Published online Aug 15, 2017. doi: 10.4251/wjgo.v9.i8.327
Peer-review started: December 20, 2016
First decision: January 18, 2017
Revised: April 24, 2017
Accepted: May 18, 2017
Article in press: May 19, 2017
Published online: August 15, 2017
To investigate predictive markers for metachronous and synchronous gastric cancer (GC), which can develop after endoscopic submucosal dissection (ESD).
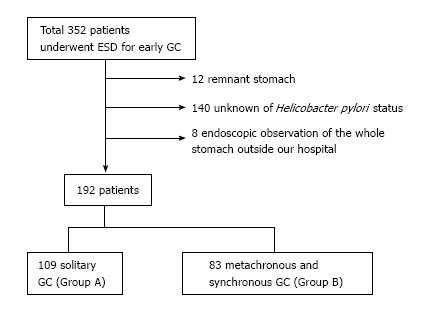
A total of 352 patients underwent ESD for early GC at NTT West Osaka Hospital between June 2006 and February 2016. Exclusion criteria were as follows: Remnant stomach, unknown Helicobacter pylori status, and endoscopic observation of the whole stomach outside our hospital. We analyzed data from 192 patients comprising 109 patients with solitary GC (Group A) and 83 with metachronous and synchronous GC (Group B). We retrospectively investigated the clinicopathological and endoscopic characteristics, and endoscopic risk score as predictive markers for GC.
The median age of Group B [72 years (interquartile range 63-78)] was significantly higher than that of Group A [66 years (interquartile range 61-74), respectively, P = 0.0009]. The prevalence of intestinal metaplasia in Group B tended to be higher than that in Group A (57.8% vs 45.0%, P = 0.08). The prevalence of gastric xanthoma (GX) in Group B was significantly higher than that in Group A (54.2% vs 32.1%, P = 0.003). The atrophy score in Group B was significantly higher than that in Group A (P = 0.005). Multivariate analysis revealed that higher age and the presence of GX were independently related to metachronous and synchronous GC [OR = 1.04 (1.01-1.08), P = 0.02; and OR = 2.11 (1.14-3.99), P = 0.02, respectively].
The presence of GX is a useful predictive marker for metachronous and synchronous GC.
Core tip: This was a retrospective observational study to identify predictive markers for metachronous and synchronous gastric cancer (GC). Multivariate analysis revealed that higher age and the presence of gastric xanthoma were independently related to the development of metachronous and synchronous GC. Additional large prospective studies are necessary to investigate this important issue further.
- Citation: Shibukawa N, Ouchi S, Wakamatsu S, Wakahara Y, Kaneko A. Gastric xanthoma is a predictive marker for metachronous and synchronous gastric cancer. World J Gastrointest Oncol 2017; 9(8): 327-332
- URL: https://www.wjgnet.com/1948-5204/full/v9/i8/327.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v9.i8.327
Endoscopic submucosal dissection (ESD) is currently accepted as an effective and minimally invasive treatment for early gastric cancer (GC)[1-7]. However, the development of metachronous and synchronous GC remains a possibility after ESD[8-14]. Predictive markers for the development of metachronous and synchronous GC have not been extensively studied. In addition, it is often difficult to clearly distinguish metachronous GC from synchronous GC because of missed detection of synchronous GC.
The cumulative incidence of metachronous GC after endoscopic resection for early GC ranges from 5.2% to 22.7%[8-14]. The incidence of missed detection of synchronous GC was reported to be between 1.2% and 7.7%[15,16]. In 2015, the Kyoto global consensus report on Helicobacter pylori (H. pylori) gastritis was published[17]. At the same time, an endoscopic score for GC risk was also announced[18]. Also, gastric xanthoma (GX) has been reported as the predictive marker of early GC[19,20].
The usefulness of endoscopic score for GC risk as an accurate predictor of GC risk remains unclear. And the utility of GX as a predictive marker of metachronous and synchronous GC also remains unknown.
Therefore, we performed a retrospective study to investigate predictive markers of the development of metachronous and synchronous GC, including GX and endoscopic score for GC risk.
This study was a retrospective, single-center, observational study. Between June 2006 and February 2016, a total of 352 patients underwent ESD for early GC at NTT West Osaka Hospital. Exclusion criteria for this study were as follows: Remnant stomach, unknown H. pylori status, and endoscopic observation of the whole stomach outside our hospital. Finally, 192 patients including 109 with solitary GC (Group A) and 83 with metachronous and synchronous GC (Group B), were included in the study (Figure 1). Solitary GC was defined as no past history of GC and only one GC that developed during the study period. Metachronous and synchronous GC were defined as a new GC that developed in an area other than the site of primary GC and multiple GC that developed at the same endoscopic examination. The following factors were examined: Age, sex, complicated with diabetes mellitus, complicated with other malignant disease, H. pylori status, tumor location, tumor size, macroscopic type, histological type, tumor depth, endoscopic score for GC risk, and presence of severe atrophy, intestinal metaplasia, and GX. Presence of H. pylori infection was determined by serum antibody, rapid urease test, immunohistochemistry, 13C urea breath test, or stool antigen test. Endoscopic images were evaluated at the time of GC diagnosis or ESD. Endoscopic images were reviewed by one expert endoscopist. We classified the severity of gastric atrophy according to the criteria of Kimura and Takemoto[21]. Severe atrophy was classified as O-2, 3, and P. O-P was the state that gastric atrophy progressed in the whole stomach. We diagnosed presence of GX as yellowish-white flat or slight elevated lesion by white light imaging. Endoscopic score for GC risk was calculated as follows: (1) atrophy: 0 if C-0 and 1, 1 if C-2 and 3, 2 if O-1, 2, 3, and P; (2) intestinal metaplasia: 0 if absent, 1 if present at gastric antrum, 2 if present at gastric antrum and body; (3) fold swelling: 0 if absent, 1 if present; (4) nodular gastritis: 0 if absent, 1 if present; and (5) diffuse redness: 0 if absent, 1 if mild, 2 if severe. For assessment of intestinal metaplasia, image-enhanced endoscopy with distinct white light imaging was necessary. In this study, we evaluated intestinal metaplasia as grayish-white flat elevated lesion by white light imaging, without image-enhanced endoscopy. This study was carried out with the approval of the NTT West Osaka Hospital Ethics Committee. Because of the anonymous nature of the date obtained after each patient had provided written informed consent for ESD, the requirement for informed consent was waived.
All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). More precisely, it is a modified version of R commander designed to add statistical functions frequently used in biostatistics[22]. Fisher’s exact test was performed to investigate the relationships between the two groups. Differences between the two groups were analyzed by Mann-Whitney U test when the data was not parametric. Multivariate logistic analysis was used to identify predictive markers of metachronous and synchronous GC. Age, sex, and baseline variates with P < 0.2 in univariate analysis were included in the multivariate logistic analysis. The threshold for significance was P < 0.05.
The characteristics of the two patient groups are shown in Table 1.
| Group A (n = 109) | Group B (n = 83) | P value | |
| Age, median [IQR], yr | 66 [61-74] | 72 [63-78] | 0.0009 |
| Male | 92 (84.4) | 72 (86.7) | 0.7 |
| Diabetes mellitus | 24 (22.0) | 12 (14.5) | 0.2 |
| Complicated other malignant disease | 27 (24.8) | 22 (26.5) | 0.9 |
| Helicobacter pylori status | 0.4 | ||
| Positive | 82 | 57 | |
| Post eradicated | 23 | 26 | |
| Negative | 4 | 0 | |
| Tumor location | 0.9 | ||
| Upper | 10 | 11 | |
| Middle | 57 | 39 | |
| Lower | 42 | 33 | |
| Tumor size, median [IQR], mm | 15 [10-20] | 15 [12-22] | 0.3 |
| Macroscopic type | 0.5 | ||
| 0-I | 9 | 3 | |
| 0-IIa | 67 | 52 | |
| 0-IIb | 26 | 25 | |
| 0-IIc | 7 | 3 | |
| Differentiated type | 109 (100) | 81 (97.6) | 0.2 |
| Tumor depth | 0.1 | ||
| M | 97 | 80 | |
| SM | 12 | 3 |
The median ages in Group A and B were 66 [interquartile range (IQR) 61-74] and 72 (IQR 63-78) years, respectively (P = 0.0009). The proportion of male patients was high in both groups (84.4% vs 86.7%, P = 0.7). H. pylori status was positive for many patients in Group A and B (75.2% vs 68.7%, respectively, P = 0.4).
Regarding tumor location, lesion of the upper part of the stomach was least frequent. Median tumor size was 15 mm in both groups. The most frequent macroscopic type was 0-IIa and the most frequent histological type was the differentiated type.
Endoscopic characteristics are shown in Table 2. Although the difference was not significant, the prevalence of severe atrophy and intestinal metaplasia in Group B tended to be higher than that in Group A (89.2% vs 79.8%, P = 0.1 and 57.8% vs 45.0%, P = 0.08). The prevalence of GX in Group B was significantly higher than that in Group A (54.2% vs 32.1%, P = 0.003). The number and size of GX were not significantly different between the two groups.
| Group A (n = 109) | Group B (n = 83) | P value | |
| Severe atrophy | 87 (79.8) | 74 (89.2) | 0.1 |
| Intestinal metaplasia | 49 (45.0) | 48 (57.8) | 0.08 |
| Gastric xanthoma | 35 (32.1) | 45 (54.2) | 0.003 |
The endoscopic score for GC risk is reported in Table 3. The atrophy score in Group B was significantly higher than that in Group A (P = 0.005). The scores for intestinal metaplasia, fold swelling, and diffuse redness, and the total score were not significantly different between the two groups. Nodular gastritis was absent in all patients in this study.
| Group A (n = 109) | Group B (n = 83) | P value | |
| Atrophy | 0.005 | ||
| 0 | 4 | 0 | |
| 1 | 6 | 0 | |
| 2 | 99 | 83 | |
| Intestinal metaplasia | 0.1 | ||
| 0 | 60 | 35 | |
| 1 | 33 | 34 | |
| 2 | 16 | 14 | |
| Fold swelling | 0.6 | ||
| 0 | 99 | 73 | |
| 1 | 10 | 10 | |
| Nodular gastritis | 1.0 | ||
| 0 | 109 | 83 | |
| 1 | 0 | 0 | |
| Diffuse redness | 0.5 | ||
| 0 | 4 | 0 | |
| 1 | 23 | 25 | |
| 2 | 82 | 58 | |
| Total score, median [IQR] | 4 [4-5] | 4 [4-5] | 0.1 |
Age, male sex, severe atrophy, presence of intestinal metaplasia, and presence of GX were subjected to multivariate logistic analysis. As shown in Table 4, higher age and the presence of GX were independently related to metachronous and synchronous GC.
| Odds ratio | P value | |
| Age | 1.04 (1.01-1.08) | 0.02 |
| Male | 1.38 (0.57-3.34) | 0.47 |
| Severe atrophy | 1.68 (0.70-4.05) | 0.25 |
| Intestinal metaplasia | 1.35 (0.71-2.54) | 0.36 |
| Gastric xanthoma | 2.11 (1.14-3.99) | 0.02 |
In the present study, we compared the characteristics of 109 patients with solitary GC to those of 83 patients with metachronous and synchronous GC in order to identify predictive markers for metachronous and synchronous GC. Multivariate logistic analysis revealed that high age and the presence of GX were independently related to metachronous and synchronous GC.
The results of recent studies indicated that male sex, multiple initial GC, severe atrophy, and multiple GC before successful H. pylori eradication were independent risk factors for metachronous GC[13,14]. After performing univariate analysis, we carried out multivariate logistic analysis using male sex and severe atrophy as variates, but our results revealed that these two markers were not predictive of metachronous and synchronous GC. This finding may reflect the fact that most patients in this study were male and showed severe atrophy in both groups.
Endoscopic findings related to the development of GC have been previously reported[23-27]. Of these, five endoscopic findings were confirmed; atrophy, intestinal metaplasia, fold swelling, nodular gastritis, and diffuse redness. A subsequent study reported the endoscopic score for GC risk[18]; however, the usefulness of this score remains unclear. After performing univariate analysis, we carried out multivariate logistic analysis using severe atrophy and intestinal metaplasia as variates. However, our results indicated that these two markers were not predictive of metachronous and synchronous GC. Further investigations are necessary to evaluate the usefulness of the endoscopic score for GC risk.
GX, a localized non-neoplastic accumulation of foamy histiocytes in the lamina propia of the inflamed gastric mucosa, is occasionally found during EGD[28]. GX is a positive indicator of H. pylori and persists after H. pylori eradication therapy. GX has received little attention clinically, perhaps because it is considered a benign entity[20]. A retrospective cohort study reported that the presence of GX was significantly associated with the presence of GC[19]. Another cohort study performed at the same hospital reported that GX was a useful marker for predicting the development of GC by performing endoscopic follow-up examinations[20]. However, both these studies did not investigate GX as a predictive marker for metachronous and synchronous GC. In our study, univariate analysis revealed that GX was significantly more prevalent in Group B than in Group A. In addition, results of multivariate logistic analysis indicated that GX was a predictive marker for metachronous and synchronous GC. To our knowledge, this is first report of the presence of GX as a useful predictive marker for metachronous and synchronous GC.
Why does GC develop more frequently in patients with GX? Increased release of oxygen free radicals may be involved in the formation of GX[29]. On the other hand, the presence of GX may reflect the severity and long duration of chronic gastritis[20], which is a risk factor for GC development. Because of the same reason, GX may be more frequent in Group B than Group A. However, further studies are required to elucidate this link.
Our study has some limitations. First, it was a retrospective single-center study. Second, the sample size was small. Finally, we did not analyze inter-observer variability in the assessment of endoscopic images.
In summary, our results revealed that higher age and the presence of GX were independently related to metachronous and synchronous GC. These findings, especially the predictive value of the presence of GX, could improve the timely detection and treatment of metachronous and synchronous GC. Further investigations are necessary to confirm the predictive value of these markers.
Predictive markers for the development of metachronous and synchronous gastric cancer (GC) have not been extensively studied. In addition, it is often difficult to clearly distinguish metachronous GC from synchronous GC because of missed detection of synchronous GC. And the usefulness of endoscopic score for GC risk and gastric xanthoma (GX) as the predictive markers of metachronous and synchronous GC remains unknown.
This study investigated the predictive markers for metachronous and synchronous GC.
Higher age and the presence of GX were independently related to the development of metachronous and synchronous GC.
GX is a useful predictive marker for metachronous and synchronous GC.
Solitary GC is defined as no past history of GC and only one GC that developed during the study period. Metachronous and synchronous GC are defined as a new GC that developed in an area other than the site of primary GC and multiple GC that developed at the same endoscopic examination.
This is a retrospective study to investigate predictive markers for metachronous and synchronous GC developing after endoscopic submucosal dissection. This is good idea for clinics.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chen JQ, Iizuka T, Nishida T S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
| 1. | Isomoto H, Shikuwa S, Yamaguchi N, Fukuda E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J, Kohno S. Endoscopic submucosal dissection for early gastric cancer: a large-scale feasibility study. Gut. 2009;58:331-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 470] [Cited by in F6Publishing: 496] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 2. | Goto O, Fujishiro M, Kodashima S, Ono S, Omata M. Outcomes of endoscopic submucosal dissection for early gastric cancer with special reference to validation for curability criteria. Endoscopy. 2009;41:118-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Gotoda T, Iwasaki M, Kusano C, Seewald S, Oda I. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg. 2010;97:868-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 193] [Cited by in F6Publishing: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Odagaki T, Taniguchi H, Kushima R, Saito Y. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy. 2013;45:703-707. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Oda I, Oyama T, Abe S, Ohnita K, Kosaka T, Hirasawa K, Ishido K, Nakagawa M, Takahashi S. Preliminary results of multicenter questionnaire study on long-term outcomes of curative endoscopic submucosal dissection for early gastric cancer. Dig Endosc. 2014;26:214-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Ohnita K, Isomoto H, Shikuwa S, Yajima H, Minami H, Matsushima K, Akazawa Y, Yamaguchi N, Fukuda E, Nishiyama H. Early and long-term outcomes of endoscopic submucosal dissection for early gastric cancer in a large patient series. Exp Ther Med. 2014;7:594-598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Suzuki H, Oda I, Abe S, Sekiguchi M, Mori G, Nonaka S, Yoshinaga S, Saito Y. High rate of 5-year survival among patients with early gastric cancer undergoing curative endoscopic submucosal dissection. Gastric Cancer. 2016;19:198-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 162] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 8. | Nasu J, Doi T, Endo H, Nishina T, Hirasaki S, Hyodo I. Characteristics of metachronous multiple early gastric cancers after endoscopic mucosal resection. Endoscopy. 2005;37:990-993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 9. | Nakajima T, Oda I, Gotoda T, Hamanaka H, Eguchi T, Yokoi C, Saito D. Metachronous gastric cancers after endoscopic resection: how effective is annual endoscopic surveillance? Gastric Cancer. 2006;9:93-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 10. | Kobayashi M, Narisawa R, Sato Y, Takeuchi M, Aoyagi Y. Self-limiting risk of metachronous gastric cancers after endoscopic resection. Dig Endosc. 2010;22:169-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Kato M, Nishida T, Yamamoto K, Hayashi S, Kitamura S, Yabuta T, Yoshio T, Nakamura T, Komori M, Kawai N. Scheduled endoscopic surveillance controls secondary cancer after curative endoscopic resection for early gastric cancer: a multicentre retrospective cohort study by Osaka University ESD study group. Gut. 2013;62:1425-1432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 12. | Shiotani A, Haruma K, Graham DY. Metachronous gastric cancer after successful Helicobacter pylori eradication. World J Gastroenterol. 2014;20:11552-11559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 23] [Cited by in F6Publishing: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Nakajima T, Sekiguchi M, Mori G, Taniguchi H, Sekine S. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy. 2015;47:1113-1118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Mori G, Nakajima T, Asada K, Shimazu T, Yamamichi N, Maekita T, Yokoi C, Fujishiro M, Gotoda T, Ichinose M. Incidence of and risk factors for metachronous gastric cancer after endoscopic resection and successful Helicobacter pylori eradication: results of a large-scale, multicenter cohort study in Japan. Gastric Cancer. 2016;19:911-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Kim HH, Cho EJ, Noh E, Choi SR, Park SJ, Park MI, Moon W. Missed synchronous gastric neoplasm with endoscopic submucosal dissection for gastric neoplasm: experience in our hospital. Dig Endosc. 2013;25:32-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Gweon TG, Park JM, Lim CH, Kim JS, Cho YK, Lee IS, Kim SW, Choi MG. Increased incidence of secondary gastric neoplasia in patients with early gastric cancer and coexisting gastric neoplasia at the initial endoscopic evaluation. Eur J Gastroenterol Hepatol. 2014;26:1209-1216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 899] [Cited by in F6Publishing: 948] [Article Influence: 105.3] [Reference Citation Analysis (0)] |
| 18. | Haruma K. Kyoto classification of gastritis. Japan: Kawasaki Medical School 2014; 97-110. [Cited in This Article: ] |
| 19. | Sekikawa A, Fukui H, Maruo T, Tsumura T, Kanesaka T, Okabe Y, Osaki Y. Gastric xanthelasma may be a warning sign for the presence of early gastric cancer. J Gastroenterol Hepatol. 2014;29:951-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Sekikawa A, Fukui H, Sada R, Fukuhara M, Marui S, Tanke G, Endo M, Ohara Y, Matsuda F, Nakajima J. Gastric atrophy and xanthelasma are markers for predicting the development of early gastric cancer. J Gastroenterol. 2016;51:35-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87-97. [Cited in This Article: ] |
| 22. | Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452-458. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9275] [Cited by in F6Publishing: 10636] [Article Influence: 966.9] [Reference Citation Analysis (0)] |
| 23. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3126] [Cited by in F6Publishing: 3021] [Article Influence: 131.3] [Reference Citation Analysis (0)] |
| 24. | Kato I, Tominaga S, Ito Y, Kobayashi S, Yoshii Y, Matsuura A, Kameya A, Kano T. Atrophic gastritis and stomach cancer risk: cross-sectional analyses. Jpn J Cancer Res. 1992;83:1041-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Inoue K, Fujisawa T, Chinuki D, Kushiyama Y. Characteristics of gastric mucosa in gastric cancer improvement-examination from a clinical survey by endoscopy. Stomach and Intestine. 2009;44:1367-1373. [Cited in This Article: ] |
| 26. | Kamada T, Tabaka A, Yamanaka Y. Nodular gastritis with Helicobacter pylori infection is strongly associated with diffuse-type gastric cancer in young patients. Dig Endosc. 2007;19:180-184. [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Nishibayashi H, Kanayama S, Kiyohara T, Yamamoto K, Miyazaki Y, Yasunaga Y, Shinomura Y, Takeshita T, Takeuchi T, Morimoto K. Helicobacter pylori-induced enlarged-fold gastritis is associated with increased mutagenicity of gastric juice, increased oxidative DNA damage, and an increased risk of gastric carcinoma. J Gastroenterol Hepatol. 2003;18:1384-1391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Hori S, Tsutsumi Y. Helicobacter pylori infection in gastric xanthomas: immunohistochemical analysis of 145 lesions. Pathol Int. 1996;46:589-593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Kaiserling E, Heinle H, Itabe H, Takano T, Remmele W. Lipid islands in human gastric mucosa: morphological and immunohistochemical findings. Gastroenterology. 1996;110:369-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |