Published online Oct 15, 2022. doi: 10.4239/wjd.v13.i10.835
Peer-review started: May 28, 2022
First decision: August 1, 2022
Revised: August 8, 2022
Accepted: September 15, 2022
Article in press: September 15, 2022
Published online: October 15, 2022
It has been 100 years since the first successful clinical use of insulin, yet it remains the only treatment option for type 1 diabetes mellitus (T1DM) patients. Advances in diabetes care, such as insulin analogue therapies and new devices, including continuous glucose monitoring with continuous subcutaneous insulin infusion have improved the quality of life of patients but have no impact on the pathogenesis of the disease. They do not eliminate long-term complications and require several lifestyle sacrifices. A more ideal future therapy for T1DM, instead of supplementing the insufficient hormone production (a consequence of β-cell destruction), would also aim to stop or slow down the destructive autoimmune process. The discovery of the autoimmune nature of type 1 diabetes mellitus has presented several targets by which disease progression may be altered. The goal of disease-modifying therapies is to target autoimmune mechanisms and prevent β-cell destruction. T1DM patients with better β-cell function have better glycemic control, reduced incidence of long-term complications and hypoglycemic episodes. Unfortunately, at the time symptomatic T1DM is diagnosed, most of the insulin secreting β cells are usually lost. Therefore, to maximize the salvageable β-cell mass by disease-modifying therapies, detecting autoimmune markers in an early, optimally presymptomatic phase of T1DM is of great importance. Disease-modifying therapies, such as immuno- and regenerative therapies are expected to take a relevant place in diabetology. The aim of this article was to provide a brief insight into the pathogenesis and course of T1DM and present the current state of disease-modifying therapeutic interventions that may impact future diabetes treatment.
Core Tip: Our knowledge is rapidly growing about the pathomechanism of type 1 diabetes mellitus, and new and improved therapies have emerged. However, the long-term complications and the required lifestyle changes cannot be eliminated. There is a growing number of research that aims to find specific immunological markers/targets that have a role in disease development. The ultimate goal is finding new therapeutic ways to treat the disease and to delay or even prevent its development. The aim of this review was to provide a brief insight into the current state of disease-modifying therapeutic interventions that may impact future diabetes care.
- Citation: Nagy G, Szekely TE, Somogyi A, Herold M, Herold Z. New therapeutic approaches for type 1 diabetes: Disease-modifying therapies. World J Diabetes 2022; 13(10): 835-850
- URL: https://www.wjgnet.com/1948-9358/full/v13/i10/835.htm
- DOI: https://dx.doi.org/10.4239/wjd.v13.i10.835
For many years it was accepted that type 1 diabetes mellitus (T1DM) starts with the classical triad of polyuria, polydipsia and polyphagia. However, it became clear that T1DM is a long standing, progressive disease with a preclinical phase without symptoms and with the appearance of multiple T1DM-associated autoantibodies. The preclinical phase is followed by a symptomatic clinical phase[1]. The burden of living with the chronic disease is considerable for the patient, the family and the society. This minireview focused on the clinical applications of novel disease-modifying therapeutic in-tervention options in early stages of T1DM that may prevent or reverse clinically overt symptomatic T1DM. The presentation of the latest improvements available for middle and late stage disease and diabetic complications is out of the scope of the current review.
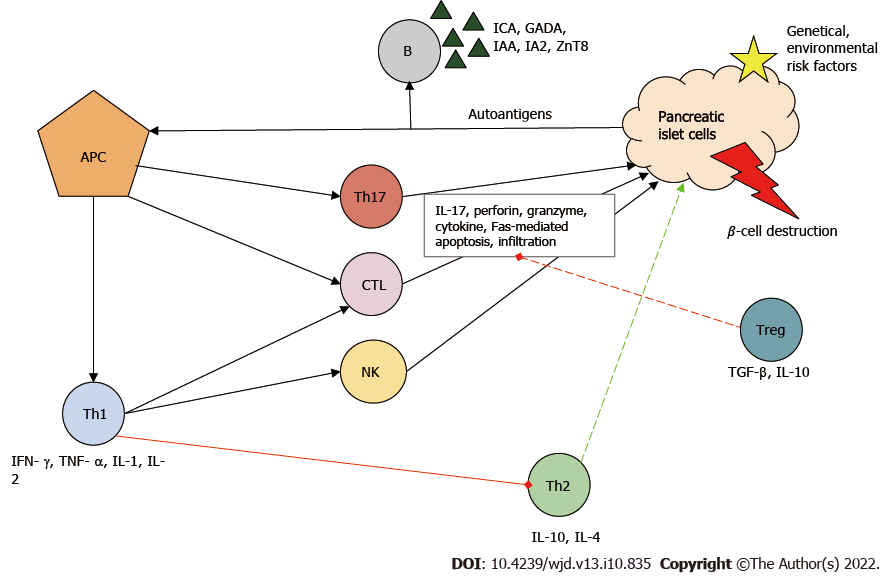
The pathogenesis of T1DM involves a complex interaction between pancreatic β cells and the innate and natural immune systems. The precise mechanism that leads to the loss of immune tolerance is still unclear. However, viral infections, nutritional factors and the perinatal environment have been associated with the disease[2-4]. It is assumed that the stability of the trimolecular complex (T cell receptor/human leukocyte antigen/peptide) during thymic selection plays a major role in the escape of autoimmune T cells[5].
In the development of T1DM, the initial step of the destructive process is considered to be the uptake and presentation of β-cell-derived peptides, autoantigens, by the antigen-presenting cells. Next, antigen-presenting cells, which can be both macrophages and dendritic cells, migrate to lymph nodes around the pancreas and activate CD4+ helper T cells (Th)[6]. Th cells differentiate into Th1, which have a proinflammatory phenotype. Th1 cells are the key effector cells in the pathogenesis of T1DM and are capable of producing interferon-γ, tumor necrosis factor α, interleukin 1 (IL-1) and IL-2. These cytokines inhibit Th2 polarization, the cells responsible for the protection of islets[7]. Th1 cells are necessary for the activation and recruitment of other autoreactive cells, such as CD8+ cytotoxic T lymphocytes (CTL), which are responsible for the lysis/apoptosis of β cells presenting the major histocompatibility complex I autoantigen complex. The cell-destructive effect of activated CTLs is due to macromolecules stored in granules (e.g., perforin, granzyme), to the cytokines and to caspase-dependent apoptosis[8].
B cells are stimulated by Th1 cells and produce autoantibodies against β cells [islet cell antibody, glutamic-acid-decarboxylase antibody (GADA), islet tyrosine phosphatase 2 antibody, insulin autoantibody and zinc transporter 8 antibodies]. These antibodies have become the biomarkers of T1DM[9]. Furthermore, Th1 cells enhance antigen presenting, costimulatory and effector functions of macrophages and dendritic cells. Natural killer cells also contribute to β-cell destruction through their cytolytic effects and antibody-dependent cellular cytotoxicity. Th17, with strong inflammatory effects, is also involved in the inflammatory process: It secretes IL-17 family cytokines and plays an important role in neutrophil granulocyte recruitment and activation[10].
Under ideal conditions, regulatory T cells (Treg) inhibit the autoreactive lymphocytes. If Treg cells are deficient, the rate of T1DM progression is increased[11]. The above-mentioned immune cells infiltrate the islets (insulitis) and eventually cause β-cell death and reduced insulin levels (Figure 1).
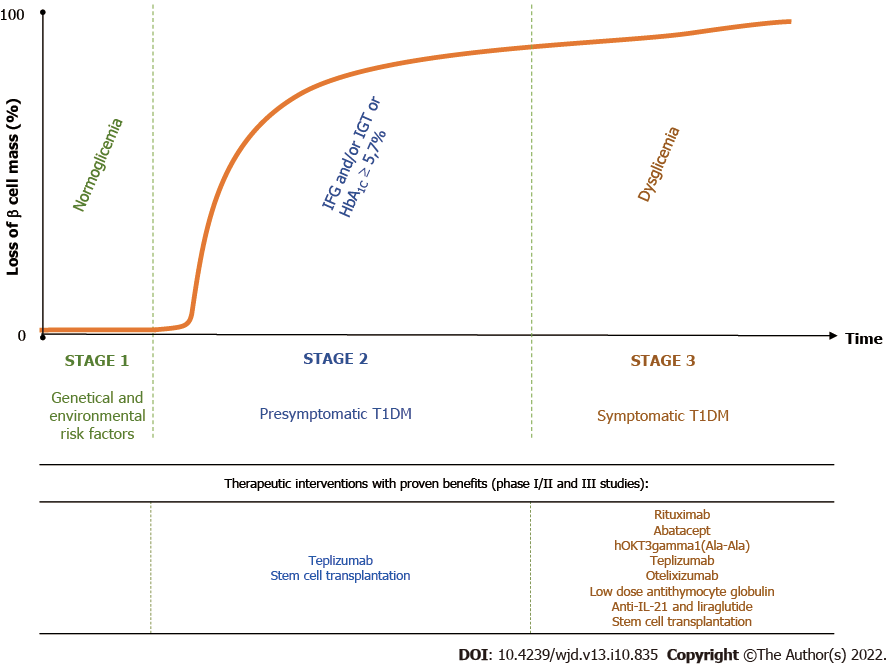
T1DM is the result of a destructive autoimmune-mediated process in which insulin-producing β cells in the islets of Langerhans are damaged. According to the novel staging classification system proposed by the Juvenile Diabetes Research Foundation, the Endocrine Society and the American Diabetes Association, there are three distinct stages in T1DM[12]. Genetic predisposing factors are present from birth. The autoimmune reaction may be initiated in genetically susceptible individuals by environmental risk factors, which are not well understood[13]. People with a first- or second-degree relative with T1DM have a 15 times greater risk of developing the disease compared to the general population[14] (Figure 2).
Stage 1 is the critical point of no return since eventually the affected individuals will develop clinical diabetes. It is characterized by the presence of immune markers, two or more of the T1DM-associated islet antibodies, such as islet cell antibodies, GADA, Islet tyrosine phosphatase 2 antibodies and zinc transporter 8 antibodies, normoglycemia and absence of diabetic symptoms. In stage 2, the β-cell volume is critically decreased, and metabolic markers become detectable in asymptomatic patients. These individuals, besides being antibody positive, display impaired fasting glycemia, impaired glucose tolerance, abnormal oral glucose tolerance test or glycated hemoglobin (HbA1C) ≥ 5.7%. Stage 3 represents the phase of clinical diagnosis and the manifestation of typical diabetic symptoms such as polyuria, polydipsia, weight loss, fatigue, diabetic ketoacidosis, etc[12]. Over time, most of the residual β cells are lost. However, sensitive C-peptide measurements have shown that 30%-80% of patients with long–standing T1DM are insulin microsecretors. This means that these patients have detectable stimulated C-peptide value of < 30 pmol/L (< 0.09 ng/mL)[15], an important consideration in therapeutic approaches targeting β-cell survival[16]. Shield et al[17] identified two clear phases of C-peptide decline after the diagnosis of T1DM: an exponential fall in the first 7 years (-47%/year) followed by a stable phase (-0.1%/year) (Figure 2).
Age has a major influence on the rate of disease progression. In children, the clinical stage develops more rapidly, and β-cell loss is more pronounced compared to adults. About 6 years to 9 years after the diagnosis of T1DM, 20% of those diagnosed in childhood and 60% of those diagnosed in adulthood had detectable C-peptide secretion, an indicator of endogenous insulin production[18]. In addition, the autoantibody titer and profile are also a determinant of β-cell loss. Most people will not develop diabetes if they have a single autoantibody. In contrast, the more autoantibodies a person has and the higher their serum concentration is, the rate of disease progression is greater[19]. Lately, stage-specific therapies have been the focus of clinical trials for modifying disease progression[13,19].
Groundbreaking studies with cyclosporin A in the 1980s showed that the disease course of T1DM can be altered with immune therapy[20] and gave rise to research in the field of definitive treatment of T1DM. Despite extensive efforts so far, immune-altering therapies could not reach approval for routine clinical use for several reasons. Some agents such as cyclosporin A have many side effects and lack long-term efficacy[21]. Other options with a more favorable tolerance profile such as the adjuvant-formulated GAD-alum vaccine, which incorporated recombinant human glutamic acid decarboxylase, had no effect on disease progression[22]. The recent development of more targeted immunotherapies, the advances in regenerative therapies and lessons learned about β-cell survival from type 2 diabetes mellitus studies gave rise to more promising therapeutic options. Of the immunotherapy agents, teplizumab has come closest to achieving success. In July 2021, the United States Food and Drug Administration considered the use of teplizumab in high-risk individuals but deemed further studies necessary before granting approval[23].
Even though T1DM is mainly a T cell-mediated autoimmune disease, antigen-presenting B lymphocytes can also play a pathogenic role by activating T-lymphocytes and triggering the autoimmune destruction of β cells. In animal models and triggered human studies, T1DM anti-B lymphocyte therapies have been shown to be effective[24].
Rituximab: Rituximab is an anti-CD20 monoclonal antibody known to cause B-cell depletion. It is widely used in clinical practice in malignant hematological diseases such as non-Hodgkins lymphomas and chronic lymphocytic leukemias. In a placebo controlled randomized trial, including 87 recent onset T1DM patients, it has been shown that rituximab treatment was associated with slower progression of β-cell dysfunction and better metabolic control during the 12-mo long study period[25]. Insulin dose requirements decreased during the study. However, none of the patients were able to become insulin free. Extensive follow-up of the patients showed a constant decline in C-peptide production. It suggests that B-cell depletion by its own is not sufficient in restoring β-cell tolerance in the long run and does not fundamentally alter the course of overt T1DM[26].
It has been reported that rituximab can suppress insulin autoantibodies, but no such effect could be found in the case of GADA, islet tyrosine phosphatase 2 antibody and zinc transporter 8 antibody[27]. Compared to placebo controls, rituximab-treated T1DM patients, whose C-peptide response was significant, have shown increased proliferative responses to islet, neuronal and disease-relevant environmental antigens ultimately resulting in increasing insulin secretory function[28]. Moreover, the combination of rituximab with CD4+ CD25high CD127- T regulatory cells[29] or therapy targeting CD4+ T cells[30] can further improve treatment efficacy. One side effect of rituximab, reported by the study of Kroll et al[31], might be the reactivation of some asymptomatic polyomavirus infections. Rituximab is currently being tested in earlier stages of T1DM (NCT03929601[32]).
T-cell co-stimulation inhibition: Abatacept is a cytotoxic T lymphocyte-associated antigen 4 immunoglobulin fusion protein designed to selectively bind to CD80/86 to inhibit the early activation and proliferation of naïve T lymphocytes. Since effector memory T cells are less dependent on CD28 costimulation, abatacept is a more selective way to inhibit T cell activation compared to general immunosuppressants. In a phase II placebo-controlled study, Orban et al[33] investigated the effect of abatacept in a population with recent onset T1DM. Patients in the treatment group received a monthly dose of 10 mg/kg intravenous abatacept for 2 years. The authors found that cytotoxic T lymphocyte-associated antigen 4 inhibition slowed the decline of β-cell function during the 2 years of the treatment and had a beneficial effect during the 1-year follow-up period without active treatment[34]. However, the observed positive effect was only temporary and declined over time. Furthermore, it was found that abatacept does not change immunogenicity of other vaccines in T1DM patients[35], but different follicular Th and central memory CD4+ T cell phenotypes might affect the efficacy of the treatment[36,37]. There are still ongoing clinical trials (e.g., NCT01773707[33], NCT04118153[38] and NCT03929601[32]) investigating whether abatacept may have a more potent impact on the disease if administered at earlier stages (e.g., at stage 2) of T1DM pathogenesis.
Anti-CD3 therapy: So far the most promising therapeutic target in modifying the course of T1DM is the ε chain of the CD3 receptor on the T cell surface, previously known as muromonab-CD3 (trade name: Orthoclone OKT3)[39]. Animal studies have shown that anti-CD3 therapy can induce diabetes remission in the models of T1DM[40]. This main effect is associated by the induction of Treg cells and immu-nosuppressive cytokines such as transforming growth factor β[41]. One of the first human trials reported significantly improved C-peptide response and other clinical parameters after a single shot of hOKT3gamma1(Ala-Ala)[42].
Teplizumab is a humanized anti-CD3 monoclonal antibody, which has been shown to be the most potent agent in slowing the progression of T1DM. In a series of human clinical trials, it was demonstrated that the treatment of teplizumab was a potent way to delay the decline of C-peptide production[43-45] and can help to preserve β-cell function[46], and its effect can be sustained by an average of 15.9 mo in T1DM[47]. Furthermore, the Protegé[48,49] study in which 516 T1DM patients were enrolled and treated with teplizumab has demonstrated that anti-CD3 therapy delayed the decline of insulin secretion and induced disease regression, and 5% of the patients became insulin independent. Even though teplizumab has shown promising results in preventing disease progression, it must be noted that significant metabolic benefits such as a significant reduction in HbA1C could not be demonstrated.
These promising results led to further trials to evaluate the effect of anti-CD3 therapy in T1DM prevention. The recently published results of a phase III follow-up trial including non-diabetic patients at high risk of developing T1DM, defined as having impaired glucose tolerance and at least two diabetes-specific autoantibodies, demonstrated the efficacy of teplizumab in delaying the onset of T1DM by 48.4 mo compared to the placebo group (24.4 mo)[50]. C-peptide levels of those who responded to treatment remained significantly better even after a 7-year follow-up period[51]. Furthermore, clinical responders to teplizumab therapy have shown significant reduction in circulating CD4+ effector memory T cells and decreased activation and regulatory gene expression in circulating CD8+ central memory T cells[52]. Currently, studies are running to investigate teplizumab in at-risk individuals[53] and recent-onset T1DM patients[54,55].
Otelixizumab is another humanized anti-CD3 antibody that has been evaluated both in the treatment of overt[56] and new-onset[57,58] T1DM. Similarly to teplizumab, a 6 d treatment of otelixizumab preserved residual β-cell function for at least 18 mo in 40 patients with recent-onset T1DM. The protective effect of otelixizumab treatment appears to be dose dependent as studies attempting to lower adverse reactions by administering lower doses could not demonstrate a benefit in C-peptide preservation[59,60]. The protective effect of otelixizumab showed the highest benefit in insulin autoantibody-positive T1DM patients[61].
Anti-CD3 therapies have been overall well tolerated among patients. Adverse reactions that were significantly more prevalent in the treatment group included: vomiting, rash, chills, cytokine release syndrome, Epstein-Barr virus reactivation[57,62] and headache. Recently, a subcutaneous formulation was also introduced[63], which significantly reduced such undesirable effects. Adverse reactions were mostly mild to moderate and self-limited; 9% of patients were not able to complete all drug doses compared to a 2% dropout rate in the placebo group. The most common cause for treatment cessation was lymphopenia, neutropenia, elevated liver enzymes and reduced platelet counts.
Low dose antithymocyte globulin: Antithymocyte globulin (ATG) is a polyclonal immunoglobulin G antibody against multiple human T cell antigens and their precursors. Only a limited number of studies are available, and their results are somewhat controversial: 6.5 mg/kg ATG alone could not preserve β-cell function, but C-peptide secretion was preserved in older participants suggesting a possible age-specific action[64,65]. In contrast, low dose ATG treatment (2.5 mg/kg administered as 0.5 mg/kg on day 1 and 2 mg/kg on day 2) in combination with pegylated granulocyte colony-stimulating factor acts by decreasing the number of activated effector T cells while relatively preserving Treg cells. T1DM patients with a diabetes onset between 4 mo and 2 years receiving low dose ATG + granulocyte colony-stimulating factor have shown a benefit in disease progression[66,67]. Patients in the treatment group have had higher C-peptide production after a mixed meal test, and lower HbA1C after 6 mo was also recorded in the treatment group compared to the placebo group.
A more recent study published by the same group indicated that low-dose ATG monotherapy without granulocyte colony-stimulating factor can delay the decline of C-peptide, can reduce the HbA1C level and affect T cell phenotypes in new-onset T1DM[68,69]. The ongoing follow-up of this study is in progress along with two additional studies[70,71], and their results will help further evaluate the potential benefits of low-dose ATG and its therapeutic potential for preventing T1DM.
Anti-IL-21 and liraglutide: A new strategy to modify the disease course in T1DM is using a drug combination that not only halts or delays the progressive autoimmune process but aims at preserving and improving residual β-cell function. This approach may have the advantage over previous monotherapies in achieving disease modification with milder immunomodulation in a safer, more sustainable way. IL-21 plays a key role in the pathomechanism of T1DM by activating and leading CD8+ T lymphocytes from lymph nodes and the exocrine pancreas to the pancreatic islets eventually leading to β-cell destruction[72,73]. Based on these findings, IL-21 inhibition has emerged as a potential disease-modifying target in preventing T1DM. 35-Liraglutide, a glucagon like peptide-1 analog that has routinely been used in type 2 diabetes therapy, has been proven to increase β-cell survival[74] and can improve glucose dependent insulin secretion not only in type 2 diabetes but in T1DM as well[75,76].
In a recent phase II clinical trial the effect of liraglutide and IL-21 inhibition was evaluated in 308 T1DM patients with recent onset disease and residual β-cell function[77]. The combination treatment was effective to preserve both fasting and postprandial endogenous insulin secretion resulting in a nonsignificant decrease in the number of hypoglycemic events and level of HbA1C for 52 wk. During the follow-up period the combination treatment was considered safe, and there were no safety concerns raised. The study included a 26 wk off-drug observation period during which the effect of the treatment deteriorated rapidly, suggesting the need for continued treatment. Overall, this combination treatment seems to be a promising candidate for further evaluations in a phase III clinical trial.
Over the past two decades, stem cell transplantation has received increased attention in clinical trials as a promising therapy within regenerative medicine for T1DM. While the treatment of T1DM with hematopoietic stem cells was more typical in the 2000s and first half of the 2010s[78], the most recent studies focus more on the treatment with mesenchymal stem cells (MSCs)[13]. This modern approach to treat T1DM has several advantages over previous treatment options. MSC transplantation is hypo-immunogenic because the cells do not express costimulatory antigens (CD80, CD86, CD40, CD40L etc) nor major histocompatibility complex II and major histocompatibility complex I. MSCs allow both autologous and allogeneic transplantation, even without conditioning treatment[79].
MSCs can be easily cultured in vitro due to their high dividing capacity, and they can be isolated from many adult and perinatal sources (e.g., bone marrow, adipose tissue, peripheral blood, dental pulp, skeletal muscle, liver, lung, umbilical cord blood, Wharton’s jelly and placenta)[80]. Of these, the umbilical cord and its derivatives stand out as they can be obtained non-invasively, are considered as ‘medical waste’ and have an exceptional differentiation capacity towards insulin-secreting cells[81]. Unlike embryonic stem cell therapy, the use of these tissue sources does not raise special ethical issues.
In addition, MSCs have no known tumorigenic effect, whereas embryonic stem cells can form teratomas and teratocarcinomas in vivo[82]. However, tumorigenesis cannot be completely ruled out as a possible adverse effect: MSCs may be a direct source of malignant cells, may maintain various cancer processes (e.g., breast and colon cancer) through paracrine factor secretion or may enhance tumor growth and progression through their immunosuppressive effects[83-85]. However, to the best of our knowledge, no similar side effects have been reported in clinical trials using MSCs[86].
MSCs are capable of generating tissue types of mesodermal origin, such as musculoskeletal, cartilaginous and adipose tissue, and may cross the boundaries of germ layers and transdifferentiate into ectodermal neurons or even endodermal islet cells[87,88]. Low amounts of MSCs in the target tissue do not explain regeneration or even wound healing. However, in vivo experiences show that MSCs have other, more pronounced therapeutic effects, such as remodeling of the diabetic microenvironment[89,90]. It should be noted that in systemic administration, MSCs are entrapped in capillaries, especially in the lungs, reducing the number of migrating cells towards the target tissue, suggesting that better outcome could be obtained through local injection[91].
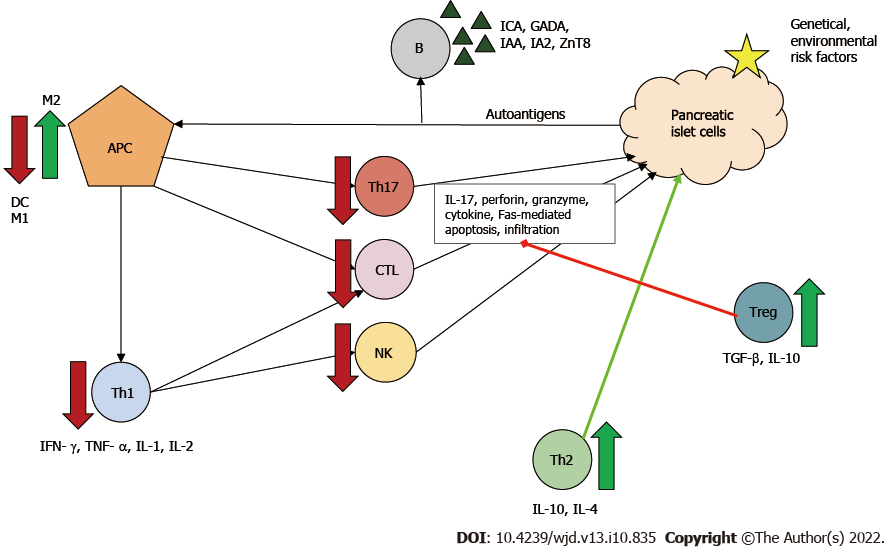
The strong immunoregulatory function of MSCs plays a key role in the regeneration of β cells. This protective effect in T1DM is due to secretion of soluble factors and cell-cell interactions. Insulin deficiency and irreversible β-cell destruction are the consequences of the autoimmune reaction in T1DM, and MSCs are able to intervene at several points in this process, modulating immune cells. MSC transplantation with its paracrine effects, due to the production of cytokines, chemokines and growth factors, can affect the local environment, inhibit apoptosis and induce proliferation. The identified bioactive factors are: IL-6, IL-8, transforming growth factor β, vascular endothelial growth factor, hepatocyte growth factor and nitric oxide[13].
Two types of MSCs are known: proinflammatory MSCs (MSC1) and anti-inflammatory MSCs (MSC2). The type of polarization depends on the inflammatory milieu. In the absence of an inflammatory environment MSCs adopt a proinflammatory phenotype and amplify T cell responses. Conversely, in an inflammatory environment (high interferon-γ and tumor necrosis factor α levels), MSCs may adopt an immunosuppressive phenotype and suppress T cell proliferation via secreted soluble factors[92]. MSCs have a regulatory function against effector T cells. In the pathogenesis of T1DM, Th1 cells are the main effector cells, and Th2 cells have been shown to be protective.
Beneficial effects of MSCs in diabetes can be attributed to: (1) Secreted IL-4; (2) Altered Th1/Th2 ratio with a Th1 to Th2 shift; and (3) Promoted maturation of naϊve T cells towards Th2[93]. Furthermore, MSCs can directly and indirectly inhibit through several pathways: (1) Th17 cell development and thus IL-17 production; (2) CTL function and thus Fas-mediated β-cell apoptosis; and (3) Both maturation and activation of antigen-presenting cells, principally dendritic cells, by secreting for example prostaglandin E2, IL-6 and macrophage colony-stimulating factor[13,94,95].
Two types of macrophages are known: M1 and M2 producing proinflammatory and anti-inflammatory cytokines, respectively. MSCs can modulate the phenotype shift, causing an M1 to M2 shift[96]. Treg cells are components of MSC-induced indirect immunosuppression. In vivo and in vitro, MSCs have been shown to enhance Treg proliferation through cell-cell interaction[13]. By producing IL-10 and transforming growth factor β, Treg cells downregulate Th1- and Th17-mediated inflammatory response and the cytotoxicity of CTLs, thereby leading to immune tolerance in the organism[97]. These mechanisms can contribute to both amelioration of auto-reactivity and of β-cell death (Figure 3).
In recent years, MSCs have attracted the attention of many researchers and clinicians as a result of encouraging preclinical animal data in T1DM. The most important advantages are: (1) Wide range of sources; (2) Self-renewal capacity; (3) Multidifferentiation capacity; and (4) Strong immunomodulatory potential. MSCs are also immunoprivileged, well-tolerated and safe[98]. The clinical studies vary in MSC origin, dose, route of transplantation, administration frequency and in eligible patients’ characteristics (Table 1).
| Ref. | Patient characteristics | Treatment | Therapeutic outcomes |
| Wu et al[105], 20221 | n = 14; aged 27-47 yr | Intrapancreatic: Allogeneic UC-MSC + autologous BM-MNC | Insulin independence: No |
| China, 8 yr | Duration of T1DM: 10-24 yr | Insulin requirement: Improvement at 1 yr but no difference at 8 yr | |
| FCP and HbA1C: Significant improvement | |||
| Significantly lower occurrence of diabetic complications | |||
| Izadi et al[108], 2022 | n = 20; aged 8-40 yr | BM-MSC | Insulin independence: No |
| Iran, 12 mo | Duration of T1DM: < 1 yr (n = 11) and > 1 yr (n = 9) | Insulin requirement, FCP, HbA1C: Significant improvement | |
| FCP (n = 11): 0.92 ± 0.57 ng/mL | Number of hypoglycemic events decreased | ||
| Patients with early onset of T1DM benefit more | |||
| Adverse effects: Possible mild injection site reactions | |||
| Lu et al[106], 2021 | n = 27; aged 8-55 yr | IV (2x): Allogeneic UC-MSC | Insulin independence: 3 subjects |
| China, 12 mo | Median duration of T1DM: 2.3 mo | Insulin requirement, HbA1C: No improvement | |
| FCP: 100 pmol/L ( 0.3 ng/mL) | SCP: improved in adult-onset T1DM subgroup | ||
| Adverse effects: Mild fever | |||
| Dantas et al[103], 20212 | N = 7; Aged 16-35 yr | Allogenic AD-MSC + 2000 UI/d cholecalciferol | Insulin independence: 1 subject |
| Brazil, 6 mo | Duration of T1DM: ≤ 4 mo | Insulin requirement: Stable at 6 mo | |
| FCP: 0.80 ± 0.38 ng/dL | FCP and HbA1C: Significant improvement | ||
| Adverse effects: Transient headache, mild local reactions, immediate tachycardia, thrombophlebitis + other mild effects | |||
| Araujo et al[102], 2020 | n = 8; aged 16-28 yr | Allogenic AD-MSC + 2000 UI/d cholecalciferol | Insulin independence: 2 subjects |
| Brazil, 3 mo | Duration of T1DM: ≤ 4 mo | Insulin requirement, HbA1C: Decreased significantly at 3 mo | |
| FCP: Only initial improvements, with the same results at the 3-mo visit | |||
| Adverse effects: Transient headache, mild local reactions, immediate tachycardia, thrombophlebitis + other mild effects | |||
| Cai et al[104], 2016 | n = 21; aged 18-10 yr | Intra-pancreatic: Allogeneic UC-MSC + autologous BM-MNC | Insulin independence: No |
| China, 12 mo | Duration of T1DM: 2-16 yr | Insulin requirement, HbA1C: Decreased significantly | |
| FCP: < 0.1 pmol/mL (< 0.3 ng/mL) | FCP: Markedly increased | ||
| Adverse effects: Transient abdominal pain, bleeding | |||
| Carlsson et al[107], 2015 | n = 9; aged 18-40 yr | IV: Autologous BM-MSC | Insulin independence: No |
| Sweden, 12 mo | Duration of T1DM: < 3 wk | Insulin requirement, HbA1C, SCP: No significant improvement | |
| SCP: > 0.1 nmol/L (> 0.3 ng/mL) | Adverse effects: No | ||
| Thakkar et al[100], 2015 | n = 20; aged 8-45 yr | Into portal + thymic circulation and subcutaneous tissue: | Insulin independence: No |
| India, 24 mo | Duration of T1DM: > 12 mo | Insulin requirement: Decreased | |
| 2 groups with a mean C-peptide: | Group 1: Autologous IS-AD-MSC+ HSC | HbA1C, C-peptide: Sustained improvement | |
| Group 1: 0.22 ng/mL | Group 2: Allogeneic IS-AD-MSC+ HSC | Adverse effects: No | |
| Group 2: 0.028 ng/mL | |||
| Dave et al[101], 20153 | n = 10; aged 9-29 yr | Into portal + thymic circulation and subcutaneous tissue: autologous IS-AD-MSC+ HSC | Insulin independence: No |
| India, 27 mo | Duration of T1DM: 2-15 yr | Insulin requirement: Decreased | |
| Pre-IV C-peptide: 0.22 ng/mL | HbA1C, C-peptide: Sustained improvement + significantly lower GADA levels | ||
| Adverse effects: No | |||
| Hu et al[99], 2013 | n = 15; aged < 25 yr | IV (2x): Allogeneic WJ-MSC | Insulin independence: 3 subjects |
| China, 24 mo | Duration of T1DM: < 6 mo | Control group: normal saline | Insulin requirement: 8 patients more than 50% reduction |
| C-peptide: ≥ 0.3 ng/mL | HbA1C: Significantly decreased; FCP: Significantly increased | ||
| Adverse effects: No |
Hu et al[99] studied the long-term effects of Wharton’s jelly-derived MSC in newly diagnosed T1DM patients. Group 1 was treated with parenteral solution of Wharton’s jelly-derived MSCs by intravenous delivery, while the control group received normal saline. In the treatment group HbA1C reached its lowest value after half a year and then began to fluctuate. Fasting C-peptide showed a progressive increase, reaching its maximum after 1 year; 3/15 patients were insulin-free, and 8 had their insulin dose halved after 2 years. As the study follow-up period lasted 2 years, exceeding the average 1.5 year honeymoon period, the therapeutic effect was due to MSCs[99]. This was one of the first studies to prove the safety and effectiveness of MSCs.
Thakkar et al[100,101] used the combination of adipose-derived insulin-secreting mesenchymal stem cells and bone marrow-derived (BM-) hematopoietic stem cells, comparing autologous (group 1) and allogeneic (group 2) stem cells. The study procedure was as follows: Resection of adipose tissue from the abdominal wall, collected in proliferation medium, bone marrow aspiration, conditioning treatment with bortezomib, methylprednisolone, ATG, and finally injection of the mixed inoculum. Autologous stem cell therapy offered better long-term control of hyperglycemia, but the two groups fairly differed in baseline mean C-peptide levels[100]. Although the two treatment methods showed significant differences in carbohydrate metabolism, the results before and after stem cell therapy were not statistically analyzed within the groups, thus lacking conclusive information about the efficacy of MSCs. As an early-result, the group reported preliminary data of 10 patients[101]. After an approximately 3-year follow-up, increased C-peptide secretion, decreased exogenous insulin requirement, improved HbA1C and significantly lower GADA levels have been found.
Similar results were obtained in the studies of Araujo et al[102] and Dantas et al[103], where a single dose of adipose-derived MSC infusion were combined with daily 2000 IU vitamin D3 supplementation. Compared to control subjects on traditional treatment, improved HbA1C levels and reduced insulin doses have been found 3-mo after MSC infusion[102], while basal C-peptide levels remained the same at first but significantly improved for the 6-mo follow-up measurement[102,103]. It has to be mentioned though that most patients reported transient headache and local reactions, and further mild but resolving adverse events were also reported by a significant amount of the study population. Although the results of this study further strengthened the efficacy and safety of adipose-derived MSCs, all positive effects were somewhat overshadowed by the significant number of adverse events.
Cai et al[104] investigated the safety and efficacy of umbilical cord-derived MSC (UC-MSC) and autologous BM-mononuclear cell cotransplantation in adult patients. The treatment group received octreotide as a prophylaxis, followed by stem cell infusion into the dorsal pancreatic artery. After 1 year, the C-peptide area under the curve during a 3-h oral glucose tolerance test increased by 105.7% in 20 of 21 responders compared to a 7.7% decrease in the control group showing the robust effect of the treatment against disease progression. Further importance of this trial was that immunological parameters were also assessed. GADA positivity remained unchanged, while IL-10 levels increased, and interferon-γ levels and adenosine triphosphate levels in CD4+ T cells decreased. While the effect of MSCs may be less pronounced in this study due to reduced inflammatory signals in long-standing disease[104], it has to be mentioned that the long-term follow-up analysis of the study population have shown a significantly decreased occurrence of various diabetic complications. Furthermore, the UC-MSC treated patients still had clinically better HbA1C and C-peptide levels, 8 years after the UC-MSC treatment, but the initial difference in insulin requirement leveled off[105]. The combined results of the original and follow-up study[104,105] indicate that UC-MSCs are good candidates for slowing down the progression of T1DM.
Lu et al[106] assessed the repeated transplantation of allogeneic UC-MSC in T1DM. The primary efficacy endpoint was clinical remission, defined as a 10% increase from baseline in the levels of fasting and/or postprandial C-peptide. After 1 year, 11 out of 27 in the UC-MSC-treated group maintained clinical remission, whereas only 3 out of 26 in the control group maintained clinical remission. The UC-MSC-treated group showed a decreasing trend in fasting and postprandial C-peptide. Three UC-MSC-treated adults became insulin independent and started using insulin again in 3-12 mo. Among adult-onset T1DM (≥ 18 years of age), UC-MSC treatment showed a protective effect on β-cell function but failed to be protective in juveniles. Three recipients had mild fever after UC-MSC infusion; all of them recovered within 24 h[106]. It seems UC-MSC therapy might be more beneficial for patients with adult-onset T1DM.
Carlsson et al[107] tested the efficacy of autologous BM-MSCs in newly diagnosed T1DM patients. Stems cells were harvested from the aspiration of the iliac crest and subsequently administered to the MSC-treated group as an intravenous infusion without premedication. HbA1C, fasting C-peptide and insulin requirement were not significantly different compared to the control group[107]. In contrast, Izadi et al[108] found improved HbA1C and C-peptide levels, a reduced number of hypoglycemic events and increased anti-inflammatory patterns. Furthermore, early BM-MSC transplantation (< 1 year after disease onset) further improved HbA1C levels and C-peptide levels compared to those who received the transplantation > 1 year after disease onset[108], similar to that of UC-MSC.
Summarizing the available clinical study results of the stem cell therapies, the results about BM-MSC and adipose-derived MSC are more controversial, suggesting that these two therapies may be less effective than UC-MSC therapy in T1DM. It should be noted, however, that based on the results of the studies so far it is recommended to apply these treatments as early as possible. The earlier these treatments are introduced, the greater the preservation of the remaining β cells, thereby the reduction of external insulin requirement and the development of long-term complications can be elongated. Adipose-derived MSCs and UC-MSCs are currently under further investigated in NCT05308836[109] and NCT04061746[110], respectively.
In the management of T1DM the focus remains on the challenges of glycemic control and long-term complications, which could not been fully overcome by new technological advances. Recently, there has been a paradigm change in the treatment of T1DM. The goal now is to cure rather than identify a lifelong ‘symptomatic treatment’ with insulin supplementation. The crucial future may lie in disease-modifying therapeutic options, which could be used to preserve β cells in the presymptomatic phase of the disease and to cease the destructive autoimmune process as well as to regenerate β-cell function in the clinical phase.
Immunotherapy appears to be a promising disease-modifying therapy in T1DM. Different agents have the potential to target the major autoreactive immune pathways leading to T1DM. Therapies interfering with T cell activation seem to be the most favorable. Regenerative therapy is developing parallel with immunotherapy. MSC therapy stands out from other cell therapies. It is safe, with its beneficial effects due to immune regulation. However, the clinical trials are limited in their conclusions due to the small patient numbers and short follow-up times. Standardized stem cell processing, transplantation protocols and dosage will be essential for future randomized, double-blinded clinical trials with large patient cohorts. Combining disease-modifying therapies with glucagon like peptide-1 analogues seem to increase efficacy and increase tolerability of interventions.
So far, neither immunotherapies nor stem cell therapies, when used alone, have had ultimate successes in altering T1DM disease course. Their common disadvantage is that their short-term therapy effects are transient. The future for disease-modifying therapies might be the individualized, long-term multimodal approach combining immune, incretin based and regenerative therapeutic options potentially by identifying biomarkers of responders for it to be used in routine clinical treatment.
We are grateful to Professor Lindner E (Memphis University) for English proofreading.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Hungary
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feng JF, China; He Z, China; Long P, China; Nong X, China S-Editor: Wu YXJ L-Editor: Filipodia P-Editor: Wu YXJ
| 1. | Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, Winkler C, Ilonen J, Veijola R, Knip M, Bonifacio E, Eisenbarth GS. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309:2473-2479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 735] [Cited by in F6Publishing: 752] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 2. | Eringsmark Regnéll S, Lernmark A. The environment and the origins of islet autoimmunity and Type 1 diabetes. Diabet Med. 2013;30:155-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Craig ME, Nair S, Stein H, Rawlinson WD. Viruses and type 1 diabetes: a new look at an old story. Pediatr Diabetes. 2013;14:149-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Stene LC, Gale EA. The prenatal environment and type 1 diabetes. Diabetologia. 2013;56:1888-1897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Bettini ML, Bettini M. Understanding Autoimmune Diabetes through the Prism of the Tri-Molecular Complex. Front Endocrinol (Lausanne). 2017;8:351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Waldron-Lynch F, Herold KC. Immunomodulatory therapy to preserve pancreatic β-cell function in type 1 diabetes. Nat Rev Drug Discov. 2011;10:439-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Hermann-Kleiter N, Baier G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood. 2010;115:2989-2997. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 8. | Arif S, Moore F, Marks K, Bouckenooghe T, Dayan CM, Planas R, Vives-Pi M, Powrie J, Tree T, Marchetti P, Huang GC, Gurzov EN, Pujol-Borrell R, Eizirik DL, Peakman M. Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated β-cell death. Diabetes. 2011;60:2112-2119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 9. | American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36 Suppl 1:S67-S74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1255] [Cited by in F6Publishing: 1422] [Article Influence: 129.3] [Reference Citation Analysis (4)] |
| 10. | Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells. 2014;6:526-539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 293] [Cited by in F6Publishing: 279] [Article Influence: 27.9] [Reference Citation Analysis (1)] |
| 11. | Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431-440. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1584] [Cited by in F6Publishing: 1549] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 12. | Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, Greenbaum CJ, Herold KC, Krischer JP, Lernmark Å, Ratner RE, Rewers MJ, Schatz DA, Skyler JS, Sosenko JM, Ziegler AG. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38:1964-1974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 610] [Cited by in F6Publishing: 572] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 13. | Xv J, Ming Q, Wang X, Zhang W, Li Z, Wang S, Li Y, Li L. Mesenchymal stem cells moderate immune response of type 1 diabetes. Cell Tissue Res. 2017;368:239-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Mahon JL, Sosenko JM, Rafkin-Mervis L, Krause-Steinrauf H, Lachin JM, Thompson C, Bingley PJ, Bonifacio E, Palmer JP, Eisenbarth GS, Wolfsdorf J, Skyler JS; TrialNet Natural History Committee; Type 1 Diabetes TrialNet Study Group. The TrialNet Natural History Study of the Development of Type 1 Diabetes: objectives, design, and initial results. Pediatr Diabetes. 2009;10:97-104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 15. | Oram RA, Jones AG, Besser RE, Knight BA, Shields BM, Brown RJ, Hattersley AT, McDonald TJ. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57:187-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 196] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 16. | DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391:2449-2462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 595] [Cited by in F6Publishing: 725] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 17. | Shields BM, McDonald TJ, Oram R, Hill A, Hudson M, Leete P, Pearson ER, Richardson SJ, Morgan NG, Hattersley AT; TIGI Consortium. C-Peptide Decline in Type 1 Diabetes Has Two Phases: An Initial Exponential Fall and a Subsequent Stable Phase. Diabetes Care. 2018;41:1486-1492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 18. | Davis AK, DuBose SN, Haller MJ, Miller KM, DiMeglio LA, Bethin KE, Goland RS, Greenberg EM, Liljenquist DR, Ahmann AJ, Marcovina SM, Peters AL, Beck RW, Greenbaum CJ; T1D Exchange Clinic Network. Prevalence of detectable C-Peptide according to age at diagnosis and duration of type 1 diabetes. Diabetes Care. 2015;38:476-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 152] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 19. | Greenbaum C, VanBuecken D, Lord S. Disease-Modifying Therapies in Type 1 Diabetes: A Look into the Future of Diabetes Practice. Drugs. 2019;79:43-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Feutren G, Papoz L, Assan R, Vialettes B, Karsenty G, Vexiau P, Du Rostu H, Rodier M, Sirmai J, Lallemand A. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet. 1986;2:119-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 336] [Cited by in F6Publishing: 350] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Martin S, Schernthaner G, Nerup J, Gries FA, Koivisto VA, Dupré J, Standl E, Hamet P, McArthur R, Tan MH. Follow-up of cyclosporin A treatment in type 1 (insulin-dependent) diabetes mellitus: lack of long-term effects. Diabetologia. 1991;34:429-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Wherrett DK, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Herold KC, Marks JB, Monzavi R, Moran A, Orban T, Palmer JP, Raskin P, Rodriguez H, Schatz D, Wilson DM, Krischer JP, Skyler JS; Type 1 Diabetes TrialNet GAD Study Group. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet. 2011;378:319-327. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 268] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 23. | Provention Bio Inc. Provention Bio Receives Complete Response Letter (CRL) to Biologics License Application (BLA) for Teplizumab for the Delay of Clinical Type 1 Diabetes (T1D) in At-risk Individuals. Cited 10 May 2022. Available from: https://investors.proventionbio.com/2021-07-06-Provention-Bio-Receives-Complete-Response-Letter-CRL-to-Biologics-License-Application-BLA-for-Teplizumab-for-the-Delay-of-Clinical-Type-1-Diabetes-T1D-in-At-risk-Individuals. [Cited in This Article: ] |
| 24. | Mariño E, Silveira PA, Stolp J, Grey ST. B cell-directed therapies in type 1 diabetes. Trends Immunol. 2011;32:287-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Pescovitz MD, Greenbaum CJ, Krause-Steinrauf H, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, McGee PF, Moran AM, Raskin P, Rodriguez H, Schatz DA, Wherrett D, Wilson DM, Lachin JM, Skyler JS; Type 1 Diabetes TrialNet Anti-CD20 Study Group. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N Engl J Med. 2009;361:2143-2152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 745] [Cited by in F6Publishing: 735] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 26. | Pescovitz MD, Greenbaum CJ, Bundy B, Becker DJ, Gitelman SE, Goland R, Gottlieb PA, Marks JB, Moran A, Raskin P, Rodriguez H, Schatz DA, Wherrett DK, Wilson DM, Krischer JP, Skyler JS; Type 1 Diabetes TrialNet Anti-CD20 Study Group. B-lymphocyte depletion with rituximab and β-cell function: two-year results. Diabetes Care. 2014;37:453-459. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 168] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 27. | Yu L, Herold K, Krause-Steinrauf H, McGee PL, Bundy B, Pugliese A, Krischer J, Eisenbarth GS; Type 1 Diabetes TrialNet Anti-CD20 Study Group. Rituximab selectively suppresses specific islet antibodies. Diabetes. 2011;60:2560-2565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Herold KC, Pescovitz MD, McGee P, Krause-Steinrauf H, Spain LM, Bourcier K, Asare A, Liu Z, Lachin JM, Dosch HM; Type 1 Diabetes TrialNet Anti-CD20 Study Group. Increased T cell proliferative responses to islet antigens identify clinical responders to anti-CD20 monoclonal antibody (rituximab) therapy in type 1 diabetes. J Immunol. 2011;187:1998-2005. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Zieliński M, Żalińska M, Iwaszkiewicz-Grześ D, Gliwiński M, Hennig M, Jaźwińska-Curyłło A, Kamińska H, Sakowska J, Wołoszyn-Durkiewicz A, Owczuk R, Młynarski W, Jarosz-Chobot P, Bossowski A, Szadkowska A, Siebert J, Myśliwiec M, Marek-Trzonkowska N, Trzonkowski P. Combined therapy with CD4+ CD25highCD127- T regulatory cells and anti-CD20 antibody in recent-onset type 1 diabetes is superior to monotherapy: Randomized phase I/II trial. Diabetes Obes Metab. 2022;24:1534-1543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Linsley PS, Greenbaum CJ, Rosasco M, Presnell S, Herold KC, Dufort MJ. Elevated T cell levels in peripheral blood predict poor clinical response following rituximab treatment in new-onset type 1 diabetes. Genes Immun. 2019;20:293-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Kroll JL, Beam C, Li S, Viscidi R, Dighero B, Cho A, Boulware D, Pescovitz M, Weinberg A; Type 1 Diabetes TrialNet Anti CD-20 Study Group. Reactivation of latent viruses in individuals receiving rituximab for new onset type 1 diabetes. J Clin Virol. 2013;57:115-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | National Institute of Diabetes and Digestive and Kidney Diseases. Rituximab and Abatacept for Prevention or Reversal of Type 1 Diabetes (TN25). [accessed 05 August 2022]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03929601 ClinicalTrials.gov Identifier: NCT03929601. [Cited in This Article: ] |
| 33. | Orban T, Bundy B, Becker DJ, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, Moran A, Raskin P, Rodriguez H, Russell WE, Schatz D, Wherrett D, Wilson DM, Krischer JP, Skyler JS; Type 1 Diabetes TrialNet Abatacept Study Group. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;378:412-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 460] [Cited by in F6Publishing: 411] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 34. | Orban T, Bundy B, Becker DJ, Dimeglio LA, Gitelman SE, Goland R, Gottlieb PA, Greenbaum CJ, Marks JB, Monzavi R, Moran A, Peakman M, Raskin P, Russell WE, Schatz D, Wherrett DK, Wilson DM, Krischer JP, Skyler JS; Type 1 Diabetes TrialNet Abatacept Study Group. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care. 2014;37:1069-1075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 35. | Weinberg A, Boulware D, Dighero B, Orban T; Type 1 Diabetes TrialNet Abatacept Study Group. Effect of abatacept on immunogenicity of vaccines in individuals with type 1 diabetes. Vaccine. 2013;31:4791-4794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | Edner NM, Heuts F, Thomas N, Wang CJ, Petersone L, Kenefeck R, Kogimtzis A, Ovcinnikovs V, Ross EM, Ntavli E, Elfaki Y, Eichmann M, Baptista R, Ambery P, Jermutus L, Peakman M, Rosenthal M, Walker LSK. Follicular helper T cell profiles predict response to costimulation blockade in type 1 diabetes. Nat Immunol. 2020;21:1244-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 37. | Orban T, Beam CA, Xu P, Moore K, Jiang Q, Deng J, Muller S, Gottlieb P, Spain L, Peakman M; Type 1 Diabetes TrialNet Abatacept Study Group. Reduction in CD4 central memory T-cell subset in costimulation modulator abatacept-treated patients with recent-onset type 1 diabetes is associated with slower C-peptide decline. Diabetes. 2014;63:3449-3457. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 38. | Greenbaum C. Early Markers of Disease and Response to Therapy. [accessed 05 August 2022]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04118153 ClinicalTrials.gov Identifier: NCT04118153. [Cited in This Article: ] |
| 39. | Alegre ML, Peterson LJ, Xu D, Sattar HA, Jeyarajah DR, Kowalkowski K, Thistlethwaite JR, Zivin RA, Jolliffe L, Bluestone JA. A non-activating "humanized" anti-CD3 monoclonal antibody retains immunosuppressive properties in vivo. Transplantation. 1994;57:1537-1543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 132] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A. 1994;91:123-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 463] [Cited by in F6Publishing: 467] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 41. | Kuhn C, You S, Valette F, Hale G, van Endert P, Bach JF, Waldmann H, Chatenoud L. Human CD3 transgenic mice: preclinical testing of antibodies promoting immune tolerance. Sci Transl Med. 2011;3:68ra10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Herold KC, Gitelman SE, Masharani U, Hagopian W, Bisikirska B, Donaldson D, Rother K, Diamond B, Harlan DM, Bluestone JA. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763-1769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 488] [Cited by in F6Publishing: 459] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 43. | Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med. 2002;346:1692-1698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 900] [Cited by in F6Publishing: 853] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 44. | Herold KC, Gitelman S, Greenbaum C, Puck J, Hagopian W, Gottlieb P, Sayre P, Bianchine P, Wong E, Seyfert-Margolis V, Bourcier K, Bluestone JA; Immune Tolerance Network ITN007AI Study Group. Treatment of patients with new onset Type 1 diabetes with a single course of anti-CD3 mAb Teplizumab preserves insulin production for up to 5 years. Clin Immunol. 2009;132:166-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 45. | Herold KC, Gitelman SE, Willi SM, Gottlieb PA, Waldron-Lynch F, Devine L, Sherr J, Rosenthal SM, Adi S, Jalaludin MY, Michels AW, Dziura J, Bluestone JA. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia. 2013;56:391-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 46. | Lebastchi J, Deng S, Lebastchi AH, Beshar I, Gitelman S, Willi S, Gottlieb P, Akirav EM, Bluestone JA, Herold KC. Immune therapy and β-cell death in type 1 diabetes. Diabetes. 2013;62:1676-1680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 47. | Herold KC, Gitelman SE, Ehlers MR, Gottlieb PA, Greenbaum CJ, Hagopian W, Boyle KD, Keyes-Elstein L, Aggarwal S, Phippard D, Sayre PH, McNamara J, Bluestone JA; AbATE Study Team. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766-3774. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 241] [Cited by in F6Publishing: 258] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 48. | Sherry N, Hagopian W, Ludvigsson J, Jain SM, Wahlen J, Ferry RJ Jr, Bode B, Aronoff S, Holland C, Carlin D, King KL, Wilder RL, Pillemer S, Bonvini E, Johnson S, Stein KE, Koenig S, Herold KC, Daifotis AG; Protégé Trial Investigators. Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo-controlled trial. Lancet. 2011;378:487-497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 343] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 49. | Hagopian W, Ferry RJ Jr, Sherry N, Carlin D, Bonvini E, Johnson S, Stein KE, Koenig S, Daifotis AG, Herold KC, Ludvigsson J; Protégé Trial Investigators. Teplizumab preserves C-peptide in recent-onset type 1 diabetes: two-year results from the randomized, placebo-controlled Protégé trial. Diabetes. 2013;62:3901-3908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 50. | Herold KC, Bundy BN, Long SA, Bluestone JA, DiMeglio LA, Dufort MJ, Gitelman SE, Gottlieb PA, Krischer JP, Linsley PS, Marks JB, Moore W, Moran A, Rodriguez H, Russell WE, Schatz D, Skyler JS, Tsalikian E, Wherrett DK, Ziegler AG, Greenbaum CJ; Type 1 Diabetes TrialNet Study Group. An Anti-CD3 Antibody, Teplizumab, in Relatives at Risk for Type 1 Diabetes. N Engl J Med. 2019;381:603-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 513] [Article Influence: 102.6] [Reference Citation Analysis (0)] |
| 51. | Perdigoto AL, Preston-Hurlburt P, Clark P, Long SA, Linsley PS, Harris KM, Gitelman SE, Greenbaum CJ, Gottlieb PA, Hagopian W, Woodwyk A, Dziura J, Herold KC; Immune Tolerance Network. Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis. Diabetologia. 2019;62:655-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 52. | Tooley JE, Vudattu N, Choi J, Cotsapas C, Devine L, Raddassi K, Ehlers MR, McNamara JG, Harris KM, Kanaparthi S, Phippard D, Herold KC. Changes in T-cell subsets identify responders to FcR-nonbinding anti-CD3 mAb (teplizumab) in patients with type 1 diabetes. Eur J Immunol. 2016;46:230-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Provention Bio Inc. At-Risk for Type 1 Diabetes Extension Study. [accessed 05 August 2022]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04270942 ClinicalTrials.gov Identifier: NCT04270942. [Cited in This Article: ] |
| 54. | Provention Bio Inc. Recent-Onset Type 1 Diabetes Trial Evaluating Efficacy and Safety of Teplizumab (PROTECT). [accessed 05 August 2022]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT03875729 ClinicalTrials.gov Identifier: NCT03875729. [Cited in This Article: ] |
| 55. | Provention Bio Inc. Recent-Onset Type 1 Diabetes Extension Study Evaluating the Long-Term Safety of Teplizumab. [accessed 05 August 2022]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04598893 ClinicalTrials.gov Identifier: NCT04598893. [Cited in This Article: ] |
| 56. | Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Engl J Med. 2005;352:2598-2608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 855] [Cited by in F6Publishing: 807] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 57. | Keymeulen B, van Maurik A, Inman D, Oliveira J, McLaughlin R, Gittelman RM, Roep BO, Gillard P, Hilbrands R, Gorus F, Mathieu C, Van de Velde U, Wisniacki N, Napolitano A. A randomised, single-blind, placebo-controlled, dose-finding safety and tolerability study of the anti-CD3 monoclonal antibody otelixizumab in new-onset type 1 diabetes. Diabetologia. 2021;64:313-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 58. | Vlasakakis G, Napolitano A, Barnard R, Brown K, Bullman J, Inman D, Keymeulen B, Lanham D, Leirens Q, MacDonald A, Mezzalana E, Page K, Patel M, Savage CO, Zamuner S, van Maurik A. Target engagement and cellular fate of otelixizumab: a repeat dose escalation study of an anti-CD3ε mAb in new-onset type 1 diabetes mellitus patients. Br J Clin Pharmacol. 2019;85:704-714. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Aronson R, Gottlieb PA, Christiansen JS, Donner TW, Bosi E, Bode BW, Pozzilli P; DEFEND Investigator Group. Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes Care. 2014;37:2746-2754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 60. | Ambery P, Donner TW, Biswas N, Donaldson J, Parkin J, Dayan CM. Efficacy and safety of low-dose otelixizumab anti-CD3 monoclonal antibody in preserving C-peptide secretion in adolescent type 1 diabetes: DEFEND-2, a randomized, placebo-controlled, double-blind, multi-centre study. Diabet Med. 2014;31:399-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 61. | Demeester S, Keymeulen B, Kaufman L, Van Dalem A, Balti EV, Van de Velde U, Goubert P, Verhaeghen K, Davidson HW, Wenzlau JM, Weets I, Pipeleers DG, Gorus FK. Preexisting insulin autoantibodies predict efficacy of otelixizumab in preserving residual β-cell function in recent-onset type 1 diabetes. Diabetes Care. 2015;38:644-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Keymeulen B, Candon S, Fafi-Kremer S, Ziegler A, Leruez-Ville M, Mathieu C, Vandemeulebroucke E, Walter M, Crenier L, Thervet E, Legendre C, Pierard D, Hale G, Waldmann H, Bach JF, Seigneurin JM, Pipeleers D, Chatenoud L. Transient Epstein-Barr virus reactivation in CD3 monoclonal antibody-treated patients. Blood. 2010;115:1145-1155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 63. | MacDonald A, Ambery P, Donaldson J, Hicks K, Keymeulen B, Parkin J. Subcutaneous Administration of Otelixizumab is Limited by Injection Site Reactions: Results of an Exploratory Study in Type 1 Diabetes Mellitus Patients. Exp Clin Endocrinol Diabetes. 2016;124:288-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Gitelman SE, Gottlieb PA, Rigby MR, Felner EI, Willi SM, Fisher LK, Moran A, Gottschalk M, Moore WV, Pinckney A, Keyes-Elstein L, Aggarwal S, Phippard D, Sayre PH, Ding L, Bluestone JA, Ehlers MR; START Study Team. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2013;1:306-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 65. | Gitelman SE, Gottlieb PA, Felner EI, Willi SM, Fisher LK, Moran A, Gottschalk M, Moore WV, Pinckney A, Keyes-Elstein L, Harris KM, Kanaparthi S, Phippard D, Ding L, Bluestone JA, Ehlers MR; ITN START Study Team. Antithymocyte globulin therapy for patients with recent-onset type 1 diabetes: 2 year results of a randomised trial. Diabetologia. 2016;59:1153-1161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 66. | Haller MJ, Gitelman SE, Gottlieb PA, Michels AW, Rosenthal SM, Shuster JJ, Zou B, Brusko TM, Hulme MA, Wasserfall CH, Mathews CE, Atkinson MA, Schatz DA. Anti-thymocyte globulin/G-CSF treatment preserves β cell function in patients with established type 1 diabetes. J Clin Invest. 2015;125:448-455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 67. | Haller MJ, Gitelman SE, Gottlieb PA, Michels AW, Perry DJ, Schultz AR, Hulme MA, Shuster JJ, Zou B, Wasserfall CH, Posgai AL, Mathews CE, Brusko TM, Atkinson MA, Schatz DA. Antithymocyte Globulin Plus G-CSF Combination Therapy Leads to Sustained Immunomodulatory and Metabolic Effects in a Subset of Responders With Established Type 1 Diabetes. Diabetes. 2016;65:3765-3775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 68. | Haller MJ, Schatz DA, Skyler JS, Krischer JP, Bundy BN, Miller JL, Atkinson MA, Becker DJ, Baidal D, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Herold KC, Marks JB, Moran A, Rodriguez H, Russell W, Wilson DM, Greenbaum CJ; Type 1 Diabetes TrialNet ATG-GCSF Study Group. Low-Dose Anti-Thymocyte Globulin (ATG) Preserves β-Cell Function and Improves HbA1c in New-Onset Type 1 Diabetes. Diabetes Care. 2018;41:1917-1925. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 69. | Haller MJ, Long SA, Blanchfield JL, Schatz DA, Skyler JS, Krischer JP, Bundy BN, Geyer SM, Warnock MV, Miller JL, Atkinson MA, Becker DJ, Baidal DA, DiMeglio LA, Gitelman SE, Goland R, Gottlieb PA, Herold KC, Marks JB, Moran A, Rodriguez H, Russell WE, Wilson DM, Greenbaum CJ; Type 1 Diabetes TrialNet ATG-GCSF Study Group. Low-Dose Anti-Thymocyte Globulin Preserves C-Peptide, Reduces HbA1c, and Increases Regulatory to Conventional T-Cell Ratios in New-Onset Type 1 Diabetes: Two-Year Clinical Trial Data. Diabetes. 2019;68:1267-1276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 70. | National Institute of Diabetes and Digestive and Kidney Diseases. Low Dose Antithymocyte Globulin (ATG) to Delay or Prevent Progression to Stage 3 T1D (TN28). [accessed 05 August 2022]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04291703 ClinicalTrials.gov Identifier: NCT04291703. [Cited in This Article: ] |
| 71. | Mathieu C. MELD-ATG: Phase II, Dose Ranging, Efficacy Study of Anti-thymocyte Globulin (ATG) Within 6 Weeks of Diagnosis of Type 1 Diabetes (T1D). [accessed 05 August 2022]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04509791 ClinicalTrials.gov Identifier: NCT04509791. [Cited in This Article: ] |
| 72. | Ferreira RC, Simons HZ, Thompson WS, Cutler AJ, Dopico XC, Smyth DJ, Mashar M, Schuilenburg H, Walker NM, Dunger DB, Wallace C, Todd JA, Wicker LS, Pekalski ML. IL-21 production by CD4+ effector T cells and frequency of circulating follicular helper T cells are increased in type 1 diabetes patients. Diabetologia. 2015;58:781-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 73. | Van Belle TL, Nierkens S, Arens R, von Herrath MG. Interleukin-21 receptor-mediated signals control autoreactive T cell infiltration in pancreatic islets. Immunity. 2012;36:1060-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 74. | Wang W, Wu RD, Chen P, Xu XJ, Shi XZ, Huang LH, Shao ZL, Guo W. Liraglutide combined with human umbilical cord mesenchymal stem cell transplantation inhibits beta-cell apoptosis via mediating the ASK1/JNK/BAX pathway in rats with type 2 diabetes. Diabetes Metab Res Rev. 2020;36:e3212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 75. | Wang L, Liu Y, Yang J, Zhao H, Ke J, Tian Q, Zhang L, Wen J, Wei R, Hong T. GLP-1 analog liraglutide enhances proinsulin processing in pancreatic β-cells via a PKA-dependent pathway. Endocrinology. 2014;155:3817-3828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 76. | Rondas D, Bugliani M, D'Hertog W, Lage K, Masini M, Waelkens E, Marchetti P, Mathieu C, Overbergh L. Glucagon-like peptide-1 protects human islets against cytokine-mediated β-cell dysfunction and death: a proteomic study of the pathways involved. J Proteome Res. 2013;12:4193-4206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 77. | von Herrath M, Bain SC, Bode B, Clausen JO, Coppieters K, Gaysina L, Gumprecht J, Hansen TK, Mathieu C, Morales C, Mosenzon O, Segel S, Tsoukas G, Pieber TR; Anti-IL-21–liraglutide Study Group investigators and contributors. Anti-interleukin-21 antibody and liraglutide for the preservation of β-cell function in adults with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2021;9:212-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 78. | Nikoonezhad M, Lasemi MV, Alamdari S, Mohammadian M, Tabarraee M, Ghadyani M, Hamidpour M, Roshandel E. Treatment of insulin-dependent diabetes by hematopoietic stem cell transplantation. Transpl Immunol. 2022;101682. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 79. | Schu S, Nosov M, O'Flynn L, Shaw G, Treacy O, Barry F, Murphy M, O'Brien T, Ritter T. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med. 2012;16:2094-2103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 80. | Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 841] [Cited by in F6Publishing: 937] [Article Influence: 104.1] [Reference Citation Analysis (0)] |
| 81. | Wu LF, Wang NN, Liu YS, Wei X. Differentiation of Wharton's jelly primitive stromal cells into insulin-producing cells in comparison with bone marrow mesenchymal stem cells. Tissue Eng Part A. 2009;15:2865-2873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 82. | Lee AS, Tang C, Rao MS, Weissman IL, Wu JC. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat Med. 2013;19:998-1004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 426] [Cited by in F6Publishing: 460] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 83. | Neri S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int J Mol Sci. 2019;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 84. | Liu S, Ginestier C, Ou SJ, Clouthier SG, Patel SH, Monville F, Korkaya H, Heath A, Dutcher J, Kleer CG, Jung Y, Dontu G, Taichman R, Wicha MS. Breast cancer stem cells are regulated by mesenchymal stem cells through cytokine networks. Cancer Res. 2011;71:614-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 439] [Cited by in F6Publishing: 479] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 85. | Tsai KS, Yang SH, Lei YP, Tsai CC, Chen HW, Hsu CY, Chen LL, Wang HW, Miller SA, Chiou SH, Hung MC, Hung SC. Mesenchymal stem cells promote formation of colorectal tumors in mice. Gastroenterology. 2011;141:1046-1056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 86. | Kamal MM, Kassem DH. Therapeutic Potential of Wharton's Jelly Mesenchymal Stem Cells for Diabetes: Achievements and Challenges. Front Cell Dev Biol. 2020;8:16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 30] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 87. | Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, Cooper DR, Sanberg PR. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1157] [Cited by in F6Publishing: 1212] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 88. | Chen LB, Jiang XB, Yang L. Differentiation of rat marrow mesenchymal stem cells into pancreatic islet beta-cells. World J Gastroenterol. 2004;10:3016-3020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 207] [Cited by in F6Publishing: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 89. | Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16:203-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 438] [Cited by in F6Publishing: 448] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 90. | Wang J, Liao L, Tan J. Mesenchymal-stem-cell-based experimental and clinical trials: current status and open questions. Expert Opin Biol Ther. 2011;11:893-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 91. | Galderisi U, Giordano A. The gap between the physiological and therapeutic roles of mesenchymal stem cells. Med Res Rev. 2014;34:1100-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 92. | Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 798] [Cited by in F6Publishing: 887] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 93. | Li FR, Wang XG, Deng CY, Qi H, Ren LL, Zhou HX. Immune modulation of co-transplantation mesenchymal stem cells with islet on T and dendritic cells. Clin Exp Immunol. 2010;161:357-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 47] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 94. | Ghannam S, Pène J, Moquet-Torcy G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 412] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 95. | Rasmusson I, Uhlin M, Le Blanc K, Levitsky V. Mesenchymal stem cells fail to trigger effector functions of cytotoxic T lymphocytes. J Leukoc Biol. 2007;82:887-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 117] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 96. | Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 903] [Cited by in F6Publishing: 971] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 97. | Luz-Crawford P, Kurte M, Bravo-Alegría J, Contreras R, Nova-Lamperti E, Tejedor G, Noël D, Jorgensen C, Figueroa F, Djouad F, Carrión F. Mesenchymal stem cells generate a CD4+CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 286] [Cited by in F6Publishing: 321] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 98. | Päth G, Perakakis N, Mantzoros CS, Seufert J. Stem cells in the treatment of diabetes mellitus - Focus on mesenchymal stem cells. Metabolism. 2019;90:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 99. | Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H, Chen Y, Zhao W, Jia Z, Yan S, Wang Y. Long term effects of the implantation of Wharton's jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr J. 2013;60:347-357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 144] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 100. | Thakkar UG, Trivedi HL, Vanikar AV, Dave SD. Insulin-secreting adipose-derived mesenchymal stromal cells with bone marrow-derived hematopoietic stem cells from autologous and allogenic sources for type 1 diabetes mellitus. Cytotherapy. 2015;17:940-947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 101. | Dave SD, Vanikar AV, Trivedi HL, Thakkar UG, Gopal SC, Chandra T. Novel therapy for insulin-dependent diabetes mellitus: infusion of in vitro-generated insulin-secreting cells. Clin Exp Med. 2015;15:41-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 102. | Araujo DB, Dantas JR, Silva KR, Souto DL, Pereira MFC, Moreira JP, Luiz RR, Claudio-Da-Silva CS, Gabbay MAL, Dib SA, Couri CEB, Maiolino A, Rebelatto CLK, Daga DR, Senegaglia AC, Brofman PRS, Baptista LS, Oliveira JEP, Zajdenverg L, Rodacki M. Allogenic Adipose Tissue-Derived Stromal/Stem Cells and Vitamin D Supplementation in Patients With Recent-Onset Type 1 Diabetes Mellitus: A 3-Month Follow-Up Pilot Study. Front Immunol. 2020;11:993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 103. | Dantas JR, Araújo DB, Silva KR, Souto DL, de Fátima Carvalho Pereira M, Luiz RR, Dos Santos Mantuano M, Claudio-da-Silva C, Gabbay MAL, Dib SA, Couri CEB, Maiolino A, Rebelatto CLK, Daga DR, Senegaglia AC, Brofman PRS, Baptista LS, de Oliveira JEP, Zajdenverg L, Rodacki M. Adipose tissue-derived stromal/stem cells + cholecalciferol: a pilot study in recent-onset type 1 diabetes patients. Arch Endocrinol Metab. 2021;65:342-351. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 104. | Cai J, Wu Z, Xu X, Liao L, Chen J, Huang L, Wu W, Luo F, Wu C, Pugliese A, Pileggi A, Ricordi C, Tan J. Umbilical Cord Mesenchymal Stromal Cell With Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes: A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. Diabetes Care. 2016;39:149-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 105. | Wu Z, Xu X, Cai J, Chen J, Huang L, Wu W, Pugliese A, Li S, Ricordi C, Tan J. Prevention of chronic diabetic complications in type 1 diabetes by co-transplantation of umbilical cord mesenchymal stromal cells and autologous bone marrow: a pilot randomized controlled open-label clinical study with 8-year follow-up. Cytotherapy. 2022;24:421-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 106. | Lu J, Shen SM, Ling Q, Wang B, Li LR, Zhang W, Qu DD, Bi Y, Zhu DL. One repeated transplantation of allogeneic umbilical cord mesenchymal stromal cells in type 1 diabetes: an open parallel controlled clinical study. Stem Cell Res Ther. 2021;12:340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 107. | Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64:587-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 108. | Izadi M, Sadr Hashemi Nejad A, Moazenchi M, Masoumi S, Rabbani A, Kompani F, Hedayati Asl AA, Abbasi Kakroodi F, Jaroughi N, Mohseni Meybodi MA, Setoodeh A, Abbasi F, Hosseini SE, Moeini Nia F, Salman Yazdi R, Navabi R, Hajizadeh-Saffar E, Baharvand H. Mesenchymal stem cell transplantation in newly diagnosed type-1 diabetes patients: a phase I/II randomized placebo-controlled clinical trial. Stem Cell Res Ther. 2022;13:264. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 109. | Vinmec Research Institute of Stem Cell and Gene Technology. Evaluate Safety of Adipose Derived Mesenchymal Stem Cell Transplantation for Type 1 Diabetes Treatment. [accessed 05 August 2022]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT05308836 Cited: ClinicalTrials.gov Identifier: NCT05308836. [Cited in This Article: ] |
| 110. | National Institute of Diabetes and Digestive and Kidney Diseases. Cellular Therapy for Type 1 Diabetes Using Mesenchymal Stem Cells. [accessed 05 August 2022]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04061746 ClinicalTrials.gov Identifier: NCT04061746. [Cited in This Article: ] |