MS, Weller NJ, Ives FJ, Carne CL, Murray K, Vanden Driesen RI, Nguyen TP, Robins PD, Bulsara M, Davis EA. Prevalence of structural central nervous system abnormalities in early-onset type 1 diabetes mellitus. J Pediatr. 2008;153:385-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 61. | Golden MP, Ingersoll GM, Brack CJ, Russell BA, Wright JC, Huberty TJ. Longitudinal relationship of asymptomatic hypoglycemia to cognitive function in IDDM. Diabetes Care. 1989;12:89-93. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 62. | Perantie DC, Lim A, Wu J, Weaver P, Warren SL, Sadler M, White NH, Hershey T. Effects of prior hypoglycemia and hyperglycemia on cognition in children with type 1 diabetes mellitus. Pediatr Diabetes. 2008;9:87-95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 63. | Chico A, Vidal-Ríos P, Subirà M, Novials A. The continuous glucose monitoring system is useful for detecting unrecognized hypoglycemias in patients with type 1 and type 2 diabetes but is not better than frequent capillary glucose measurements for improving metabolic control. Diabetes Care. 2003;26:1153-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 64. | Hay LC, Wilmshurst EG, Fulcher G. Unrecognized hypo- and hyperglycemia in well-controlled patients with type 2 diabetes mellitus: the results of continuous glucose monitoring. Diabetes Technol Ther. 2003;5:19-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 108] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 65. | Desouza C, Salazar H, Cheong B, Murgo J, Fonseca V. Association of hypoglycemia and cardiac ischemia: a study based on continuous monitoring. Diabetes Care. 2003;26:1485-1489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 261] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 66. | Tanenberg RJ, Newton CA, Drake AJ. Confirmation of hypoglycemia in the “dead-in-bed” syndrome, as captured by a retrospective continuous glucose monitoring system. Endocr Pract. 2010;16:244-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 67. | Johnston SS, Conner C, Aagren M, Smith DM, Bouchard J, Brett J. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes Care. 2011;34:1164-1170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 68. | Miller DR, Fincke G, Lafrance JP, Palnati M, Shao Q, Zhang Q, Fonseca V, Riddle M, Vijan S, Christiansen CI. Hypoglycaemia and risk of myocardial infarction in US veterans with diabetes. Diabetologia. 2009;52:S63. [Cited in This Article: ] |
| 69. | Holstein A, Egberts EH. Risk of hypoglycaemia with oral antidiabetic agents in patients with Type 2 diabetes. Exp Clin Endocrinol Diabetes. 2003;111:405-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 70. | Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in Type 2 diabetes. Diabet Med. 2008;25:245-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 350] [Cited by in F6Publishing: 373] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 71. | Marrett E, Radican L, Davies MJ, Zhang Q. Assessment of severity and frequency of self-reported hypoglycemia on quality of life in patients with type 2 diabetes treated with oral antihyperglycemic agents: A survey study. BMC Res Notes. 2011;4:251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 72. | Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009;301:1565-1572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 764] [Cited by in F6Publishing: 692] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 73. | Allen KV, Frier BM. Nocturnal hypoglycemia: clinical manifestations and therapeutic strategies toward prevention. Endocr Pract. 2003;9:530-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 74. | Alagiakrishnan K, Mereu L. Approach to managing hypoglycemia in elderly patients with diabetes. Postgrad Med. 2010;122:129-137. [PubMed] [Cited in This Article: ] |
| 75. | Bruce DG, Casey GP, Grange V, Clarnette RC, Almeida OP, Foster JK, Ives FJ, Davis TM. Cognitive impairment, physical disability and depressive symptoms in older diabetic patients: the Fremantle Cognition in Diabetes Study. Diabetes Res Clin Pract. 2003;61:59-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 76. | Bree AJ, Puente EC, Daphna-Iken D, Fisher SJ. Diabetes increases brain damage caused by severe hypoglycemia. Am J Physiol Endocrinol Metab. 2009;297:E194-E201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 77. | Abbaszadeh Ahranjani S, Tabatabaei-Malazy O, Pajouhi M. Diabetes in old age, a review. Iranian J Diabetes and Lipid Disorders. 2009;8:113-128. [Cited in This Article: ] |
| 78. | Aung PP, Strachan MW, Frier BM, Butcher I, Deary IJ, Price JF. Severe hypoglycaemia and late-life cognitive ability in older people with Type 2 diabetes: the Edinburgh Type 2 Diabetes Study. Diabet Med. 2012;29:328-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 79. | Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011;365:2002-2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1224] [Cited by in F6Publishing: 1155] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 80. | Evers IM, ter Braak EW, de Valk HW, van Der Schoot B, Janssen N, Visser GH. Risk indicators predictive for severe hypoglycemia during the first trimester of type 1 diabetic pregnancy. Diabetes Care. 2002;25:554-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 82] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Nielsen LR, Pedersen-Bjergaard U, Thorsteinsson B, Johansen M, Damm P, Mathiesen ER. Hypoglycemia in pregnant women with type 1 diabetes: predictors and role of metabolic control. Diabetes Care. 2008;31:9-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 82. | Robertson H, Pearson DW, Gold AE. Severe hypoglycaemia during pregnancy in women with Type 1 diabetes is common and planning pregnancy does not decrease the risk. Diabet Med. 2009;26:824-826. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | Rossi G, Lapaczewski P, Diamond MP, Jacob RJ, Shulman GI, Sherwin RS. Inhibitory effect of pregnancy on counterregulatory hormone responses to hypoglycemia in awake rat. Diabetes. 1993;42:1440-1445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 84. | Rosenn BM, Miodovnik M, Khoury JC, Siddiqi TA. Counterregulatory hormonal responses to hypoglycemia during pregnancy. Obstet Gynecol. 1996;87:568-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 85. | Ringholm L, Pedersen-Bjergaard U, Thorsteinsson B, Damm P, Mathiesen ER. Hypoglycaemia during pregnancy in women with Type 1 diabetes. Diabet Med. 2012;29:558-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 86. | Heller S, Damm P, Mersebach H, Skjøth TV, Kaaja R, Hod M, Durán-García S, McCance D, Mathiesen ER. Hypoglycemia in type 1 diabetic pregnancy: role of preconception insulin aspart treatment in a randomized study. Diabetes Care. 2010;33:473-477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 87. | Barendse S, Singh H, Frier BM, Speight J. The impact of hypoglycaemia on quality of life and related patient-reported outcomes in Type 2 diabetes: a narrative review. Diabet Med. 2012;29:293-302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 88. | Davis RE, Morrissey M, Peters JR, Wittrup-Jensen K, Kennedy-Martin T, Currie CJ. Impact of hypoglycaemia on quality of life and productivity in type 1 and type 2 diabetes. Curr Med Res Opin. 2005;21:1477-1483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 89. | Williams SA, Pollack MF, Dibonaventura M. Effects of hypoglycemia on health-related quality of life, treatment satisfaction and healthcare resource utilization in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2011;91:363-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 90. | Lundkvist J, Berne C, Bolinder B, Jönsson L. The economic and quality of life impact of hypoglycemia. Eur J Health Econ. 2005;6:197-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 91. | Fidler C, Elmelund Christensen T, Gillard S. Hypoglycemia: an overview of fear of hypoglycemia, quality-of-life, and impact on costs. J Med Econ. 2011;14:646-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 92. | Gold AE, Deary IJ, Frier BM. Hypoglycaemia and non-cognitive aspects of psychological function in insulin-dependent (type 1) diabetes mellitus (IDDM). Diabet Med. 1997;14:111-118. [PubMed] [DOI] [Cited in This Article: ] |
| 93. | Strachan MW, Deary IJ, Ewing FM, Frier BM. Recovery of cognitive function and mood after severe hypoglycemia in adults with insulin-treated diabetes. Diabetes Care. 2000;23:305-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 94. | King P, Kong MF, Parkin H, Macdonald IA, Tattersall RB. Well-being, cerebral function, and physical fatigue after nocturnal hypoglycemia in IDDM. Diabetes Care. 1998;21:341-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Ritholz MD, Jacobson AM. Living with hypoglycemia. J Gen Intern Med. 1998;13:799-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 96. | Eadington DW, Frier BM. Type 1 diabetes and driving experience: an eight-year cohort study. Diabet Med. 1989;6:137-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 97. | Lave LB, Songer TJ, LaPorte RE. Should persons with diabetes be licensed to drive trucks?--Risk management. Risk Anal. 1993;13:327-334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 98. | Cox DJ, Kovatchev B, Vandecar K, Gonder-Frederick L, Ritterband L, Clarke W. Hypoglycemia preceding fatal car collisions. Diabetes Care. 2006;29:467-468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 99. | Lawton J, Rankin D, Elliott J, Heller SR, Rogers HA, De Zoysa N, Amiel S. Experiences, views, and support needs of family members of people with hypoglycemia unawareness: interview study. Diabetes Care. 2014;37:109-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 100. | Gonder-Frederick LA, Schmidt KM, Vajda KA, Greear ML, Singh H, Shepard JA, Cox DJ. Psychometric properties of the hypoglycemia fear survey-ii for adults with type 1 diabetes. Diabetes Care. 2011;34:801-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 101. | Tupola S, Rajantie J, Akerblom HK. Experience of severe hypoglycaemia may influence both patient’s and physician’s subsequent treatment policy of insulin-dependent diabetes mellitus. Eur J Pediatr. 1998;157:625-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 102. | Leese GP, Wang J, Broomhall J, Kelly P, Marsden A, Morrison W, Frier BM, Morris AD. Frequency of severe hypoglycemia requiring emergency treatment in type 1 and type 2 diabetes: a population-based study of health service resource use. Diabetes Care. 2003;26:1176-1180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 398] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 103. | Zhang Y, Wieffer H, Modha R, Balar B, Pollack M, Krishnarajah G. The burden of hypoglycemia in type 2 diabetes: a systematic review of patient and economic perspectives. J Clin Outcomes Manage. 2010;17:547-557. [Cited in This Article: ] |
| 104. | Brod M, Christensen T, Thomsen TL, Bushnell DM. The impact of non-severe hypoglycemic events on work productivity and diabetes management. Value Health. 2011;14:665-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 105. | Fanelli CG, Epifano L, Rambotti AM, Pampanelli S, Di Vincenzo A, Modarelli F, Lepore M, Annibale B, Ciofetta M, Bottini P. Meticulous prevention of hypoglycemia normalizes the glycemic thresholds and magnitude of most of neuroendocrine responses to, symptoms of, and cognitive function during hypoglycemia in intensively treated patients with short-term IDDM. Diabetes. 1993;42:1683-1689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 106. | Klonoff DC. Continuous glucose monitoring: roadmap for 21st century diabetes therapy. Diabetes Care. 2005;28:1231-1239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 489] [Cited by in F6Publishing: 394] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 107. | Bode B, Gross K, Rikalo N, Schwartz S, Wahl T, Page C, Gross T, Mastrototaro J. Alarms based on real-time sensor glucose values alert patients to hypo- and hyperglycemia: the guardian continuous monitoring system. Diabetes Technol Ther. 2004;6:105-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 108. | Garg S, Zisser H, Schwartz S, Bailey T, Kaplan R, Ellis S, Jovanovic L. Improvement in glycemic excursions with a transcutaneous, real-time continuous glucose sensor: a randomized controlled trial. Diabetes Care. 2006;29:44-50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 299] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 109. | Streja D. Can continuous glucose monitoring provide objective documentation of hypoglycemia unawareness? Endocr Pract. 2005;11:83-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 110. | Ly TT, Hewitt J, Davey RJ, Lim EM, Davis EA, Jones TW. Improving epinephrine responses in hypoglycemia unawareness with real-time continuous glucose monitoring in adolescents with type 1 diabetes. Diabetes Care. 2011;34:50-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 111. | Choudhary P, Ramasamy S, Green L, Gallen G, Pender S, Brackenridge A, Amiel SA, Pickup JC. Real-time continuous glucose monitoring significantly reduces severe hypoglycemia in hypoglycemia-unaware patients with type 1 diabetes. Diabetes Care. 2013;36:4160-4162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 112. | The Clinical Trials gov. The effect of RT-CGM on glycemia and Qol in patients with T1DM and IHA (IN CONTROL). [Updated 2013 Mar 6]. Available from: https://clinicaltrials.gov/ct2/show/NCT01787903. [Cited in This Article: ] |
| 113. | Schrot RJ. Targeting plasma glucose: preprandial versus postprandial. Clin Diabetes. 2004;22:169-172. [DOI] [Cited in This Article: ] |
| 114. | Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER, Service FJ. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94:709-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 712] [Cited by in F6Publishing: 672] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 115. | Qaseem A, Vijan S, Snow V, Cross JT, Weiss KB, Owens DK. Glycemic control and type 2 diabetes mellitus: the optimal hemoglobin A1c targets. A guidance statement from the American College of Physicians. Ann Intern Med. 2007;147:417-422. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 116. | Pampanelli S, Fanelli C, Lalli C, Ciofetta M, Sindaco PD, Lepore M, Modarelli F, Rambotti AM, Epifano L, Di Vincenzo A. Long-term intensive insulin therapy in IDDM: effects on HbA1c, risk for severe and mild hypoglycaemia, status of counterregulation and awareness of hypoglycaemia. Diabetologia. 1996;39:677-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 117. | Hamdan M, Brunet P, Wagner J. [Metastatic jejunal perforation of cancer of the larynx]. J Chir (Paris). 2008;128:332. [PubMed] [Cited in This Article: ] |
| 118. | Hermanns N, Kulzer B, Kubiak T, Krichbaum M, Haak T. The effect of an education programme (HyPOS) to treat hypoglycaemia problems in patients with type 1 diabetes. Diabetes Metab Res Rev. 2007;23:528-538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 119. | de Zoysa N, Rogers H, Stadler M, Gianfrancesco C, Beveridge S, Britneff E, Choudhary P, Elliott J, Heller S, Amiel SA. A psychoeducational program to restore hypoglycemia awareness: the DAFNE-HART pilot study. Diabetes Care. 2014;37:863-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 120. | Leelarathna L, Little SA, Walkinshaw E, Tan HK, Lubina-Solomon A, Kumareswaran K, Lane AP, Chadwick T, Marshall SM, Speight J. Restoration of self-awareness of hypoglycemia in adults with long-standing type 1 diabetes: hyperinsulinemic-hypoglycemic clamp substudy results from the HypoCOMPaSS trial. Diabetes Care. 2013;36:4063-4070. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 121. | Cryer PE, Davis SN, Shamoon H. Hypoglycemia in diabetes. Diabetes Care. 2003;26:1902-1912. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 881] [Cited by in F6Publishing: 780] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 122. | Hirsch IB. Insulin analogues. N Engl J Med. 2005;352:174-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 581] [Cited by in F6Publishing: 517] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 123. | Yki-Järvinen H, Dressler A, Ziemen M. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130-1136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 423] [Cited by in F6Publishing: 370] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 124. | Rosenstock J, Schwartz SL, Clark CM, Park GD, Donley DW, Edwards MB. Basal insulin therapy in type 2 diabetes: 28-week comparison of insulin glargine (HOE 901) and NPH insulin. Diabetes Care. 2001;24:631-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 351] [Cited by in F6Publishing: 367] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 125. | Mensing C, Boucher J, Cypress M, Weinger K, Mulcahy K, Barta P, Hosey G, Kopher W, Lasichak A, Lamb B. National standards for diabetes self-management education. Diabetes Care. 2006;29 Suppl 1:S78-S85. [PubMed] [Cited in This Article: ] |
| 126. | Garber AJ, King AB, Del Prato S, Sreenan S, Balci MK, Muñoz-Torres M, Rosenstock J, Endahl LA, Francisco AM, Hollander P. Insulin degludec, an ultra-longacting basal insulin, versus insulin glargine in basal-bolus treatment with mealtime insulin aspart in type 2 diabetes (BEGIN Basal-Bolus Type 2): a phase 3, randomised, open-label, treat-to-target non-inferiority trial. Lancet. 2012;379:1498-1507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 248] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 127. | Heise T, Nosek L, Bøttcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab. 2012;14:944-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 236] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 128. | Ratner RE, Gough SC, Mathieu C, Del Prato S, Bode B, Mersebach H, Endahl L, Zinman B. Hypoglycaemia risk with insulin degludec compared with insulin glargine in type 2 and type 1 diabetes: a pre-planned meta-analysis of phase 3 trials. Diabetes Obes Metab. 2013;15:175-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 251] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 129. | Giménez M, Lara M, Conget I. Sustained efficacy of continuous subcutaneous insulin infusion in type 1 diabetes subjects with recurrent non-severe and severe hypoglycemia and hypoglycemia unawareness: a pilot study. Diabetes Technol Ther. 2010;12:517-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 130. | Chantelau E, Spraul M, Mühlhauser I, Gause R, Berger M. Long-term safety, efficacy and side-effects of continuous subcutaneous insulin infusion treatment for type 1 (insulin-dependent) diabetes mellitus: a one centre experience. Diabetologia. 1989;32:421-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 91] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 131. | Bendtson I, Kverneland A, Pramming S, Binderboland C. Incidence of nocturnal hypoglycaemia in insulin-dependent diabetic patients on intensive therapy. Acta Med Scand. 1988;223:543-548. [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 132. | Fanelli CG, Porcellati F, Pampanelli S, Bolli GB. Insulin therapy and hypoglycaemia: the size of the problem. Diabetes Metab Res Rev. 2004;20 Suppl 2:S32-S42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 133. | Skyler JS, Ponder S, Kruger DF, Matheson D, Parkin CG. Is there a place for insulin pump therapy in your practice? Clinical Diabetes. 2007;25:50-56. [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 134. | Bode BW, Steed RD, Davidson PC. Reduction in severe hypoglycemia with long-term continuous subcutaneous insulin infusion in type I diabetes. Diabetes Care. 1996;19:324-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 197] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 135. | Kanc K, Janssen MM, Keulen ET, Jacobs MA, Popp-Snijders C, Snoek FJ, Heine RJ. Substitution of night-time continuous subcutaneous insulin infusion therapy for bedtime NPH insulin in a multiple injection regimen improves counterregulatory hormonal responses and warning symptoms of hypoglycaemia in IDDM. Diabetologia. 1998;41:322-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 136. | Castle JR, Engle JM, El Youssef J, Massoud RG, Yuen KC, Kagan R, Ward WK. Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care. 2010;33:1282-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 137. | Hirsch IB, Boyle PJ, Craft S, Cryer PE. Higher glycemic thresholds for symptoms during beta-adrenergic blockade in IDDM. Diabetes. 1991;40:1177-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 138. | White JR, Campbell RK. Dangerous and common drug interactions in patients with diabetes mellitus. Endocrinol Metab Clin North Am. 2000;29:789-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 139. | Fritsche A, Stumvoll M, Häring HU, Gerich JE. Reversal of hypoglycemia unawareness in a long-term type 1 diabetic patient by improvement of beta-adrenergic sensitivity after prevention of hypoglycemia. J Clin Endocrinol Metab. 2000;85:523-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 140. | De Galan BE, De Mol P, Wennekes L, Schouwenberg BJ, Smits P. Preserved sensitivity to beta2-adrenergic receptor agonists in patients with type 1 diabetes mellitus and hypoglycemia unawareness. J Clin Endocrinol Metab. 2006;91:2878-2881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 141. | Kerr D, Sherwin RS, Pavalkis F, Fayad PB, Sikorski L, Rife F, Tamborlane WV, During MJ. Effect of caffeine on the recognition of and responses to hypoglycemia in humans. Ann Intern Med. 1993;119:799-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 142. | Rosenthal MJ, Smith D, Yaguez L, Giampietro V, Kerr D, Bullmore E, Brammer M, Williams SC, Amiel SA. Caffeine restores regional brain activation in acute hypoglycaemia in healthy volunteers. Diabet Med. 2007;24:720-727. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 143. | Watson JM, Jenkins EJ, Hamilton P, Lunt MJ, Kerr D. Influence of caffeine on the frequency and perception of hypoglycemia in free-living patients with type 1 diabetes. Diabetes Care. 2000;23:455-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 144. | de Galan BE, Tack CJ, Lenders JW, Pasman JW, Elving LD, Russel FG, Lutterman JA, Smits P. Theophylline improves hypoglycemia unawareness in type 1 diabetes. Diabetes. 2002;51:790-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 145. | Kerr D, Everett J. Coffee, diabetes and insulin sensitivity. Diabetologia. 2005;48:1418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 146. | Sawka AM, Burgart V, Zimmerman D. Loss of awareness of hypoglycemia temporally associated with selective serotonin reuptake inhibitors. Diabetes Care. 2001;24:1845-1846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 147. | Fan X, Ding Y, Cheng H, Gram DX, Sherwin RS, McCrimmon RJ. Amplified hormonal counterregulatory responses to hypoglycemia in rats after systemic delivery of a SUR-1-selective K(+) channel opener? Diabetes. 2008;57:3327-3334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 148. | Raju B, Cryer PE. Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. Diabetes. 2005;54:757-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 149. | Froud T, Ricordi C, Baidal DA, Hafiz MM, Ponte G, Cure P, Pileggi A, Poggioli R, Ichii H, Khan A. Islet transplantation in type 1 diabetes mellitus using cultured islets and steroid-free immunosuppression: Miami experience. Am J Transplant. 2005;5:2037-2046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 327] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 150. | Rickels MR, Schutta MH, Mueller R, Kapoor S, Markmann JF, Naji A, Teff KL. Glycemic thresholds for activation of counterregulatory hormone and symptom responses in islet transplant recipients. J Clin Endocrinol Metab. 2007;92:873-879. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 151. | Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care. 1995;18:517-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 437] [Cited by in F6Publishing: 431] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 152. | Leitão CB, Tharavanij T, Cure P, Pileggi A, Baidal DA, Ricordi C, Alejandro R. Restoration of hypoglycemia awareness after islet transplantation. Diabetes Care. 2008;31:2113-2115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 153. | Gabriely I, Hawkins M, Vilcu C, Rossetti L, Shamoon H. Fructose amplifies counterregulatory responses to hypoglycemia in humans. Diabetes. 2002;51:893-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |