Published online Nov 15, 2023. doi: 10.4239/wjd.v14.i11.1693
Peer-review started: February 26, 2023
First decision: April 20, 2023
Revised: May 18, 2023
Accepted: October 11, 2023
Article in press: October 11, 2023
Published online: November 15, 2023
In contrast to overt diabetes mellitus (DM), gestational DM (GDM) is defined as impaired glucose tolerance induced by pregnancy, which may arise from exaggerated physiologic changes in glucose metabolism. GDM prevalence is reported to be as high as 20% among pregnancies depending on the screening method, gestational age, and the population studied. Maternal and fetal effects of uncontrolled GDM include stillbirth, macrosomia, neonatal diabetes, birth trauma, and subsequent postpartum hemorrhage. Therefore, it is essential to find the potential target population and associated predictive and preventive measures for future intensive peripartum care.
To review studies that explored the cellular and molecular mechanisms of GDM as well as predictive measures and prevention strategies.
The search was performed in the Medline and PubMed databases using the terms “gestational diabetes mellitus,” “overt diabetes mellitus,” and “insulin resistan
Finally, a total of 79 articles were collected for review. Reported risk factors for GDM included maternal obesity or overweight, pre-existing DM, and polycystic ovary syndrome. The pathophysiology of GDM involves genetic variants responsible for insulin secretion and glycemic control, pancreatic β cell depletion or dysfunction, aggravated insulin resistance due to failure in the plasma membrane translocation of glucose transporter 4, and the effects of chronic, low-grade inflammation. Currently, many antepartum measurements including adipokines (leptin), body mass ratio (waist circumference and waist-to-hip ratio], and biomarkers (microRNA in extracellular vesicles) have been studied and confirmed to be useful markers for predicting GDM. For preventing GDM, physical activity and dietary approaches are effective interventions to control body weight, improve glycemic control, and reduce insulin resistance.
This review explored the possible factors that influence GDM and the underlying molecular and cellular mechanisms of GDM and provided predictive measures and prevention strategies based on results of clinical studies.
Core Tip: Maternal and fetal effects of uncontrolled gestational diabetes mellitus (GDM) include stillbirth, macrosomia, neonatal diabetes, and birth trauma. Risk factors are maternal obesity or overweight, pre-existing diabetes mellitus, and polycystic ovary syndrome. The complex pathophysiology involves genetic variants, pancreatic β cell depletion or dysfunction, aggravated insulin resistance due to glucose transporter 4 translocation failure, and chronic, low-grade inflammation. Antepartum measurements including adipokines (leptin), body mass ratio (waist circumference and waist-to-hip ratio), and biomarkers (microRNA in extracellular vesicles) are useful markers for predicting GDM. For preventing GDM, physical activity and diet (such as the Mediterranean diet) control are effective interventions.
- Citation: Lim PQ, Lai YJ, Ling PY, Chen KH. Cellular and molecular overview of gestational diabetes mellitus: Is it predictable and preventable? World J Diabetes 2023; 14(11): 1693-1709
- URL: https://www.wjgnet.com/1948-9358/full/v14/i11/1693.htm
- DOI: https://dx.doi.org/10.4239/wjd.v14.i11.1693
Gestational diabetes mellitus (GDM) poses a severe and neglected threat to maternal and neonatal health. According to data from the International Diabetes Federation, approximately 223 million women globally are living with diabetes[1]. By 2045, this number is projected to increase to 343 million, and it is estimated that 1 in 6 births will be affected by GDM[1]. The majority of hyperglycemia cases during pregnancy occur in low-income and middle-income countries where access to maternal care is limited[1]. The rising incidence of obesity has contributed to an increase in the occurrence of GDM and related complications during pregnancy and the perinatal period[2]. Various risk factors for GDM include excessive weight gain during early adulthood and before 24 wk gestation, advanced maternal age, a history of prior GDM, a family history of overt diabetes, prepregnancy body mass index (BMI) ≥ 30 kg/m2, prepregnancy polycystic ovary syndrome, and prior delivery of a newborn more than 4000 g[3-6]. Several races/ethnicities (Native and Hispanic American, Native Hawaiian, Native Alaskan, East or South Asian, and Pacific Islander) are also at a greater risk for the development of GDM[7-9].
Recommendations for universal screening of GDM advise conducting the screening between 24 wk and 28 wk of gestation, based on randomized controlled trials (RCTs) demonstrating improved maternal and perinatal outcomes with GDM treatment[10]. However, there is currently no consensus on the optimal diagnostic method for gestational diabetes. Two screening approaches (one-step vs two-step) are considered acceptable. Proposed by the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) and used by the American Diabetes Association (ADA), the one-step approach employs a 2-h oral glucose tolerance test (OGTT) in all pregnant women. Despite the advantage of concurrent screening and diagnosis within a single visit, pregnant women have to fast before the examination and wait for a subsequent 2-h examination[10]. Recommended by the American College of Obstetricians and Gynecologists (ACOG), the two-step approach involves a non-fasting 1-h glucose challenge test and seems more convenient for the participants. If the patient passes the first step, further screening is usually unnecessary. However, those who fail the initial screening undergo the 3-h 100 g OGTT to confirm the diagnosis of GDM[10]. Despite the availability of these two screening options, there is currently a lack of consensus regarding the preferred approach. The ACOG has not fully adopted the one-step process due to insufficient evidence regarding its impact on pregnancy outcomes[2]. Hillier et al[10] conducted a RCT and concluded that even the one-step approach can detect more cases of GDM in comparison with the two-step approach. There was no significant difference between the two groups regarding the risks of maternal and perinatal complications.
Maternal hyperglycemia stimulates fetal hyperglycemia, which in turn triggers the release of fetal insulin. As insulin possesses both metabolic and anabolic effects, fetal hyperinsulinemia can induce excessive fetal growth and subsequent adverse perinatal outcomes including neonatal shoulder dystocia, birth injury, prematurity, and death and an increased likelihood of cesarean section[11]. It was observed that 93% of women who exceeded the gestational weight gain guidelines set by the Institute of Medicine experienced excessive early gestational weight gain[11]. Excessive early gestational weight gain in low-risk nulliparous women was associated with the development of GDM and increased fetal growth[11]. The results of the Hyperglycemia and Adverse Pregnancy Outcomes (HAPO) study revealed close relations between maternal hyperglycemia and adverse perinatal outcomes, including preterm delivery, shoulder dystocia, or other birth injury, conversion to cesarean delivery, neonatal hypoglycemia or hyperbilirubinemia, and the need for intensive neonatal care. These associations were attributed to the clinical consequences of maternal hyperglycemia, namely fetal overgrowth and hyperinsulinemia[12]. The aforementioned findings further supported the role of glucose-lowering therapy as a priority for women who were diagnosed with GDM[12].
This review explored the underlying molecular and cellular mechanisms of GDM as well as the potential influencing factors. Additionally, we discussed predictive and preventive measures based on both basic and clinical studies that have investigated the etiology, pathophysiology, and management of GDM. Understanding the cellular and molecular mechanisms involved in GDM will enhance our knowledge of the current principles of drug action and may provide insights into identifying new treatment targets.
The objective of this review was to identify relevant studies in the literature that investigated the underlying cellular and molecular mechanisms of GDM as well as potential influencing factors, predictive measures, and preventive strategies. A systematic search was conducted in the Medline and PubMed databases using keywords such as “gestational diabetes mellitus,” “overt diabetes mellitus,” and “insulin resistance.” The flowchart in Figure 1 illustrates the search terms, strategy, and the process of study screening and selection. Initially, only full-text articles were considered for further analysis, followed by the exclusion of articles published prior to 1997 and any duplicates. Out of the 237 articles initially identified, 111 articles published between 1997 and 2023 met the inclusion criteria.
Following the article selection process, two experts in the field conducted independent reviews of the selected articles, evaluating various aspects such as demographics, research designs, and outcomes to identify relevant basic and clinical studies for inclusion. Articles with inadequate research designs, questionable sampling methods, or incongruent outcomes were excluded at this stage. Any discrepancies between the experts were resolved through mutual communication to establish a consensus. All eligible studies included in this review adhered to the predefined search terms and strategy, which encompassed database identification, study screening, article selection, and final inclusion. In the end, a total of 79 articles were included in the review out of the initial 237 identified articles. Among these selected articles (n = 79), approximately 60% (n = 47) were published between 2018 and 2023, ensuring the novelty of the review.
GDM is a worldwide health problem that threatens both maternal and fetal health, and its prevalence is increasing. Both global and regional prevalence of GDM are affected by different race/ethnicities, age, body composition, and geological regions[13,14]. Prevalence is also affected by variations between different screening strategies and diagnostic criteria[15]. Noted international organizations, including the ADA, Federation International of Gynecology and Obstetrics, and the World Health Organization, have all adopted the diagnostic criteria for GDM adopted by the IADPSG after the landmark HAPO study. According to the statistics and data collected by the International Diabetes Federation using the 2021 IADPSG diagnostic criteria, the global prevalence of GDM was 14%[15,16]. The regional prevalence of GDM was 20.7% in North America and Caribbean, 15.0% in Europe, 15.8% in South America and Central America, 13.0% in Africa, 14.0% in the Western Pacific, 25.9% in South-East Asia, and 14.1% in the Middle East and North Africa. A high prevalence of GDM was noted especially in Asia and North America[16].
The specific and detailed cause of GDM is not fully known, although theories have been proposed for its etiology and pathophysiology. During pregnancy, the placenta supplies not only nutrition to the fetus but also a variety of hormones to maintain the pregnancy, such as human placental lactogen and estrogen. These hormones can cause insulin resistance, starting usually from 20-24 wk of pregnancy and becoming more prominent in the later stages of pregnancy. Thus, the β cells of the pancreas need to produce more insulin to overcome insulin resistance. Once this additional production of insulin is not enough to overcome the resistance, GDM occurs[17].
Many well-established risk factors are associated with GDM. Each of these risk factors is either directly or indirectly associated with impaired β cell function and insulin resistance. Several risk factors increase concurrently with the rising prevalence of GDM, including pregestational overweight and obesity, excessive gestational weight gain, and advanced maternal age[18-21]. Other risk factors include a previous history of GDM, a family history of DM, and endocrine diseases such as hypothyroidism and polycystic ovary syndrome (PCOS)[18,19]. Most of the identified factors can be categorized into several groups.
Obesity is one of the key factors causing overt DM and GDM. BMI is commonly used to measure the severity of obesity. Therefore, pregestational BMI is one of the most important risk factors associated with GDM. According to a recent umbrella review and other studies, a convincing correlation was shown between BMI and GDM[18,19]. A meta-analysis in Asia has also shown that pregestational BMI ≥ 25 kg/m2 increased the risk of GDM by more than three-fold compared to the risk in those with normal BMI before pregnancy[19]. Moreover, pregestational BMI and gestational weight gain, both of which result in elevated gestational BMI, are major risk factors for GDM. High rates of bodyweight gain, particularly during early pregnancy, has been shown to increase the risk of GDM. This is also more prominent in those who are already overweight/obese before pregnancy[22,23].
Advanced maternal age is another important risk factor for GDM. The risk is more prominent for women with a maternal age of 35 years or older, by more than three-fold. According to a systematic review and meta-analysis of over 120 million participants, for each 1-year increase in maternal age from 18 years, the risk of GDM increases by 7.90% for the overall population[24]. According to previous reports, the odds ratios (ORs) and 95% confidence intervals (CIs) for the risk of GDM acquired from this large-scale population of women aged 30-34 years, 35-39 years, and ≥ 40 years were 2.73 (95%CI: 2.28-3.27), 3.54 (95%CI: 2.88-4.34) and 4.86 (95%CI: 3.78-6.24), respectively[24,25]. These age-related results were attributed to decreased insulin sensitivity and pancreatic β cell function, which eventually led to glucose and lipid metabolism abnormalities during pregnancy.
Experiencing a previous pregnancy complicated by GDM is a well-established risk factor for developing GDM in subsequent pregnancies, as highlighted in various studies, systematic reviews, and meta-analyses[19,26]. A meta-analysis focusing on Asian populations found that pregnant women with a history of GDM were at least eight times more likely to develop GDM in their subsequent pregnancy compared to those without such a history[19].
In addition to a personal history of GDM, having a family history of DM is also significant as a risk factor. Females with a family history of DM, particularly in first-degree relatives, have a genetic predisposition and increased susceptibility to GDM. Multiple studies, including a systematic review and meta-analysis, have reported that pregnant women with a family history of DM are at least twice as likely to develop GDM compared to those without a family history[18,26-28].
PCOS is a complex condition characterized by irregular menstruation/oligo-ovulation, or anovulation, and excess androgens and/or small cysts on one or both ovaries[29]. Due to the hormone imbalance of hyperandrogenism, the majority of women with PCOS also manifest insulin resistance. This gives females with PCOS a higher risk of developing GDM. A recent systematic review and meta-analysis displayed the positive relationship between PCOS and GDM with an OR of 2.33 (95%CI: 1.72-3.17), consistent with the underlying pathophysiology[19]. This finding was further supported by an umbrella review in 2019[18]. Our previous study of a large population (> 1000000 nationals) also reported that prepregnancy PCOS was significantly associated with subsequent GDM (adjusted OR: 2.15; 95%CI: 1.96-2.37)[30].
Hypothyroidism disorders and GDM are among the most common endocrinopathies during pregnancy. A meta-analysis of 5995 cases revealed a significant association between hypothyroidism and GDM[31]. A recent umbrella review also provided convincing evidence to support this point of view[18]. Based on these results, routine screening of thyroid function appears to be necessary in pregnant women.
Several risk factors that are less frequently documented yet identified by a number of studies and reviews include previous congenital anomalies of fetuses, previous macrosomia, a history of stillbirth, previous abortion, multiparity ≥ 2, and previous preterm delivery with ORs ranging from 1.37 to 4.25[19]. Other reported influence factors include ethnicity, lifestyles, and socioeconomic status[32,33].
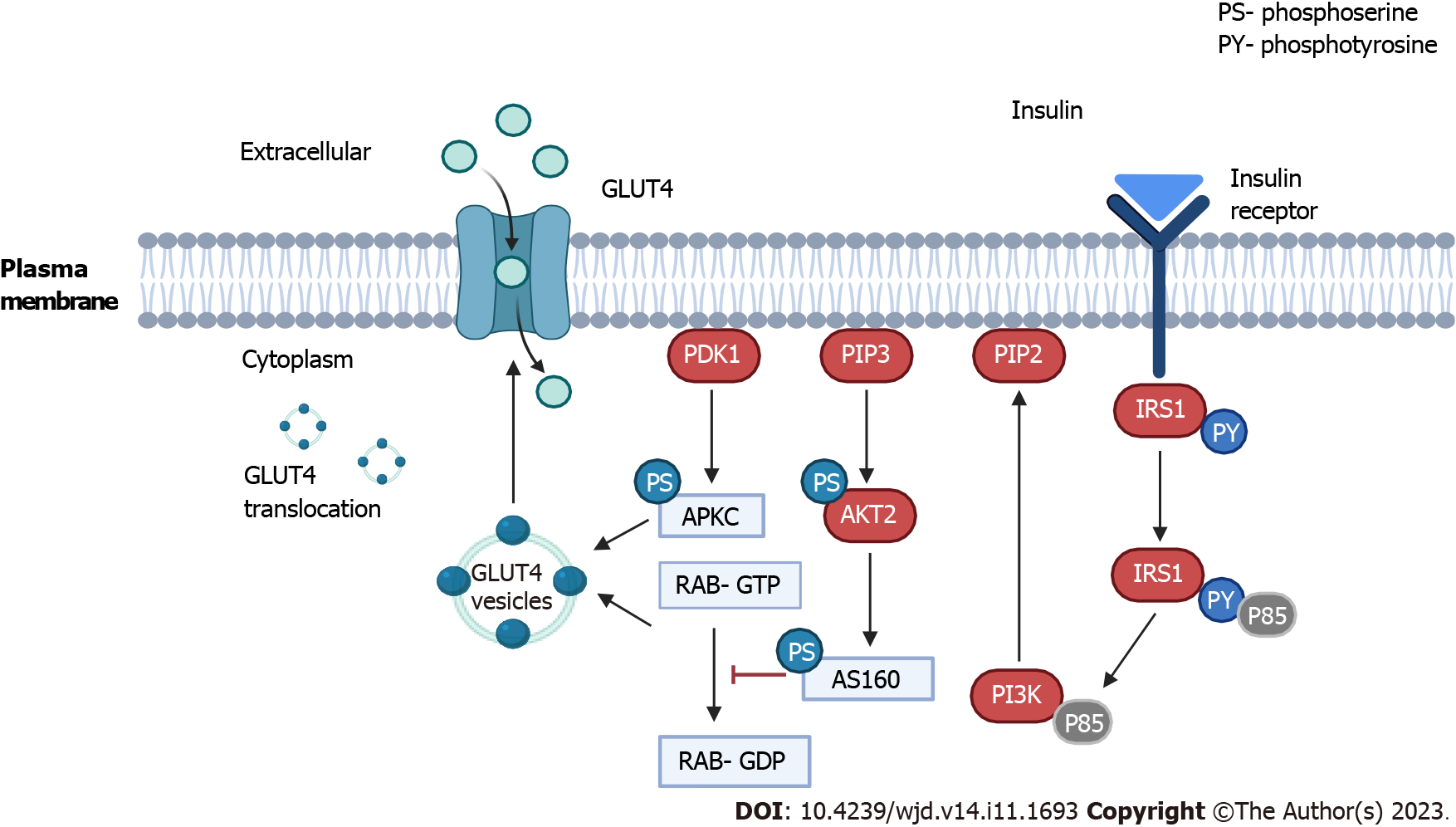
GDM is characterized by an imbalance in glucose control during pregnancy, involving complex interactions among insulin sensitivity, glucose production, and the body’s cells. Throughout pregnancy, insulin requirements naturally increase due to factors such as heightened maternal caloric intake, maternal weight gain, and the influence of placental hormones like placental growth hormone and human placental lactogen[2]. Additionally, there is an approximate 50% decrease in maternal insulin sensitivity, which is compensated by a 250% increase in maternal insulin production to maintain normal blood sugar levels during pregnancy[34]. Figure 2 provides a concise overview of insulin signaling and subsequent translocation of glucose transporter 4 (GLUT4), illustrating the cellular and molecular mechanisms implicated in GDM.
GDM is influenced by a combination of genetic variants, nutritional factors, and environmental influences. The pathophysiological changes observed in GDM are similar to those found in type 2 DM (T2DM), characterized by impaired insulin secretion and increased insulin resistance. This indicates a strong association between the two forms of diabetes. Consequently, research on GDM has focused on investigating genetic variants associated with T2DM susceptibility. Several case-control studies and meta-analyses using single nucleotide polymorphisms have revealed common pathogenic pathways shared by GDM and T2DM. A genome-wide association study conducted on a South Korean cohort showed a significant association between GDM and specific single nucleotide polymorphisms in genes already known to be associated with T2DM susceptibility, highlighting the genetic overlap between the two forms of diabetes. Many of the genetic loci associated with GDM primarily impact β cell functions. Various genome-wide association studies have identified variants in the HKDC1 and BACE2 genes, which influence glycemic traits during pregnancy and exhibit specific associations with GDM in different ethnic groups[34].
The mechanisms underlying β cell dysfunction and inadequate insulin production in GDM are intricate and diverse. Defects can occur at various stages of the process, including pro-insulin synthesis, post-translational modifications, granule storage, glucose sensing, and granule exocytosis. However, the majority of susceptibility genes associated with GDM primarily relate to β cell functions, such as potassium voltage-gated channel KQT-like 1 and glucokinase. Minor deficiencies in β cell machinery may only become evident during metabolic stress, such as pregnancy. Animal studies have demonstrated that the quantity of β cells plays a crucial role in glucose homeostasis. A reduction in β cell mass, associated with the epigenetic downregulation of pancreatic homeobox transcription factor, has been linked to GDM. Additionally, adequate β cell proliferation relies on prolactin, as evidenced in mouse models with prolactin receptor knockouts (Prlr-/-). Glucotoxicity is also believed to contribute to β cell apoptosis over time. Pancreatic samples from patients with T2DM showed a reduction in β cell mass ranging from 40% to 60%, while a loss of less than 24% has been reported after 5 years of the disease. Animal studies and limited post-mortem human studies suggest that reduced β cell hyperplasia may also play a role in the pathophysiology of GDM. Overall, a reduced β cell mass, decreased number of β cells, β cell dysfunction, or a combination of these factors contribute to GDM, depending on the individual[17].
In clinical practice, insulin resistance refers to a state in which a given concentration of insulin is associated with a subnormal glucose response. At the molecular level, GLUT4 serves as the primary transporter responsible for bringing glucose into the cell to use as an energy source. Insulin resistance is usually a failure of insulin signaling, which is associated with inadequate plasma membrane translocation of GLUT4 (Figure 2)[17]. GLUT4 has the unique characteristic of a mostly intracellular disposition in the unstimulated state in storage vesicles called GLUT4 storage vesicles that are acutely redistributed in the plasma membrane in response to insulin and other stimuli, like exercise. The major insulin signaling pathway involved in GLUT4 storage vesicles translocation is the phosphatidylinositol 3 kinase/phosphoinositide-dependent protein kinase 1/protein kinase B pathway through phosphorylation of the AS160 substrate. AS160 is a guanosine triphosphatase-activating protein that blocks the exchange of guanosine triphosphate for guanosine diphosphate when small G proteins called RAB that are involved in membrane trafficking are activated by phos-phorylation. Atypical protein kinases C isoforms also appear to be involved downstream of phosphoinositide-dependent protein kinase 1 but not through protein kinase B[35].
Another notable etiology that is responsible for GDM is dysfunction of the leptin signaling pathways (Figure 3). Leptin signaling is mediated by the JAK2/signal transducer and activator of transcription 3 (STAT3) pathway to exert its anorexigenic effect. Binding to the leptin receptor, leptin activates JAK2, STAT3, and MAPK via phosphorylation of different sites on the leptin receptor and subsequent binding to downstream molecules[36,37]. Thus, JAK2 and STAT3 are phosphorylated. The activated STAT3 translocates to the nucleus and activates the transcription of the target genes, which mediates the anorexigenic effect of leptin. The negative regulators of JAK2, including suppressor of cytokine signaling 3 and protein tyrosine phosphatase 1B, which act as feedback inhibitors of leptin signaling, have been reported to promote obesity and diabetes. On the other hand, leptin also regulates MAPK and phosphatidylinositol 3 kinase signaling through phosphorylation of insulin receptor substrate. In addition, leptin inhibits appetite-stimulators neuropeptide Y and agouti-related peptide, thus activating the anorexigenic polypeptide pro-opiomelanocortin[36,37].
During pregnancy, a regulated inflammatory profile is necessary for successful implantation and fetal development. However, conditions like GDM, obesity, and metabolic diseases are associated with chronic low-grade inflammation, leading to altered immune profiles and the promotion of a proinflammatory environment in various tissues, including adipose tissue, liver, kidney, heart, and pancreas. This chronic inflammation, characterized by increased levels of proinflammatory cytokines (IL-1β, IL-6, TNFα, and leptin) and decreased levels of anti-inflammatory molecules (IL-4, IL-10, and adiponectin), plays a significant role in the pathophysiology of GDM. Studies have shown that inflammation in pregnant women with obesity or GDM can affect fetal development. Experimental animal models and clinical studies provide evidence that maternal and placental inflammation associated with GDM and obesity can influence neurodevelopment and alter inflammatory responses in offspring[34].
The nuclear factor kappa B (NF-κB) signaling pathway is a central detailed mechanism in the inflammatory process (Figure 4). The proteins in the NF-κB family combine with each other to form homodimers or heterodimers to exert stimulative or repressive functions after transcription. As suppressors, the inhibitory regulators of NF-κB bind to the NF-κB dimers to form a complex that remains sequestered and inactive in the cytoplasm of non-stimulated cells[38]. Under the status of insulin resistance, proinflammatory cytokines, including IL-1β, IL-6, and TNFα, are increased to initiate the NF-κB signaling pathway. After receiving stimulation from the aforementioned cytokines and toll-like receptors, the inhibitory regulators of NF-κB s are rapidly phosphorylated, ubiquitinated, and then degraded, exposing a nuclear localization sequence on the NF-κB proteins. Thus, the NF-κB dimers translocate to the nucleus to regulate gene transcription and induce inflammatory cascades[38].
Recently, many antepartum measurements, including adipokines (leptin), body mass ratio [waist circumference (WC) and waist-to-hip ratio], and biomarkers (microRNA in extracellular vesicles) have been studied and confirmed to be useful as markers for predicting development of GDM during pregnancy. The HAPO study has provided compelling evidence of the associations between maternal BMI, hyperglycemia, and pregnancy complications. Both factors are correlated with higher rates of excessive fetal growth, primary cesarean birth, clinical neonatal hypoglycemia, fetal adiposity, neonatal hyperinsulinemia, and hypertensive disorders of pregnancy. The relationship between hyperglycemia and adverse outcomes generally follows a linear pattern, while BMI exhibits a quadratic pattern with diminishing increments in the highest BMI categories. Moreover, considering BMI and GDM together, the HAPO study has shown that they are associated with pregnancy complications.
GDM often serves as a precursor to the later development of T2DM and indicates a higher risk of (premature) cardiovascular disease in females. McIntyre et al[39] concluded that predictive models incorporating multiple clinical characteristics and early pregnancy glucose measurements exhibited the best performance. Research has also indicated that interventions initiated early in pregnancy can reduce the incidence of GDM in overweight and obese pregnant women[40].
Adipokines, such as leptin, play a crucial role in regulating various processes in the human body, including glucose and lipid metabolism, insulin sensitivity, appetite, immune response, and inflammation, and may serve as potential targets for predictive and therapeutic strategies in different medical conditions[17]. Leptin, primarily secreted by adipocytes in response to adequate fuel stores, acts on neurons within the hypothalamic arcuate nucleus to decrease appetite and increase energy expenditure. During normal pregnancy, a certain degree of leptin resistance develops to ensure sufficient fat stores. However, in GDM, leptin resistance is further exacerbated, leading to hyperleptinemia. The placenta becomes the primary source of leptin during pregnancy and increases its production, facilitating the passage of amino acids to the fetus. Elevated leptin levels have been associated with obesity, GDM, and the risk of fetal macrosomia. Therefore, hyperleptinemia can serve as a marker for predicting the occurrence of GDM. Additionally, leptin has been implicated in placental neoformation, functioning as a growth factor, angiogenic factor, and immunomodulator[40].
GDM represents a stage of subclinical inflammation and serves as a risk factor for the subsequent development of T2DM and cardiovascular diseases. Leptin has been associated with vascular and metabolic changes in GDM, although findings regarding its involvement in maternal, perinatal, and future complications have been inconsistent and varied. Several studies have demonstrated significantly higher leptin levels in participants with GDM compared to controls. Moreover, leptin has been incorporated into first-trimester risk prediction models for GDM, and elevated levels have been observed in females who later develop GDM, confirming its predictive value. Leptin has also been identified as a promising diagnostic biomarker for GDM, showing high sensitivity and specificity in adequate sample sizes. During pregnancy, circulating leptin concentrations increase, reaching a peak around 28 wk of gestation and returning to prepregnancy levels postpartum. Pregnant women with GDM exhibit even higher leptin levels, further highlighting its potential as a predictive marker for GDM[40].
Maternal obesity contributes to increased risks during pregnancy, including the development of GDM. Women with a BMI of 30 kg/m2 or higher are considered at risk and should receive additional care. Heslehurst et al[41], in a systematic review, presented comprehensive evidence on the relationship between adiposity measures in early pregnancy and maternal health outcomes. WC was the most commonly studied adiposity measure during early pregnancy. Both meta-analyses and narrative syntheses consistently demonstrated that WC is a robust predictor of adverse maternal health outcomes. It consistently exhibits a significant association with GDM, hypertensive disorders, delivery-related outcomes, metabolic syndrome, and composite adverse pregnancy outcomes. Waist-to-hip ratios also show potential associations with these conditions, as they are significantly linked to GDM, hypertensive disorders, and delivery-related outcomes. Furthermore, several other measures such as fat mass, neck circumference, skinfold thickness, visceral fat measures, arm circumference, and waist-to-height ratio exhibit significant associations with various adverse outcomes[41].
Meta-analyses have indicated significantly increased odds of GDM in higher WC categories (OR: 1.40, 95%CI: 1.04-1.88) and per unit increase in WC (OR: 1.31, 95%CI: 1.03-1.67), suggesting that women with GDM have larger WC measurements compared to controls (mean difference: 6.18 cm, 95%CI: 3.92-8.44 cm). Although data on other adiposity measures are limited, fat mass, neck circumference, skinfold thickness, and visceral fat exhibit significant associations with adverse outcomes. This study emphasized the importance of exploring the utility of adiposity measures in predicting the risk of GDM during pregnancy beyond BMI alone, highlighting the need for personalized care based on specific requirements of pregnant women[41].
GDM is linked to abnormal placentation and early pregnancy markers commonly used for predicting aneuploidy, such as pregnancy-associated plasma protein A and free β human chorionic gonadotropin. These markers have been integrated into predictive models. Proteomic screening during early pregnancy has identified potential protein markers, including a cluster related to insulin secretion, binding, resistance, and signaling, which may have implications for the development of GDM later on.
Additionally, extracellular vesicles (ECVs) have been investigated as potential markers for GDM. ECVs primarily contain microRNAs and are associated with glucose metabolism. These circulating particles originate mainly from the placenta and adipose tissue during pregnancy and carry various protein and RNA molecules that can be transported to specific sites. James-Allan et al[42] demonstrated that specific small ECVs are associated with GDM and that infusing ECVs from females with GDM induced insulin resistance and reduced insulin secretion in rodents, aligning with the pathophysiology of GDM. Yoffe et al[43] conducted a case-control study and suggested that microRNA-223 and microRNA-23a in first-trimester blood samples strongly predict the development of GDM (area under the receiver operating characteristic curve: 0.91)[39]. Similarly, another recent cohort study confirmed similar findings for microRNA-233[36,39]. These cohort studies have examined various clinical, demographic, and molecular biomarkers, including early pregnancy glycemic measurements, to uncover complex networks of predictive factors. For molecular biomarkers to be clinically useful, they must outperform clinical risk factors and simple glucose measurements in predicting GDM and pregnancy outcomes[39].
Cohort studies have examined single or multiple clinical and demographic measures, including early pregnancy glycemic measurements, and have expanded their focus to include complex networks of molecular biomarkers. To be applicable in routine clinical practice, molecular biomarkers need to demonstrate superior performance compared to clinical risk factors and simple glucose measurements in predicting GDM and pregnancy outcomes[39].
Numerous reviews and studies have revealed that early intervention and prevention of GDM reduces the risk of perinatal and long-term complications. Therefore, it is important to provide early intervention to achieve optimal outcomes. Several meta-analyses have emphasized the effectiveness of lifestyle interventions, including both dietary interventions and physical exercise for prevention of GDM.
One meta-analysis involving 29 RCTs with around 11500 participants demonstrated that either diet or physical activity may result in an overall 18% reduction in the risk of GDM, especially in early pregnancy before the 15th gestational week [relative risk (RR): 0.80, 95%CI: 0.66-0.97][44]. A meta-analysis involving 47 RCTs with more than 15000 individuals showed that diet and exercise during pregnancy were preventive factors for GDM (RR: 0.77, 95%CI: 0.69-0.87)[45]. A review indicated that moderate-intensity exercise for 50-60 min twice a week reduced the incidence of GDM by about 24%[46]. Another systematic review and meta-analysis soliciting 45 studies also reported that dietary management and physical activity during pregnancy reduced the risk of GDM by 44% (RR: 0.56, 95%CI: 0.36-0.87) and 38% (RR: 0.62, 95%CI: 0.50-0.78), respectively[46].
In contrast, another systematic review and network meta-analysis pointed out that neither diet, physical activity, nor a combination of both offered significant benefit in preventing GDM in overweight/obese women, despite that these measures were all remarkably beneficial for gestational weight gain restriction. Although most studies concluded that combining diet with physical activity may still tend to prevent GDM development, more studies are required to compensate for the inconsistency between studies. Table 1 summarizes the preventive strategies for GDM.
| Ref. | Study design | Patients | Intervention | Result |
| Song et al[44], 2016 | Meta-analysis of 29 RCTs | 11487 pregnant women | Diet or physical activity during pregnancy | (1) Intervention groups resulted in 18% (95%CI: 5%-30%) reduction in the risk of GDM (P = 0.0091); and (2) Intervention was effective especially before the 15th gestational week (RR: 0.80, 95%CI: 0.66-0.97) |
| Guo et al[45], 2019 | Meta-analysis of 47 RCTs | 15745 participants | Diet and exercise during pregnancy | (1) Combination intervention were preventive of GDM (RR 0.77, 95%CI: 0.69-0.87); and (2) Exercise of moderate intensity for 50-60 min twice a week could lead to an approximately 24% reduction in GDM |
| Bennett et al[46], 2018 | Systematic review and meta-analysis of 45 studies | 15293 participants | Diet and exercise during pregnancy | (1) Diet intervention reduced GDM risk by 44% (RR: 0.56, 95%CI: 0.36-0.87); (2) PA intervention reduced GDM risk by 38% (RR: 0.62, 95%CI: 0.50-0.78); (3) PA interventions in southern European group reduced GDM risk by 37% (RR: 0.63, 95%CI: 0.50-0.80); and (4) Diet and PA interventions in Asian group reduced GDM risk by 62% (RR: 0.38, 95%CI: 0.24-0.59) and 32% (RR: 0.68, 95%CI: 0.54-0.86), respectively |
| Sanabria-Martínez et al[48], 2015 | Meta-analysis of 13 RCTs | 2873 pregnant women | Physical exercise during pregnancy | (1) Physical exercise decreased the risk of GDM by 31% (RR = 0.69; P = 0.009); and (2) Decreases were also observed in maternal weight (WMD = -1.14 kg; 95%CI: -1.50 to -0.78; P < 0.001) |
| Davenport et al[49], 2018 | A systematic review and meta-analysis including 106 studies | 273182 participants | Exercise, alone or in combination (dietary + exercise) | (1) Exercise-only interventions, but not exercise + cointerventions, reduced odds of GDM (n = 6934; OR: 0.62, 95%CI: 0.52 to 0.75); and (2) Also reduced odds of gestational hypertension (n = 5316; OR: 0.61, 95%CI: 0.43 to 0.85) and pre-eclampsia (n = 3322; OR: 0.59, 95%CI: 0.37 to 0.9) compared with no exercise |
| Martínez-Vizcaíno et al[50], 2022 | An umbrella review: 23 systematic reviews and meta-analyses and 63 RCTs were included | Single exercise interventions | (1) Single exercise interventions reduced GDM incidence in most systematic reviews and meta-analyses around 39%; (2) Single exercise interventions also reduced gestational hypertension incidence in most systematic reviews and meta-analyses around 47%; and (3) Particularly when supervised, with low-to-moderate intensity level, and initiated early during the first trimester of pregnancy | |
| Griffith et al[55], 2020 | An overview of Cochrane Reviews (11 Cochrane Reviews with 71 trials) | 23154 women | Diet, exercise, and combination; dietary supplements such as vitamin D and probiotics, pharmaceuticals such as metformin | (1) A combination of exercise and diet of possible benefit in reducing the risk of GDM; (2) Unknown benefit or harm of a low glycemic index diet vs a moderate-high glycemic index diet on the risk of GDM; (3) Supplementation with vitamin D and metformin were of possible benefit in reducing the risk of GDM; and (4) There was insufficient high-quality evidence to establish the effect on the risk of GDM of diet or exercise alone, probiotics, vitamin D with calcium or other vitamins and minerals |
| Melero et al[61], 2020 | RCT | 284 participants | MedDiet vs control | MedDiet intervention reduced GDM rate with adjusted RR 0.77 (95%CI: 0.61-0.97, P = 0.008) |
| Assaf-Balut et al[62], 2017 | RCT | 874 participants | MedDiet vs control | MedDiet intervention reduced GDM rate with adjusted RR 0.75 (95%CI: 0.57-0.98; P = 0.039) |
| Al Wattar et al[63], 2019 | Multicenter RCT | 1252 participants | MedDiet vs control | MedDiet intervention reduced GDM rate with adjusted RR 0.65 (95%CI: 0.47-0.91, P = 0.01) |
| Tobias et al[64], 2012 | Retrospective cohort | 15254 participants | MedDiet, DASH | (1) MedDiet was associated with a 24% lower risk (RR: 0.76; 95%CI: 0.60-0.95; P = 0.004); and (2) DASH with a 34% lower risk (RR: 0.66; 95%CI: 0.53-0.82; P = 0.0005) |
| Tobias et al[64], 2012 | Retrospective cohort | 4413 participants | MedDiet, DASH | (1) MedDiet was associated with a 40% lower risk (RR: 0.60; 95%CI: 0.44-0.82; P = 0.002); and (2) DASH with a 46% lower risk (RR: 0.54; 95%CI: 0.39-0.73; P < 0.001) |
| Luoto et al[67], 2016 | RCT | 256 participants | Probiotic with/without dietary counseling vs control | One probiotic intervention reduced the frequency of GDM; 13% (diet/probiotics), 36% (diet/placebo), and 34% (control); P = 0.003 |
| Callaway et al[68], 2019 | RCT | 411 overweight and obese pregnant women | Probiotic vs placebo | (1) GDM occurred in 12.3% in the placebo arm and 18.4% in the probiotics arm (P = 0.10); and (2) Probiotics did not prevent GDM in overweight and obese pregnant women |
| Davidson et al[69], 2021 | 7 RCTs | 1647 participants | Probiotic with either placebo or diet | (1) It was uncertain if probiotics had any effect on the risk of GDM compared to placebo (RR: 0.80, 95%CI: 0.54-1.20) due to low-certainty evidence; and (2) High-certainty evidence suggested an increased risk of pre-eclampsia with probiotic administration (RR: 1.85, 95%CI: 1.04- 3.29) and increased the risk of hypertensive disorders of pregnancy (RR: 1.39, 95%CI: 0.96-2.01) |
| Mijatovic-Vukas et al[70], 2018 | A systematic review and meta-analysis of 40 trials | 30871 pregnant women | A variety of dietary regimens, prepregnancy and early pregnancy PA | (1) MedDiet, DASH were associated with 15%-38% reduced RR of GDM; and (2) Compared to no PA, any prepregnancy and early pregnancy PA was associated with 30% and 21%, respectively, reduced odds of GDM |
| De-Regil et al[71], 2016 | Cochrane database systematic. review with 15 RCTs | 2833 females | Vitamin D alone or in combination with other micronutrients during pregnancy | (1) Similar risk of GDM among those taking vitamin D supplements or no intervention/placebo (RR: 0.43, 95%CI: 0.05-3.45) very low quality evidence; and (2) Vitamin D supplements may lower risk of pre-eclampsia risk (RR: 0.52, 95%CI: 0.25-1.05) low quality evidence |
| Pérez-López et al[72], 2015 | A systematic review and meta-analysis of 13 RCTs | 2299 females | Vitamin D supplementation during pregnancy | Incidence of pre-eclampsia, GDM, weight, preterm birth, and cesarean section were not influenced by vitamin D supplementation |
Physical activity is an effective intervention for controlling body weight, improving glycemic control, and reducing insulin resistance. At one time, physical activity alone was considered not effective enough in reducing the risk of developing GDM. However, the American Dietetic Association and the American Nutrition Society published a study in 2009 that overweight and obese females who practiced moderate exercise during pregnancy not only gained significantly less fat but also reduced the risk of GDM[47]. Two meta-analyses in 2015 and 2016, which analyzed 2873 pregnant women from 13 RCTs and 11487 pregnant women from 29 RCTs, respectively, revealed that physical exercise during pregnancy decreased the risk of GDM by 31% (RR: 0.69; P = 0.009)[48] and 20% (RR: 0.80, 95%CI: 0.66-0.97)[42], respectively, especially during the early stage of pregnancy (before the 15th week)[42].
Moreover, a 2018 systematic review and meta-analysis that examined a total of 106 RCTs including 273182 participants with moderate-to-high-quality evidence revealed that exercise-only interventions were effective in reducing the risk of GDM by a remarkable 38% (OR: 0.62, 95%CI: 0.52-0.75)[49]. A recent umbrella review in 2022 focusing on the relationship between exercise during pregnancy and GDM prevention further confirmed the effectiveness of early initiated (first trimester), low-to-moderate intensity exercise in reducing the incidence of GDM[50].
In addition to the advantage of preventing GDM, physiological studies have reported that exercise in pregnant women improved cardiovascular functions, resulting in improved fitness, blood pressure, and peripheral edema. An ACOG report also showed that exercise improved the symptoms of constipation, bloating, fatigue, and insomnia in pregnant women. For the fetus, the benefits of moderate maternal exercise included an increase in amniotic fluid, increases in placenta viability and volume, improvement in neurological system development, and reduction in body fat percentage. However, excessive exercise may elevate the incidence of antepartum hemorrhage and the risk of preterm birth. Considering these disadvantages of excess exercise, physical exercise should be avoided in pregnant women with restrictive lung disease, pre-eclampsia, persistent bleeding in the second or third trimester, incompetent cervix, placenta previa, hemodynamically significant heart disease, and higher-order multiple gestations (≥ triplets)[51].
Several types of diets have been devised and studied to reduce the risk of GDM. These diets included a low glycemic index (GI) diet, energy restriction diet, low carbohydrate content with high fat substitute, the Mediterranean diet (MedDiet), and Dietary Approaches to Stop Hypertension (DASH)[52-54]. The low GI diet ranks foods on a scale from 0 to 100 based on their effects on blood sugar. A food with a low GI was less likely to impact the blood sugar levels significantly. Although the diet was introduced to reduce insulin resistance and to decrease the risk of GDM, a meta-analysis from 2016 and an overview of Cochrane Reviews from 2020 displayed conflicting results of benefit from the low GI diet on reducing the risk of GDM[55,56]. Therefore, the effect of a low GI diet on reducing the incidence of GDM still remains inconclusive.
The restricted-energy diet and low carbohydrate diet have been suggested to minimize weight gain during pregnancy, while some approaches tried to replace dietary carbohydrates with fat. Excessive fat or a ketogenic diet may have an impact on maternal insulin resistance and will result in excessive fat accumulation in the fetus[57]. To date, no sufficient data are available to support the safety of the ketogenic diet in the GDM population[58]. In addition, these diets are not practical as there is still a minimum requirement of daily carbohydrates of at least 175 g to ensure appropriate fetal growth and development. The Institute of Medicine has recommended that 46%-65% of calories come from carbohydrates[59,60].
The MedDiet is considered one of the healthiest forms of nutrition and is confirmed safe for GDM[58]. It is composed of a high vegetable and fruit content along with legumes, olive oil, cereals, fish, and limited animal products. Olive oil is the main source of additional fat, and the consumption of processed meat is low. Several RCTs that studied 284, 874, and 1252 participants, respectively, have reported the positive impact of the MedDiet on lowering the incidence of GDM to around 35%[61-63].
DASH was originally developed for reducing hypertension. It consists of a high intake of vegetables, fruits, legumes, and nuts, with low consumption of sodium and sweets, moderate low-fat dairy products, and limited processed meat and animal protein. It was also shown to be effective in reducing the risk of GDM because the majority of its components overlapped with the MedDiet. In some studies, it was even considered superior to the MedDiet[64-66].
Two double-blind controlled studies have shown that probiotics may reduce the risk of GDM[67,68]. That conclusion was based on the hypothesis that probiotics affect intestinal microflora, thus they may influence host inflammatory pathways and result in better control of glucose and lipid metabolism. However, a recent Cochrane Review concluded that due to low-certainty evidence and limited trials no definitive benefit of probiotics was identified for GDM control[69,70].
Previous evidence indicated that insufficient supplementation of vitamin D in early pregnancy may correlate with an increased risk of GDM. However, the mechanism of reducing GDM risk by supplementing vitamin D was not fully understood. Similar to probiotics, it was suggested that vitamin D supplements in early pregnancy may also reduce the risk of GDM. Based on the available studies discussed in several meta-analyses, no compelling evidence was found to support this practice[70-72].
The functional advantages of low GI foods include lowering postprandial glucose, preventing excessive increases in postprandial insulin, and inducing satiety, all of which may contribute to weight loss. To date, however, low GI foods have shown no marked influence on obstetric, maternal, or fetal outcomes, including maternal weight gain, neonatal birth weight or the proportion of large-for-gestational-age, and macrosomia[73]. Current evidence has suggested that the low GI nutritional approach is reasonably safe in GDM. However, further research is needed to develop tools to facilitate patient adherence to treatment goals, individualize interventions and improve outcomes[74].
The low-carbohydrate diet results in a lower postprandial glucose, lower daytime mean glucose concentrations, lower area under the curve for 2-h postprandial glucose, and lower 24-h total glucose area under the curve when compared with the 60% carbohydrate diet. However, study results have revealed that lower carbohydrate intake will often lead to an increased intake of fat, which outside of pregnancy has been associated with an increase in serum fatty acids, insulin resistance, increased fetal fat accretion, and infant adiposity[59]. These disadvantages may further limit the use of the low-carbohydrate diet as a means of dietary intervention.
The MedDiet may reduce the risk of GDM by alleviating systemic oxidative stress[75]. Further, the MedDiet may downregulate circulating inflammatory biomarkers and favor glucose homeostasis, improving insulin sensitivity and glycemic postprandial response. Moreover, the MedDiet has a high fiber content, which increases satiety and controls weight gain[76]. Similar to the low GI MedDiet, the DASH diet in pregnant women with GDM is associated with a decreased number of macrosomic infants. The DASH diet also led to lower mean weight and head circumference and ponderal index newborns but did not affect the infants’ body lengths and Apgar scores[77]. However, the disadvantages of dietary control, as mentioned above, lie in the degree of adherence to dietary interventions by pregnant women.
GDM is a complex condition of pregnancy with significant implications for both the mother and the fetus. Currently, no scientific consensus has been reached on how best to diagnose GDM. Expert professional organizations acknowledge two acceptable options: the IADPSG one-step screening approach (currently preferred by the ADA); and the two-step Carpenter-Coustan screening approach (recommended by the ACOG). Both organizations have noted the need for additional evidence related to outcomes. Each approach has advantages and disadvantages. The 1-step approach involves a 2-h OGTT for all participants; while screening and diagnosis can be completed in a single visit, all women must fast before screening and allow time for a 2-h visit. The 2-step approach includes an initial non-fasting 1-h glucose challenge test, which is logistically simpler for gravidas, and can easily be done as part of a scheduled prenatal visit; most women do not require further screening. However, approximately 20% of pregnant women fail this initial screening and must return for a 3-h fasting diagnostic OGTT. In addition, these two methods have different diagnostic cutoffs. In comparison to the 2-step approach, the 1-step approach identifies women with milder hyperglycemia as having GDM and expands the target population that will be further treated under the diagnosis of GDM. Although a clear linear relationship is shown between maternal hyperglycemia and maternal and perinatal outcomes, the specific effects on these outcomes of identifying and treating milder cases of GDM are not known[10].
The pathophysiology of GDM is complex and probably involves genetic variants that are responsible for insulin secretion and glycemic control, pancreatic β cell depletion or dysfunction, aggravated insulin resistance due to failure in the plasma membrane translocation of GLUT4, and the actions of chronic, low-grade inflammation. In practice, each factor or a combination of the aforementioned factors may contribute to the development of GDM.
Currently, many antepartum measurements, including adipokines (leptin), body mass ratio (WC and waist-to-hip ratio), and biomarkers (microRNA in ECVs) have been studied and confirmed as appropriate markers for predicting the occurrence of GDM during pregnancy. Leptin resistance is further augmented in GDM. Thus, the resultant hyperleptinemia can be used as a marker in predicting the occurrence of GDM.
For preventing GDM, physical activity is an effective intervention to control body weight, improve glycemic control, and reduce insulin resistance. On the other hand, several RCTs have reported the positive impact of dietary control (such as the MedDiet) on lowering the incidence of GDM to around 35%[61-63].
To update this overview of GDM, the current treatment for GDM is very frequently suboptimal. The most common oral pharmacological interventions that have been assessed include metformin, probiotics, and vitamin D administration. However, no single intervention appears to be universally superior to placebo/no intervention for preventing GDM[78]. Currently, insulin injection is the preferred medication for treating hyperglycemia in GDM. Metformin and glyburide are not regarded as first-line agents since both cross the placenta to the fetus. Even though sufficient data are available confirming the safety and effectiveness of metformin in pregnant women with GDM, data are very limited concerning the long-term effects of metformin on the offspring. Furthermore, glyburide should be used with caution, as it increases the risk of neonatal hypoglycemia. Some studies have also shown that glyburide increases the risk of macrosomia. Overall, oral agents may be a therapeutic option in women with GDM after the known risks have been reviewed along with the need for more long-term safety data in the offspring[78].
The INTERCOVID study investigated the role of overt or GDM and high BMI as risk factors for coronavirus disease 2019 (COVID-19) infection during pregnancy[79]. The study, conducted between March 2020 and February 2021 across 43 institutions in 18 countries, included 2184 pregnant women aged ≥ 18 years. Each pregnant woman diagnosed with COVID-19 was matched with two females without COVID-19 who received antenatal care or delivered at the same institution. The findings of the study indicated that COVID-19 was associated with pre-existing DM (RR: 1.94, 95%CI: 1.55-2.42), overweight or obesity (RR: 1.20, 95%CI: 1.06-1.37), and GDM (RR: 1.21, 95%CI: 0.99-1.46). Specifically, the association between COVID-19 and GDM was observed among women requiring insulin, regardless of their weight (RR: 1.79, 95%CI: 1.06-3.01) or being overweight or obese (RR: 1.77, 95%CI: 1.28-2.45). A somewhat stronger association with COVID-19 diagnosis was observed among women with pre-existing DM, regardless of their weight (RR: 1.93, 95%CI: 1.18-3.17) or being overweight or obese (RR: 2.32, 95%CI: 1.82-2.97). The study concluded that DM and overweight or obesity were risk factors for COVID-19 diagnosis during pregnancy, with insulin-dependent GDM being particularly associated with the disease. Therefore, it is crucial to prioritize the vaccination of women with these comorbidities[79].
Subsequently, a large prospective, observational study (INTERCOVID-2022) involving 41 hospitals across 18 countries, enrolled 4618 pregnant women from November 27, 2021 to June 30, 2022[80]. During pregnancy, each woman with a COVID-19 diagnosis was matched with two females without COVID-19. Overall, females with a COVID-19 diagnosis had an increased risk for morbidity and mortality index (RR: 1.16, 95%CI: 1.03-1.31), severe perinatal morbidity and mortality index (RR: 1.21, 95%CI: 1.00-1.46), and severe neonatal morbidity index (RR: 1.23, 95%CI: 0.88-1.71) compared to those without a COVID-19 diagnosis[80]. Moreover, severe COVID-19 symptoms in the total sample increased the risk of severe maternal complications (RR: 2.51, 95%CI: 1.84-3.43), perinatal complications (RR: 1.84, 95%CI: 1.02-3.34), and referral, intensive care unit admission, or death (RR: 11.83, 95%CI: 6.67-20.97). Notably, vaccine effectiveness (all vaccines combined) for severe complications of COVID-19 in all females who completed the regimen was 48% (95%CI: 22-65) and 76% (47-89) after a booster dose[80]. The study conclusion stated that COVID-19 in pregnancy was associated with increased risk of severe maternal morbidity and mortality, especially among symptomatic and unvaccinated women. Females with complete or boosted vaccine doses had a reduced risk for severe symptoms, complications, and death. Accordingly, COVID-19 vaccination coverage among pregnant women remains a priority[80].
The present review of the literature has identified several cellular and molecular mechanisms associated with GDM as well as influence factors and risk factors that can affect its development. Additionally, we have identified a number of potential predictive and preventive measures that can be employed to help reduce the incidence of GDM. These measures include lifestyle modifications, such as dietary changes and increased physical activity, and screening for risk factors and early diagnosis. Advances in our understanding of the pathophysiology of GDM have enabled the development of more effective preventive strategies, which can have a significant impact on the health of both mother and child. It is our hope that the findings of this review will contribute to the ongoing effort to improve the management of GDM and ultimately lead to better health outcomes for women and their offspring.
GDM is a complex condition of pregnancy with significant implications for both the mother and the fetus. Currently, no scientific consensus has been reached on how best to diagnose GDM, either by one-step or two-step OGTT. The pathophysiology of GDM undoubtedly involves genetic variants, pancreatic β cell depletion or dysfunction, and aggravated insulin resistance due to failure in the plasma membrane translocation of GLUT4. GDM pathophysiology is also associated with the negative regulation of leptin signaling pathways and the actions of chronic low-grade inflammation, which involve the NF-κB signaling pathway. Currently, leptin and body mass ratio are used as markers for predicting the occurrence of GDM during pregnancy. For preventing GDM, physical activity and dietary control are substantial interventions. Nevertheless, many detailed cellular and molecular mechanisms underlying GDM, as well as prediction and prevention, remain unexplored and warrant further investigation.
Defined as impaired glucose tolerance induced by pregnancy, gestational diabetes mellitus (GDM) may arise from exaggerated physiologic changes in glucose metabolism. Maternal and fetal effects of uncontrolled GDM include stillbirth, macrosomia, neonatal diabetes, birth trauma, and subsequent postpartum hemorrhage.
It is essential to find the potential target population and associated predictive and preventive measures for future intensive peripartum care.
To review studies that explored the cellular and molecular mechanisms of GDM as well as predictive measures and prevention strategies.
The search was performed in the Medline and PubMed databases using the terms “gestational diabetes mellitus,” “overt diabetes mellitus,” and “insulin resistance.” In the literature, only full-text articles were considered for inclusion (237 articles). Furthermore, articles published before 1997 and duplicate articles were excluded. After a final review by two experts, all studies (1997-2023) included in the review met the search terms and search strategy (identification from the database, screening of the studies, selection of potential articles, and final inclusion).
A total of 79 articles were collected for review. Reported risk factors for GDM included maternal obesity or overweight, pre-existing DM, and polycystic ovary syndrome. The pathophysiology of GDM involves genetic variants responsible for insulin secretion and glycemic control, pancreatic β cell depletion or dysfunction, aggravated insulin resistance due to failure in the plasma membrane translocation of glucose transporter 4, and the effects of chronic, low-grade inflammation. Currently, many antepartum measurements including adipokines (leptin), body mass ratio (waist circumference and waist-to-hip ratio], and biomarkers (microRNA in extracellular vesicles) have been studied and confirmed to be useful markers for predicting GDM. For preventing GDM, physical activity and dietary approaches are effective interventions to control body weight, improve glycemic control, and reduce insulin resistance.
This review explored the possible factors that influence GDM and the underlying molecular and cellular mechanisms of GDM and provided predictive measures and prevention strategies based on results of clinical studies.
Many detailed cellular and molecular mechanisms underlying GDM, as well as prediction and prevention, remain unexplored and warrant further investigation.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Cavoretto PI, Italy; Geng TY, China; Zhang Y, China S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Yuan YY
| 1. | International Diabetes Federation. IDF Diabetes Atlas, 9th edit. Brussels, Belgium: International Diabetes Federation, 2019. [Cited in This Article: ] |
| 2. | Lende M, Rijhsinghani A. Gestational Diabetes: Overview with Emphasis on Medical Management. Int J Environ Res Public Health. 2020;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 3. | ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol. 2018;131:e49-e64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 641] [Cited by in F6Publishing: 905] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 4. | Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, Stampfer MJ, Speizer FE, Spiegelman D, Manson JE. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278:1078-1083. [PubMed] [Cited in This Article: ] |
| 5. | Kiani F, Ghare Naz S, Sayehmiri M, Sayehmiri F, Zali K. The Risk Factors of Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis Study. Int J Women's Health Reprod Sci. 2017;5:253-263. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Kim C, Liu T, Valdez R, Beckles GL. Does frank diabetes in first-degree relatives of a pregnant woman affect the likelihood of her developing gestational diabetes mellitus or nongestational diabetes? Am J Obstet Gynecol. 2009;201:576.e1-576.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Hedderson MM, Williams MA, Holt VL, Weiss NS, Ferrara A. Body mass index and weight gain prior to pregnancy and risk of gestational diabetes mellitus. Am J Obstet Gynecol. 2008;198:409.e1-409.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol. 2010;115:597-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 378] [Cited by in F6Publishing: 377] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 9. | Gibson KS, Waters TP, Catalano PM. Maternal weight gain in women who develop gestational diabetes mellitus. Obstet Gynecol. 2012;119:560-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 10. | Hillier TA, Pedula KL, Ogasawara KK, Vesco KK, Oshiro CES, Lubarsky SL, Van Marter J. A Pragmatic, Randomized Clinical Trial of Gestational Diabetes Screening. N Engl J Med. 2021;384:895-904. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 130] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 11. | Carreno CA, Clifton RG, Hauth JC, Myatt L, Roberts JM, Spong CY, Varner MW, Thorp JM Jr, Mercer BM, Peaceman AM, Ramin SM, Carpenter MW, Sciscione A, Tolosa JE, Saade GR, Sorokin Y; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal-Fetal Medicine Units (MFMU) Network. Excessive early gestational weight gain and risk of gestational diabetes mellitus in nulliparous women. Obstet Gynecol. 2012;119:1227-1233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 110] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 12. | Fu J, Retnakaran R. The life course perspective of gestational diabetes: An opportunity for the prevention of diabetes and heart disease in women. EClinicalMedicine. 2022;45:101294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 13. | Choudhury AA, Devi Rajeswari V. Gestational diabetes mellitus - A metabolic and reproductive disorder. Biomed Pharmacother. 2021;143:112183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 71] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 14. | Kim HY, Kim J, Noh E, Ahn KH, Cho GJ, Hong SC, Oh MJ, Kim HJ. Prepregnancy hemoglobin levels and gestational diabetes mellitus in pregnancy. Diabetes Res Clin Pract. 2021;171:108608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, Hoegfeldt CA, Elise Powe C, Immanuel J, Karuranga S, Divakar H, Levitt N, Li C, Simmons D, Yang X; IDF Diabetes Atlas Committee Hyperglycaemia in Pregnancy Special Interest Group. IDF Diabetes Atlas: Estimation of Global and Regional Gestational Diabetes Mellitus Prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group's Criteria. Diabetes Res Clin Pract. 2022;183:109050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 224] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 16. | International Diabetes Federation. IDF Diabetes Atlas. 10th edition. 2021. Available from: https://diabetesatlas.org/en/. [Cited in This Article: ] |
| 17. | Plows JF, Stanley JL, Baker PN, Reynolds CM, Vickers MH. The Pathophysiology of Gestational Diabetes Mellitus. Int J Mol Sci. 2018;19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 817] [Cited by in F6Publishing: 480] [Article Influence: 80.0] [Reference Citation Analysis (0)] |
| 18. | Giannakou K, Evangelou E, Yiallouros P, Christophi CA, Middleton N, Papatheodorou E, Papatheodorou SI. Risk factors for gestational diabetes: An umbrella review of meta-analyses of observational studies. PLoS One. 2019;14:e0215372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 19. | Lee KW, Ching SM, Ramachandran V, Yee A, Hoo FK, Chia YC, Wan Sulaiman WA, Suppiah S, Mohamed MH, Veettil SK. Prevalence and risk factors of gestational diabetes mellitus in Asia: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2018;18:494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 20. | Mnatzaganian G, Woodward M, McIntyre HD, Ma L, Yuen N, He F, Nightingale H, Xu T, Huxley RR. Trends in percentages of gestational diabetes mellitus attributable to overweight, obesity, and morbid obesity in regional Victoria: an eight-year population-based panel study. BMC Pregnancy Childbirth. 2022;22:95. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Yen IW, Lee CN, Lin MW, Fan KC, Wei JN, Chen KY, Chen SC, Tai YY, Kuo CH, Lin CH, Hsu CY, Chuang LM, Lin SY, Li HY. Overweight and obesity are associated with clustering of metabolic risk factors in early pregnancy and the risk of GDM. PLoS One. 2019;14:e0225978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Juan J, Yang H. Prevalence, Prevention, and Lifestyle Intervention of Gestational Diabetes Mellitus in China. Int J Environ Res Public Health. 2020;17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 23. | Shi P, Liu A, Yin X. Association between gestational weight gain in women with gestational diabetes mellitus and adverse pregnancy outcomes: a retrospective cohort study. BMC Pregnancy Childbirth. 2021;21:508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 24. | Li Y, Ren X, He L, Li J, Zhang S, Chen W. Maternal age and the risk of gestational diabetes mellitus: A systematic review and meta-analysis of over 120 million participants. Diabetes Res Clin Pract. 2020;162:108044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 90] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 25. | Lamminpää R, Vehviläinen-Julkunen K, Gissler M, Selander T, Heinonen S. Pregnancy outcomes in women aged 35 years or older with gestational diabetes - a registry-based study in Finland. J Matern Fetal Neonatal Med. 2016;29:55-59. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Kouhkan A, Najafi L, Malek M, Baradaran HR, Hosseini R, Khajavi A, Khamseh ME. Gestational diabetes mellitus: Major risk factors and pregnancy-related outcomes: A cohort study. Int J Reprod Biomed. 2021;19:827-836. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Amiri FN, Faramarzi M, Bakhtiari A, Omidvar S. Risk Factors for Gestational Diabetes Mellitus: A Case-Control Study. Am J Lifestyle Med. 2021;15:184-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Zhang L, Zheng W, Huang W, Zhang L, Liang X, Li G. Differing risk factors for new onset and recurrent gestational diabetes mellitus in multipara women: a cohort study. BMC Endocr Disord. 2022;22:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Ndefo UA, Eaton A, Green MR. Polycystic ovary syndrome: a review of treatment options with a focus on pharmacological approaches. P T. 2013;38:336-355. [PubMed] [Cited in This Article: ] |
| 30. | Pan ML, Chen LR, Tsao HM, Chen KH. Relationship between Polycystic Ovarian Syndrome and Subsequent Gestational Diabetes Mellitus: A Nationwide Population-Based Study. PLoS One. 2015;10:e0140544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 31. | Gong LL, Liu H, Liu LH. Relationship between hypothyroidism and the incidence of gestational diabetes: A meta-analysis. Taiwan J Obstet Gynecol. 2016;55:171-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 32. | McIntyre HD, Catalano P, Zhang C, Desoye G, Mathiesen ER, Damm P. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5:47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 487] [Cited by in F6Publishing: 701] [Article Influence: 140.2] [Reference Citation Analysis (0)] |
| 33. | Wagnild JM, Pollard TM. Socioeconomic correlates of sedentary time during pregnancy among women at risk of gestational diabetes in the UK. J Biosoc Sci. 2022;54:876-887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Lorenzo PI, Martín-Montalvo A, Cobo Vuilleumier N, Gauthier BR. Molecular Modelling of Islet β-Cell Adaptation to Inflammation in Pregnancy and Gestational Diabetes Mellitus. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 35. | De Meyts P. The Insulin Receptor and Its Signal Transduction Network. 2016 Apr 27. In: Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000. [PubMed] [Cited in This Article: ] |
| 36. | Park HK, Ahima RS. Leptin signaling. F1000Prime Rep. 2014;6:73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 37. | Vilmi-Kerälä T, Palomäki O, Kankkunen P, Juurinen L, Uotila J, Palomäki A. Oxidized LDL, insulin resistance and central blood pressure after gestational diabetes mellitus. Acta Obstet Gynecol Scand. 2016;95:1425-1432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 38. | de Mendonça ELSS, Fragoso MBT, de Oliveira JM, Xavier JA, Goulart MOF, de Oliveira ACM. Gestational Diabetes Mellitus: The Crosslink among Inflammation, Nitroxidative Stress, Intestinal Microbiota and Alternative Therapies. Antioxidants (Basel). 2022;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 39. | McIntyre HD, Kapur A, Divakar H, Hod M. Gestational Diabetes Mellitus-Innovative Approach to Prediction, Diagnosis, Management, and Prevention of Future NCD-Mother and Offspring. Front Endocrinol (Lausanne). 2020;11:614533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 40. | Roca-Rodríguez MDM, Ramos-García P, López-Tinoco C, Aguilar-Diosdado M. Significance of Serum-Plasma Leptin Profile during Pregnancy in Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. J Clin Med. 2022;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 41. | Heslehurst N, Ngongalah L, Bigirumurame T, Nguyen G, Odeniyi A, Flynn A, Smith V, Crowe L, Skidmore B, Gaudet L, Simon A, Hayes L. Association between maternal adiposity measures and adverse maternal outcomes of pregnancy: Systematic review and meta-analysis. Obes Rev. 2022;23:e13449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 42. | James-Allan LB, Rosario FJ, Madi L, Barner K, Nair S, Lai A, Carrion F, Powell TL, Salomon C, Jansson T. A novel technique using chronic infusion of small extracellular vesicles from gestational diabetes mellitus causes glucose intolerance in pregnant mice. Clin Sci (Lond). 2022;136:1535-1549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 43. | Yoffe L, Polsky A, Gilam A, Raff C, Mecacci F, Ognibene A, Crispi F, Gratacós E, Kanety H, Mazaki-Tovi S, Shomron N, Hod M. Early diagnosis of gestational diabetes mellitus using circulating microRNAs. Eur J Endocrinol. 2019;181:565-577. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 44. | Song C, Li J, Leng J, Ma RC, Yang X. Lifestyle intervention can reduce the risk of gestational diabetes: a meta-analysis of randomized controlled trials. Obes Rev. 2016;17:960-969. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 45. | Guo XY, Shu J, Fu XH, Chen XP, Zhang L, Ji MX, Liu XM, Yu TT, Sheng JZ, Huang HF. Improving the effectiveness of lifestyle interventions for gestational diabetes prevention: a meta-analysis and meta-regression. BJOG. 2019;126:311-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 46. | Bennett CJ, Walker RE, Blumfield ML, Gwini SM, Ma J, Wang F, Wan Y, Dickinson H, Truby H. Interventions designed to reduce excessive gestational weight gain can reduce the incidence of gestational diabetes mellitus: A systematic review and meta-analysis of randomised controlled trials. Diabetes Res Clin Pract. 2018;141:69-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 47. | American Dietetic Association; American Society of Nutrition, Siega-Riz AM, King JC. Position of the American Dietetic Association and American Society for Nutrition: obesity, reproduction, and pregnancy outcomes. J Am Diet Assoc. 2009;109:918-927. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | Sanabria-Martínez G, García-Hermoso A, Poyatos-León R, Álvarez-Bueno C, Sánchez-López M, Martínez-Vizcaíno V. Effectiveness of physical activity interventions on preventing gestational diabetes mellitus and excessive maternal weight gain: a meta-analysis. BJOG. 2015;122:1167-1174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 49. | Davenport MH, Ruchat SM, Poitras VJ, Jaramillo Garcia A, Gray CE, Barrowman N, Skow RJ, Meah VL, Riske L, Sobierajski F, James M, Kathol AJ, Nuspl M, Marchand AA, Nagpal TS, Slater LG, Weeks A, Adamo KB, Davies GA, Barakat R, Mottola MF. Prenatal exercise for the prevention of gestational diabetes mellitus and hypertensive disorders of pregnancy: a systematic review and meta-analysis. Br J Sports Med. 2018;52:1367-1375. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 50. | Martínez-Vizcaíno V, Sanabria-Martínez G, Fernández-Rodríguez R, Cavero-Redondo I, Pascual-Morena C, Álvarez-Bueno C, Martínez-Hortelano JA. Exercise during pregnancy for preventing gestational diabetes mellitus and hypertensive disorders: An umbrella review of randomised controlled trials and an updated meta-analysis. BJOG. 2023;130:264-275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 14] [Reference Citation Analysis (0)] |
| 51. | Padayachee C, Coombes JS. Exercise guidelines for gestational diabetes mellitus. World J Diabetes. 2015;6:1033-1044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 52] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 52. | Koivusalo SB, Rönö K, Klemetti MM, Roine RP, Lindström J, Erkkola M, Kaaja RJ, Pöyhönen-Alho M, Tiitinen A, Huvinen E, Andersson S, Laivuori H, Valkama A, Meinilä J, Kautiainen H, Eriksson JG, Stach-Lempinen B. Gestational Diabetes Mellitus Can Be Prevented by Lifestyle Intervention: The Finnish Gestational Diabetes Prevention Study (RADIEL): A Randomized Controlled Trial. Diabetes Care. 2016;39:24-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 268] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 53. | Markovic TP, Muirhead R, Overs S, Ross GP, Louie JC, Kizirian N, Denyer G, Petocz P, Hyett J, Brand-Miller JC. Randomized Controlled Trial Investigating the Effects of a Low-Glycemic Index Diet on Pregnancy Outcomes in Women at High Risk of Gestational Diabetes Mellitus: The GI Baby 3 Study. Diabetes Care. 2016;39:31-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 54. | Facchinetti F, Dante G, Petrella E, Neri I. Dietary interventions, lifestyle changes, and dietary supplements in preventing gestational diabetes mellitus: a literature review. Obstet Gynecol Surv. 2014;69:669-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Griffith RJ, Alsweiler J, Moore AE, Brown S, Middleton P, Shepherd E, Crowther CA. Interventions to prevent women from developing gestational diabetes mellitus: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2020;6:CD012394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 56. | Wei J, Heng W, Gao J. Effects of Low Glycemic Index Diets on Gestational Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Clinical Trials. Medicine (Baltimore). 2016;95:e3792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 57. | Barbour LA. Unresolved controversies in gestational diabetes: implications on maternal and infant health. Curr Opin Endocrinol Diabetes Obes. 2014;21:264-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 58. | Mahajan A, Donovan LE, Vallee R, Yamamoto JM. Evidenced-Based Nutrition for Gestational Diabetes Mellitus. Curr Diab Rep. 2019;19:94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Rasmussen L, Poulsen CW, Kampmann U, Smedegaard SB, Ovesen PG, Fuglsang J. Diet and Healthy Lifestyle in the Management of Gestational Diabetes Mellitus. Nutrients. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 60. | Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): National Academies Press (US); 2009 . [PubMed] [Cited in This Article: ] |
| 61. | Melero V, García de la Torre N, Assaf-Balut C, Jiménez I, Del Valle L, Durán A, Bordiú E, Valerio JJ, Herraiz MA, Izquierdo N, Torrejón MJ, Runkle I, Barabash A, Rubio MA, Calle-Pascual AL. Effect of a Mediterranean Diet-Based Nutritional Intervention on the Risk of Developing Gestational Diabetes Mellitus and Other Maternal-Fetal Adverse Events in Hispanic Women Residents in Spain. Nutrients. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Assaf-Balut C, García de la Torre N, Durán A, Fuentes M, Bordiú E, Del Valle L, Familiar C, Ortolá A, Jiménez I, Herraiz MA, Izquierdo N, Perez N, Torrejon MJ, Ortega MI, Illana FJ, Runkle I, de Miguel MP, Montañez C, Barabash A, Cuesta M, Rubio MA, Calle-Pascual AL. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS One. 2017;12:e0185873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 63. | H Al Wattar B, Dodds J, Placzek A, Beresford L, Spyreli E, Moore A, Gonzalez Carreras FJ, Austin F, Murugesu N, Roseboom TJ, Bes-Rastrollo M, Hitman GA, Hooper R, Khan KS, Thangaratinam S; ESTEEM study group. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): A pragmatic multicentre randomised trial. PLoS Med. 2019;16:e1002857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 64. | Tobias DK, Zhang C, Chavarro J, Bowers K, Rich-Edwards J, Rosner B, Mozaffarian D, Hu FB. Prepregnancy adherence to dietary patterns and lower risk of gestational diabetes mellitus. Am J Clin Nutr. 2012;96:289-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 65. | Tobias DK, Hu FB, Chavarro J, Rosner B, Mozaffarian D, Zhang C. Healthful dietary patterns and type 2 diabetes mellitus risk among women with a history of gestational diabetes mellitus. Arch Intern Med. 2012;172:1566-1572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 66. | Izadi V, Tehrani H, Haghighatdoost F, Dehghan A, Surkan PJ, Azadbakht L. Adherence to the DASH and Mediterranean diets is associated with decreased risk for gestational diabetes mellitus. Nutrition. 2016;32:1092-1096. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 67. | Luoto R, Laitinen K, Nermes M, Isolauri E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br J Nutr. 2010;103:1792-1799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 68. | Callaway LK, McIntyre HD, Barrett HL, Foxcroft K, Tremellen A, Lingwood BE, Tobin JM, Wilkinson S, Kothari A, Morrison M, O'Rourke P, Pelecanos A, Dekker Nitert M. Probiotics for the Prevention of Gestational Diabetes Mellitus in Overweight and Obese Women: Findings From the SPRING Double-Blind Randomized Controlled Trial. Diabetes Care. 2019;42:364-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 69. | Davidson SJ, Barrett HL, Price SA, Callaway LK, Dekker Nitert M. Probiotics for preventing gestational diabetes. Cochrane Database Syst Rev. 2021;4:CD009951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 70. | Mijatovic-Vukas J, Capling L, Cheng S, Stamatakis E, Louie J, Cheung NW, Markovic T, Ross G, Senior A, Brand-Miller JC, Flood VM. Associations of Diet and Physical Activity with Risk for Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis. Nutrients. 2018;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 71. | De-Regil LM, Palacios C, Lombardo LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2016;CD008873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 72. | Pérez-López FR, Pasupuleti V, Mezones-Holguin E, Benites-Zapata VA, Thota P, Deshpande A, Hernandez AV. Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2015;103:1278-88.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 186] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 73. | Filardi T, Panimolle F, Crescioli C, Lenzi A, Morano S. Gestational Diabetes Mellitus: The Impact of Carbohydrate Quality in Diet. Nutrients. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 74. | Mustad VA, Huynh DTT, López-Pedrosa JM, Campoy C, Rueda R. The Role of Dietary Carbohydrates in Gestational Diabetes. Nutrients. 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 75. | Mahjoub F, Ben Jemaa H, Ben Sabeh F, Ben Amor N, Gamoudi A, Jamoussi H. Impact of nutrients and Mediterranean diet on the occurrence of gestational diabetes. Libyan J Med. 2021;16:1930346. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 76. | Assaf-Balut C, Torre N, Valle L, Valerio J, Duran A, Bordiú E, Barabash A, Rubio M, Calle-Pascual A. Gestational diabetes mellitus and Mediterranean diet principles. In: Preedy VR, Watson RR, editors. Handbook of Famine, Starvation, and Nutrient Deprivation: From Biology to Policy. Academic Press; 2020. [Cited in This Article: ] |
| 77. | Asemi Z, Samimi M, Tabassi Z, Esmaillzadeh A. The effect of DASH diet on pregnancy outcomes in gestational diabetes: a randomized controlled clinical trial. Eur J Clin Nutr. 2014;68:490-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 78. | Chatzakis C, Cavoretto P, Sotiriadis A. Gestational Diabetes Mellitus Pharmacological Prevention and Treatment. Curr Pharm Des. 2021;27:3833-3840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 79. | Eskenazi B, Rauch S, Iurlaro E, Gunier RB, Rego A, Gravett MG, Cavoretto PI, Deruelle P, García-May PK, Mhatre M, Usman MA, Elbahnasawy M, Etuk S, Napolitano R, Deantoni S, Liu B, Prefumo F, Savasi V, Marques PF, Baafi E, Zainab G, Nieto R, Serrano B, Aminu MB, Cardona-Perez JA, Craik R, Winsey A, Tavchioska G, Bako B, Oros D, Benski C, Galadanci H, Savorani M, Oberto M, Sentilhes L, Risso M, Takahashi K, Vecciarelli C, Ikenoue S, Pandey AK, Soto Conti CP, Cetin I, Nachinab VB, Ernawati E, Duro EA, Kholin A, Firlit ML, Easter SR, Sichitiu J, John-Akinola Y, Casale R, Cena H, Agyeman-Duah J, Roggero P, Langer A, Bhutta ZA, Kennedy SH, Villar J, Papageorghiou AT. Diabetes mellitus, maternal adiposity, and insulin-dependent gestational diabetes are associated with COVID-19 in pregnancy: the INTERCOVID study. Am J Obstet Gynecol. 2022;227:74.e1-74.e16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 80. | Villar J, Soto Conti CP, Gunier RB, Ariff S, Craik R, Cavoretto PI, Rauch S, Gandino S, Nieto R, Winsey A, Menis C, Rodriguez GB, Savasi V, Tug N, Deantoni S, Fabre M, Martinez de Tejada B, Rodriguez-Sibaja MJ, Livio S, Napolitano R, Maiz N, Sobrero H, Peterson A, Deruelle P, Giudice C, Teji JS, Casale RA, Salomon LJ, Prefumo F, Cheikh Ismail L, Gravett MG, Vale M, Hernández V, Sentilhes L, Easter SR, Capelli C, Marler E, Cáceres DM, Albornoz Crespo G, Ernawati E, Lipschuetz M, Takahashi K, Vecchiarelli C, Hubka T, Ikenoue S, Tavchioska G, Bako B, Ayede AI, Eskenazi B, Thornton JG, Bhutta ZA, Kennedy SH, Papageorghiou AT; INTERCOVID-2022 International Consortium. Pregnancy outcomes and vaccine effectiveness during the period of omicron as the variant of concern, INTERCOVID-2022: a multinational, observational study. Lancet. 2023;401:447-457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 54] [Article Influence: 54.0] [Reference Citation Analysis (0)] |