Published online Jul 15, 2016. doi: 10.4251/wjgo.v8.i7.573
Peer-review started: March 7, 2016
First decision: April 15, 2016
Revised: April 15, 2016
Accepted: May 17, 2016
Article in press: May 27, 2016
Published online: July 15, 2016
Processing time: 125 Days and 21.9 Hours
AIM: To verify the safety and validity of laparoscopic surgery for the treatment of colorectal cancer in elderly patients.
METHODS: A meta-analysis was performed of a systematic search of studies on an electronic database. Studies that compared laparoscopic colectomy (LAC) in elderly colorectal cancer patients with open colectomy (OC) were retrieved, and their short and long-term outcomes compared. Elderly people were defined as 65 years old or more. Inclusion criteria were set at: Resection of colorectal cancer, comparison between laparoscopic and OC and no significant difference in backgrounds between groups.
RESULTS: Fifteen studies were identified for analysis. LAC was performed on 1436 patients, and OC performed on 1810 patients. In analyses of short-term outcomes, operation time for LAC was longer than for OC (mean difference = 34.4162, 95%CI: 17.8473-50.9851, P < 0.0001). The following clinical parameters were lower in LAC than in OC: Amount of estimated blood loss (mean difference = -93.3738, 95%CI: -132.3437 to -54.4039, P < 0.0001), overall morbidity (OR = 0.5427, 95%CI: 0.4425-0.6655, P < 0.0001), incisional surgical site infection (OR = 0.6262, 95%CI: 0.4310-0.9097, P = 0.0140), bowel obstruction and ileus (OR = 0.6248, 95%CI: 0.4519-0.8638, P = 0.0044) and cardiovascular complications (OR = 0.4767, 95%CI: 0.2805-0.8101, P = 0.0062). In analyses of long-term outcomes (median follow-up period: 36.4 mo in LAC, 34.3 mo in OC), there was no significant difference in overall survival (mean difference = 0.8321, 95%CI: 0.5331-1.2990, P = 0.4187) and disease specific survival (mean difference = 1.0254, 95%CI: 0.6707-1.5675, P = 0.9209). There was also no significant difference in the number of dissected lymph nodes (mean difference = -0.1360, 95%CI: -4.0553-3.7833, P = 0.9458).
CONCLUSION: LAC in elderly colorectal cancer patients had benefits in short-term outcomes compared with OC except operation time. The long-term outcomes and oncological clearance of LAC were similar to that of OC. These results support the assertion that LAC is an effective procedure for elderly patients with colorectal cancer.
Core tip: Safety and effectiveness of laparoscopic surgery (LAC) in elderly has been unknown. A meta-analysis was performed of a systematic search of studies on an electronic database. Studies that compared LAC in elderly colorectal cancer patients with open colectomy (OC) were retrieved, and their short and long-term outcomes compared. Fifteen studies which had 1436 LAC and 1810 OC were identified. In short-term outcomes, blood loss, morbidity, incisional surgical site infection, bowel obstruction and cardiovascular complications were superior in LAC except operation time. There was no significant difference in long-term outcomes. LAC is an effective procedure for elderly with colorectal cancer.
- Citation: Fujii S, Tsukamoto M, Fukushima Y, Shimada R, Okamoto K, Tsuchiya T, Nozawa K, Matsuda K, Hashiguchi Y. Systematic review of laparoscopic vs open surgery for colorectal cancer in elderly patients. World J Gastrointest Oncol 2016; 8(7): 573-582
- URL: https://www.wjgnet.com/1948-5204/full/v8/i7/573.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v8.i7.573
People are living longer across the globe. According to the World Health Organization, 6.9% of the world was over the age of 65 in 2000 with an estimated increase to 10.4% in 2025 and a further rise to 16.4% in 2050[1]. This estimation is valid in all regions of the world. Average life expectancies in 2025 are estimated to be 77 years old in the Americas and Europe and 72 years old in Asia. Colorectal cancer is the third most common malignant neoplasm in the world and aging is assumed to be one of the risk factors for colorectal carcinogenesis[2]. Elderly patients have a higher American Society of Anesthesiologists score, higher cardiac and pulmonary comorbidity rate and lower preoperative nutritional conditioning than younger patients[3-5]. Therefore, there is a high risk associated with even minimally invasive surgery in elderly patients. Several studies have reported the benefits of laparoscopic colorectal surgery in elderly patients[6-10]. Most studies concluded that laparoscopic surgery had a lower postoperative morbidity rate and shorter length of hospital stay when compared to open surgery. Several large-scale systematic reviews that compare laparoscopic colorectal surgery with open surgery have been published in recent years[11,12]. They report that laparoscopic surgery has lower mortality, lower overall morbidity, lower cardiac and respiratory complications, lower wound infection and shorter length of hospital stay. However, they analyzed both colorectal cancer and benign diseases together. The surgical procedure for colorectal cancer differs from that for benign disease because optimal lymph node dissection and resection, with a securing safety margin, are vital in malignant neoplasm surgery. Therefore, a study analyzing laparoscopic surgery that targeted only colorectal cancer was required.
Moreover, the results of previous reviews reported only short-term outcomes. The evaluation of long-term outcomes is very important in the analysis of treatment efficacy for malignant neoplasia. The purpose of the present review is to clarify the benefits of laparoscopic surgery in elderly patients with colorectal cancer. We analyzed not only short-term but also long-term outcomes.
Elderly people were defined as 65 years old or more, as outlined by the World Health Organization[1]. All studies were limited to randomized controlled or comparative studies. The subject of each study was limited to colorectal cancer and studies that included any benign disease were excluded. Backgrounds were similar between both groups, and had at least 15 patients in one group. The results had to include a comparison between laparoscopic and open surgery.
Short-term outcomes analyzed in the present study were as follows: Operative time, amount of estimated blood loss, mortality, overall morbidity, incisional surgical site infection, deep surgical site infection, anastomotic leakage, bowel obstruction and ileus, pneumonia, cardiovascular complication, time of normal bowel function and length of postoperative hospital stay. Duration of short-term was defined after the operation within 30 d.
The overall and disease specific survival rates were measured as long-term outcomes.
The number of dissected lymph nodes was used as an indicator of oncological clearance.
The literature search was performed electronically using PubMed (MEDLINE). The search terms were as follows: Elderly or old, colorectal cancer or colon cancer, and laparoscopic surgery or laparoscopic colectomy (LAC) in combination with Boolean operators AND or OR. The language was limited to English. Studies were selected from those published after 2000 because they included the long-term results of several randomized controlled studies that compared laparoscopic and open surgery[13-18]. Moreover, developments in laparoscopic surgery instrumentation might influence short-term results in studies conducted in more recent years.
The number of randomized controlled study was only one in this meta-analysis[19]. The randomized controlled study was assessed for methodological quality using the Cochrane Handbook[20]. Five of six items were at low risk of bias. Blinding of the study was not possible.
The comparative studies were assessed by the Newcastle-Ottawa Quality Assessment Scale (NOS)[21]. Twelve of 14 studies had 6 or more star points on the NOS scale.
The odds ratios (ORs) for each study and 95%CIs were calculated from event numbers of categorical variables of short-term results. Pooled ORs were calculated using a random effect model. The mean value difference between continuous variables of short-term results and the number of dissected lymph nodes was also calculated using a random effect model. In the analysis of long-term results, 95%CIs of survival comparison and the number of patients in each study were synthesized using a random effect model. Synthesis of data was performed using the DerSirmonian-Laird method[22]. Study heterogeneity was checked by means of Cochran’s Q statistic. If the P value of the heterogeneity test was less than 0.05 in significance level, a null hypothesis of homogeneity was dismissed and study heterogeneity was proved. Publication bias among the studies was checked using the Egger test or Begg test accordingly. If the P value for publication bias was less than 0.10 a null hypothesis of no bias was dismissed and publication bias was confirmed.
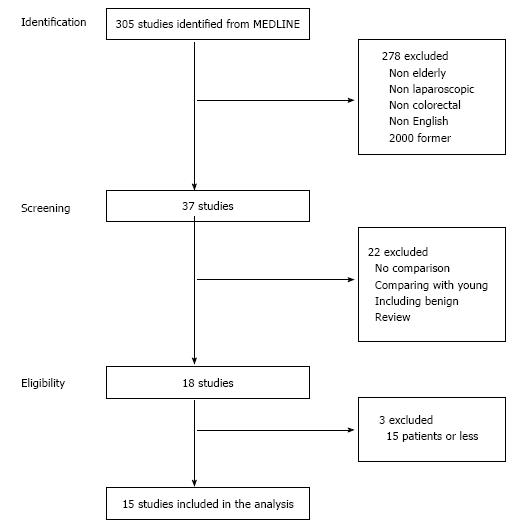
Thirty seven studies were identified by the first screening of MEDLINE. The reviews and studies that included benign disease cases or no data comparison between laparoscopic and open surgery were excluded. Finally, 15 studies were selected for analysis (Figure 1)[19,23-36]. The types of studies were as follows: 1 randomized controlled, 2 case-matched, 1 prospective comparative and 11 retrospective comparative studies. In total, 1436 laparoscopic surgeries and 1810 open surgeries were analyzed. Conversion to open surgery was described in 9 studies. The range of conversion rate was between 0% and 13.9%, and the incidence of total patients was 4.5%. A summary of study characteristics is shown in Table 1.
| Ref. | Year | Study type | Age | Age | Patient | No. of LAC | No. of OC | Convert (%) | ASA (1-2/3-4) | Gender (M/F) | NOS | |||
| LAC | OC | LAC | OC | LAC | OC | |||||||||
| Sklow et al[23] | 2003 | Case-matched | 76 | 81.4 ± 0.83 | 81.8 ± 0.91 | All cancer | 39 | 39 | 19/20 | 10/29 | 22/17 | 21/18 | 6 | |
| Vignali et al[24] | 2005 | Case-matched | 80 | 82.3 ± 2.3 | 83.1 ± 3.1 | All cancer | 61 | 61 | 4 (6.6) | 2.5 ± 0.1 | 2.6 ± 0.6 | 29/32 | 29/32 | 6 |
| Feng et al[25] | 2006 | Retro, comparative | 71 | 77.8 ± 5.1 | 76.9 ± 6.1 | All cancer | 51 | 102 | 2 (3.9) | 5 | ||||
| Tei et al[26] | 2009 | Retro, comparative | 71 | 75.5 (71-89) | 76.0 (71-93) | All cancer | 51 | 78 | 3 (5.9) | Apr-37 | 63/15 | 32/19 | 43/35 | 6 |
| Akiyoshi et al[27] | 2009 | Retro, comparative | 75 | 79 (75-90) | 79 (75-86) | Rectal cancer | 44 | 43 | 0 (0) | Jun-38 | Jul-36 | 21/23 | 23/20 | 3 |
| Tomimaru et al[28] | 2011 | Retro, comparative | 76 | 82.0 ± 4.6 | 81.9 ± 5.7 | Colon cancer | 36 | 15 | 5 (13.9) | 20/16 | 8/7 | 13/23 | 7/8 | 8 |
| Robinson et al[29] | 2011 | Retro, comparative | 65 | 74 (65-86 ) | 75 (65-91 ) | All cancer | 47 | 195 | 1/46 | 9/162 | 47/0 | 191/4 | 8 | |
| She et al[30] | 2013 | Retro, comparative | 75 | 80 (75-94) | 80 (75-95) | All cancer | 189 | 245 | 9 (4.8) | 122/66 | 134/101 | 90/99 | 120/125 | 6 |
| Scarpa et al[31] | 2013 | Retro, comparative | 70 | 77 (74-80) | 75 (72-80) | All cancer | 33 | 24 | 14/19 | 8/16 | 6 | |||
| Fujii et al[19] | 2014 | RCT | 75 | 79.8 ± 3.6 | 80.1 ± 4.2 | All cancer | 100 | 100 | 3 (3) | Sep-91 | 85/15 | 50/50 | 60/40 | NA |
| Hinoi et al[32] | 2014 | Retro, comparative | 80 | 83 (81-85) | 83 (81-85) | All cancer | 459 | 459 | 362/107 | 355/104 | 215/244 | 222/237 | 8 | |
| Miyasaka et al[33] | 2014 | Retro, comparative | 70 | 75 (70-86) | 78 (70-94) | All cancer | 28 | 79 | 6/22 | 48/31 | 13/15 | 27/52 | 6 | |
| Vallribera Valls et al[34] | 2014 | Prospective, comparative | 75 | All cancer | 134 | 133 | 59/75 | 71/62 | 88/46 | 88/45 | 8 | |||
| Zeng et al[35] | 2015 | Retro, comparative | 70 | 74 (70-87) | 74 (70-88) | Rectal cancer | 112 | 182 | 7 (6.3) | 66/46 | 92/90 | 62/50 | 98/84 | 6 |
| Shigeta et al[36] | 2015 | Retro, comparative | 80 | 82 (81-84) | 83 (81-87) | All cancer | 52 | 55 | 0 (0) | Apr-48 | Apr-81 | 28/24 | 26/29 | 7 |
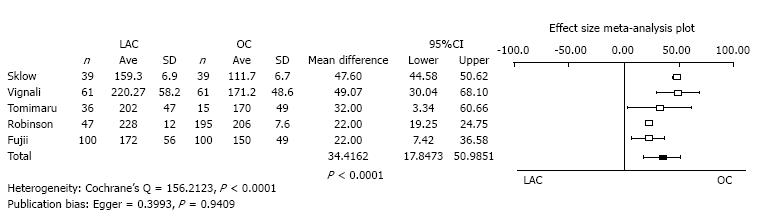
Operation time: Five studies reported operative time as the mean value with standard deviation. The operation time of LAC was significantly longer than OC (mean difference = 34.4162, 95%CI: 17.8473-50.9851, P < 0.0001). The heterogeneity was statistically significant (Cochrane’s Q = 156.2123, P < 0.0001). Publication bias was not evident (Egger = 0.3993, P = 0.9409) (Figure 2).
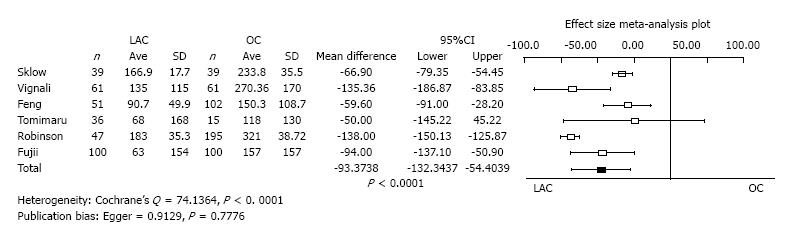
Amount of estimated blood loss: Six studies reported the amount of estimated blood loss as a mean value with standard deviation. The operation time of LAC was significantly less than OC (mean difference = -93.3738, 95%CI: -132.3437 to -54.4039, P < 0.0001). Heterogeneity was statistically evident (Cochrane’s Q = 74.1364, P < 0.0001). Publication bias was not evident (Egger = 0.9129, P = 0.7776) (Figure 3).
Mortality: Four studies reported mortality. There was no significant difference between LAC and OC in mortality (OR = 0.5052, 95%CI: 0.2438-1.0467, P = 0.0662). Heterogeneity and publication bias were not evident (Cochrane’s Q = 2.0911, P = 0.5537, Egger = -0.6646, P = 0.5883).
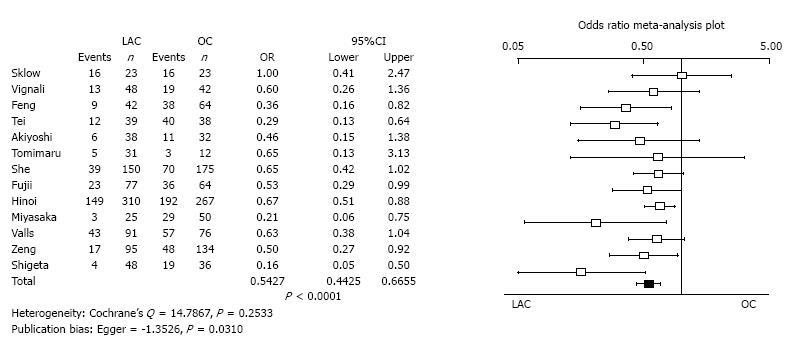
Overall morbidity: Thirteen studies reported incidence of overall morbidity. The overall morbidity of LAC was significantly less than for OC (OR = 0.5427, 95%CI: 0.4425-0.6655, P < 0.0001). Heterogeneity was not evident (Cochrane’s Q = 14.7867, P = 0.2533). Publication bias was statistically evident (Egger = -1.3526, P = 0.0310) (Figure 4).
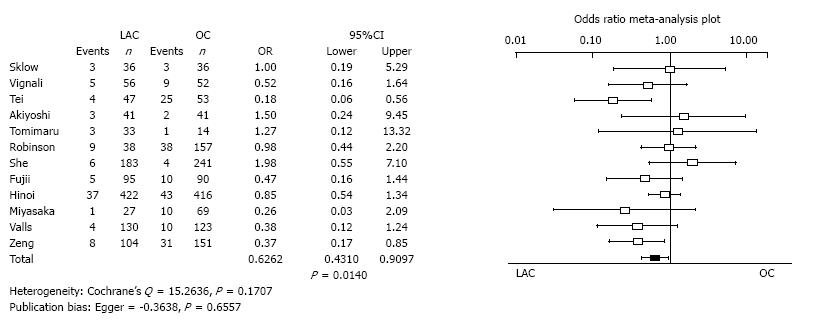
Incisional surgical site infection: Twenty studies reported the incidence of incisional surgical site infection. The incisional surgical site infection of LAC was significantly less than for OC (OR = 0.6262, 95%CI: 0.4310-0.9097, P = 0.0140). Heterogeneity and publication bias were not evident (Cochrane’s Q = 15.2636, P = 0.1707, Egger = -0.3638, P = 0.6557) (Figure 5).
Deep surgical site infection: Four studies reported the incidence of deep surgical site infection. There was no significant difference between LAC and OC in deep surgical site infection (OR = 0.8234, 95%CI: 0.3298-2.0556, P = 0.6771). Heterogeneity and publication bias were not evident (Cochrane’s Q = 6.3512, P = 0.0957, Egger = -3.0524, P = 0.1922).
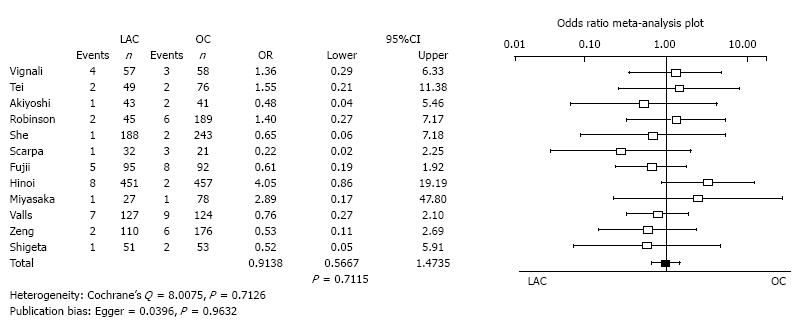
Anastomotic leakage: Twenty studies reported the incidence of anastomotic leakage. There was no significant difference between LAC and OC in anastomotic leakage (OR = 0.9138, 95%CI: 0.5667-1.4735, P = 0.7115). Heterogeneity and publication bias were not evident (Cochrane’s Q = 8.0075, P = 0.7126, Egger = 0.0396, P = 0.9632) (Figure 6).
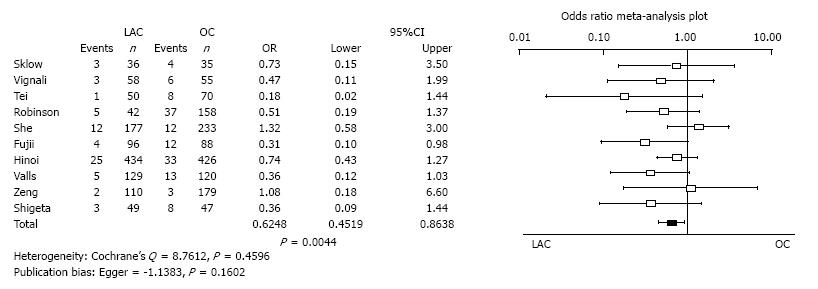
Bowel obstruction and ileus: Ten studies reported the incidence of bowel obstruction and ileus. Bowel obstruction and ileus of LAC was significantly less than for OC (OR = 0.6248, 95%CI: 0.4519-0.8638, P = 0.0044). Heterogeneity and publication bias were not evident (Cochrane’s Q = 8.7612, P = 0.4596, Egger = -1.1383, P = 0.1602) (Figure 7).
Pneumonia: Three studies reported the incidence of pneumonia. There was no significant difference between LAC and OC in the incidence of pneumonia (OR = 0.4526, 95%CI: 0.1976-1.0365, P = 0.0608). Heterogeneity and publication bias were not evident (Cochrane’s Q = 2.3251, P = 0.3127, Egger = 0.1846, P = 0.9743).
Cardiovascular complication: Eight studies reported the incidence of cardiovascular complication. Cardiovascular complications of LAC was significantly less than for OC (OR = 0.4767, 95%CI: 0.2805-0.8101, P = 0.0062). Heterogeneity was not evident (Cochrane’s Q = 6.6316, P = 0.4682). Publication bias was statistically evident (Egger = 1.5152, P = 0.0521) (Figure 8).
Recovery time of normal bowel function: Five studies reported the recovery time of normal bowel function as the mean value with standard deviation. There was no significant difference in the recovery time to normal bowel function between LAC and OC (mean difference = -0.8573, 95%CI: -1.8778 to 0.1632, P = 0.0997). Heterogeneity was statistically evident (Cochrane’s Q = 379.9427, P < 0.0001). Publication bias was not evident (Egger = 5.4503, P = 0.5226).
Length of postoperative hospital stay: Three studies reported the length of postoperative hospital stay as mean value with standard deviation. There was no significant difference in the length of postoperative hospital stay between LAC and OC (mean difference = -1.3336, 95%CI: -3.3995 to 0.7322, P = 0.2058). Heterogeneity was not evident (Cochrane’s Q = 3.9019, P = 0.1421). Publication bias was statistically evident (Egger = -1.4308, P = 0.0689).
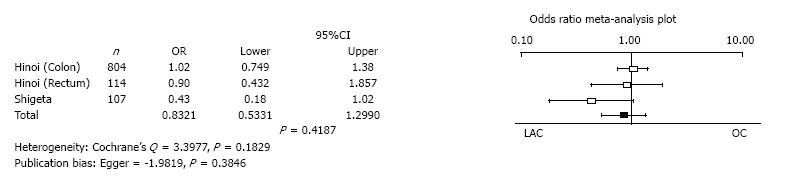
Overall survival: Hinoi et al[32] reported overall survival in colon and rectal cancer separately. Three analyses of two studies reported the overall survival with 95%CIs. There was no significant difference in the overall survival between LAC and OC (mean difference = 0.8321, 95%CI: 0.5331 to 1.2990, P = 0.4187). Heterogeneity and publication bias were not evident (Cochrane’s Q = 3.3977, P = 0.1829, Egger = -1.9819, P = 0.3846) (Figure 9).
Disease specific survival: Hinoi et al[32] reported disease specific survival in colon and rectal cancer separately. Three analyses of two studies reported the disease specific survival with 95%CIs. There was no significant difference in the disease specific survival between LAC and OC (mean difference = 1.0254, 95%CI: 0.6707 to 1.5675, P = 0.9209). Heterogeneity and publication bias were not evident (Cochrane’s Q = 0.1648, P = 0.9209, Egger = -0.4921, P = 0.1559) (Figure 10).
The number of dissected lymph nodes: Two studies reported the number of dissected lymph nodes as the mean value with standard deviation. There was no significant difference in the number of dissected lymph nodes between LAC and OC (mean difference = -0.1360, 95%CI: -4.0553 to 3.7833, P = 0.9458). Heterogeneity and publication bias were not evident (Cochrane’s Q = 3.2471, P = 0.0716, Kendall tau rank correlation coefficient by Begg test = 1.0000, P = 0.3173).
There was only one randomized controlled study[19] which contained the following; random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective reporting and other potential threats to validity.
The study which had 5 or less star points on the NOS scale related to 5 short-term outcomes; amount of estimated blood loss, overall morbidity, incisional surgical site infection, anastomotic leakage and time of normal bowel function. These outcomes were synthesized with studies which had 6 or more star points. Results were similar to the primary analyzed results and there was no conversion of interpretation.
Two systematic reviews that compare LAC with OC report benefits in short-term outcome. Grailey et al[11] report that LAC reduces the length of hospital stay, intraoperative blood loss, pneumonia, time to normal bowel function, cardiac complication and wound infection. Antoniou et al[12] report that LAC had a decreased risk for mortality, overall morbidity, plus cardiac and respiratory complications. Their results are similar to those reported in this review. However, they included analyses for both colorectal cancer and benign disease. Large scale, randomized studies and reviews that compare long-term results between LAC and OC in all generations report no difference in colon cancer patients[37]. However, long-term results of randomized studies and reviews with elderly patient have not yet been reported. This meta-analysis, which compared LAC and OC in elderly colorectal cancer patients, demonstrates advantages in short-term and equivalency with respect to long-term outcomes and oncological clearance. These results will be useful in informing the selection of operative approach in elderly patients.
In analyses of the amount of estimated blood loss, overall morbidity, incisional surgical site infection and cardiovascular complication were all reduced in LAC. These results are similar to previous reports[11,12]. It has been suggested that decreases in blood loss and postoperative pain reduce the stress of surgery, and therefore reduce overall morbidity. The reduction in cardiovascular complications might also be due to decrease in blood loss. Bowel obstruction and ileus was also reduced in LAC. Bowel obstruction and ileus were not distinguished in this analysis, because the definition was not clear in some studies and data was assigned to both conditions. This was not shown in previous reviews and it is supposed that the incidence of ileus increase is due to the extent of lymph node dissection in colorectal cancer. The exposure of intestines and major trauma to the abdominal wall might explain the increase in incidence of bowel paralysis and adhesion in OC.
The operative time of LAC was longer than OC. This result was consistent with past reports, too. However, pneumonia was not increased and overall morbidity was decreased in LAC. Mean difference in operative time was about 34 min. The increase in operative time and pneumoperitoneum may not cause adverse effects on postoperative morbidity.
In this meta-analysis, there were no significant differences in mortality, incidence of pneumonia and recovery time of normal bowel function, which is not consistent with past reports. However, all LAC results tended to be lower than OC and p-values were close to being significantly different (mortality; OR = 0.5052, 95%CI: 0.2438- 1.0467, P = 0.0662, pneumonia; OR = 0.4526, 95%CI: 0.1976-1.0365, P = 0.0608, recovery time of normal bowel function; mean difference = -0.8573, 95%CI: -1.8778 to 0.1632, P = 0.0997). These inconsistent results may be due to the fact that patients who underwent elective colorectal surgery could be considered to be at relatively low risk. The reason for there being no significant difference in recovery time of normal bowel function is unknown. There might have been a significant difference if the time period of the data collection was a number of days not hours.
The incidences of deep surgical site infection and anastomotic leakage were similar between LAC and OC (deep surgical site infection; OR = 0.8234, 95%CI: 0.3298-2.0556, P = 0.6771, anastomotic leakage; OR = 0.9138, 95%CI: 0.5667-1.4735, P = 0.7115). It has been suggested that surgical invasiveness of the retroperitoneal dissection and anastomotic procedure are similar between LAC and OC in colorectal cancer surgery.
There was no significant difference in length of postoperative hospital stay.
This may be due to differences in the standard for hospital discharge in each study and may also be related to differences in the insurance systems in each country. Thus there might be a large bias in social factors between studies.
In analyses of long-term outcomes, both overall and disease specific survival rates were similar. There was also no significant difference in the number of dissected lymph nodes. This reveals the fact that LAC had similar treatment success to OC. The results of randomized studies and Cochrane review were also supported by this meta-analysis in elderly colorectal cancer surgery patients[13-18,37].
The limitation of this review is that it consists of only one randomized controlled study. Thus there were publication biases in analyses of overall morbidity, cardiovascular complication and length of postoperative hospital stay. Analysis of high risk elderly patients with impaired cardiac and pulmonary function might be required in the future. A secondary limitation of this study is that long-term outcomes were limited to three data sets from two studies. The analysis of more long-term results, that include details on the specific form of relapse, may thus be required.
LAC in elderly colorectal cancer patients had benefits in short-term outcomes such as amount of estimated blood loss, overall morbidity, incidences of incisional surgical site infection, bowel obstruction and ileus and cardiovascular complications. The only area where LAC did not show a benefit over OC was for operative time. The long-term outcomes and oncological clearance of LAC were similar to that of OC. These results support the view that LAC is an effective and safe procedure for elderly patients with colorectal cancer.
Laparoscopic surgery for colorectal cancer is increasing rapidly, particularly among elderly patients. However, neither the safety nor the effectiveness of laparoscopic surgery in this demographic has yet been determined.
Some systematic reviews that compare laparoscopic colectomy (LAC) with open colectomy for elderly had reported benefits in short-term outcome.
However, past reports included benign diseases, and no report about long-term results. The authors analyzed for elderly colorectal cancer only and long-term outcomes.
Some short-term outcomes were superior in LAC except operation time. There was no significant difference in long-term outcomes. LAC is an effective procedure for elderly with colorectal cancer.
LAC: Laparoscopic colectomy.
Good review article, scientific and rigorous analysis.
Manuscript source: Invited manuscript
P- Reviewer: Afzal M, Jelinek F, Ryu DH, Surlin V S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | World Health Organization. Men, ageing and health: Achieving health across the life span. [World Health Organization web site]. Available from: http://whqlibdoc.who.int/hq/2001/WHO_NMH_NPH_01.2.pdf?ua=1. |
| 2. | Rasool S, Kadla SA, Rasool V, Ganai BA. A comparative overview of general risk factors associated with the incidence of colorectal cancer. Tumour Biol. 2013;34:2469-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 3. | Frasson M, Braga M, Vignali A, Zuliani W, Di Carlo V. Benefits of laparoscopic colorectal resection are more pronounced in elderly patients. Dis Colon Rectum. 2008;51:296-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 156] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Senagore AJ, Madbouly KM, Fazio VW, Duepree HJ, Brady KM, Delaney CP. Advantages of laparoscopic colectomy in older patients. Arch Surg. 2003;138:252-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Manceau G, Karoui M, Werner A, Mortensen NJ, Hannoun L. Comparative outcomes of rectal cancer surgery between elderly and non-elderly patients: a systematic review. Lancet Oncol. 2012;13:e525-e536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Tuech JJ, Pessaux P, Rouge C, Regenet N, Bergamaschi R, Arnaud JP. Laparoscopic vs open colectomy for sigmoid diverticulitis: a prospective comparative study in the elderly. Surg Endosc. 2000;14:1031-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Stocchi L, Nelson H, Young-Fadok TM, Larson DR, Ilstrup DM. Safety and advantages of laparoscopic vs. open colectomy in the elderly: matched-control study. Dis Colon Rectum. 2000;43:326-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 164] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Stewart BT, Stitz RW, Lumley JW. Laparoscopically assisted colorectal surgery in the elderly. Br J Surg. 1999;86:938-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Lian L, Kalady M, Geisler D, Kiran RP. Laparoscopic colectomy is safe and leads to a significantly shorter hospital stay for octogenarians. Surg Endosc. 2010;24:2039-2043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Law WL, Chu KW, Tung PH. Laparoscopic colorectal resection: a safe option for elderly patients. J Am Coll Surg. 2002;195:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Grailey K, Markar SR, Karthikesalingam A, Aboud R, Ziprin P, Faiz O. Laparoscopic versus open colorectal resection in the elderly population. Surg Endosc. 2013;27:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 12. | Antoniou SA, Antoniou GA, Koch OO, Pointner R, Granderath FA. Laparoscopic colorectal surgery confers lower mortality in the elderly: a systematic review and meta-analysis of 66,483 patients. Surg Endosc. 2015;29:322-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, Brown JM. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 495] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 14. | Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, Pique JM. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008;248:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 436] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 15. | Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW, Hellinger M, Flanagan R, Peters W, Nelson H. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246:655-662; discussion 662-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 798] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 16. | Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 1053] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 17. | Ng SS, Leung KL, Lee JF, Yiu RY, Li JC, Hon SS. Long-term morbidity and oncologic outcomes of laparoscopic-assisted anterior resection for upper rectal cancer: ten-year results of a prospective, randomized trial. Dis Colon Rectum. 2009;52:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 18. | Morris EJ, Jordan C, Thomas JD, Cooper M, Brown JM, Thorpe H, Cameron D, Forman D, Jayne D, Quirke P. Comparison of treatment and outcome information between a clinical trial and the National Cancer Data Repository. Br J Surg. 2011;98:299-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Fujii S, Ishibe A, Ota M, Yamagishi S, Watanabe K, Watanabe J, Kanazawa A, Ichikawa Y, Oba M, Morita S. Short-term results of a randomized study between laparoscopic and open surgery in elderly colorectal cancer patients. Surg Endosc. 2014;28:466-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 20. | Higgins JPT, Green S. Assessing risk of bias in included studies Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. [accessed May 12]. 2015; Available from: http://handbook.cochrane.org. |
| 21. | Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital/Rearch Institute web site, 2003. [accessed May 12]. 2015; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 22. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30347] [Article Influence: 778.1] [Reference Citation Analysis (0)] |
| 23. | Sklow B, Read T, Birnbaum E, Fry R, Fleshman J. Age and type of procedure influence the choice of patients for laparoscopic colectomy. Surg Endosc. 2003;17:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Vignali A, Di Palo S, Tamburini A, Radaelli G, Orsenigo E, Staudacher C. Laparoscopic vs. open colectomies in octogenarians: a case-matched control study. Dis Colon Rectum. 2005;48:2070-2075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 99] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 25. | Feng B, Zheng MH, Mao ZH, Li JW, Lu AG, Wang ML, Hu WG, Dong F, Hu YY, Zang L. Clinical advantages of laparoscopic colorectal cancer surgery in the elderly. Aging Clin Exp Res. 2006;18:191-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Tei M, Ikeda M, Haraguchi N, Takemasa I, Mizushima T, Ishii H, Yamamoto H, Sekimoto M, Doki Y, Mori M. Postoperative complications in elderly patients with colorectal cancer: comparison of open and laparoscopic surgical procedures. Surg Laparosc Endosc Percutan Tech. 2009;19:488-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Akiyoshi T, Kuroyanagi H, Oya M, Konishi T, Fukuda M, Fujimoto Y, Ueno M, Yamaguchi T. Short-term outcomes of laparoscopic rectal surgery for primary rectal cancer in elderly patients: is it safe and beneficial? J Gastrointest Surg. 2009;13:1614-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Tomimaru Y, Ide Y, Murata K. Outcome of laparoscopic surgery for colon cancer in elderly patients. Asian J Endosc Surg. 2011;4:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Robinson CN, Balentine CJ, Marshall CL, Wilks JA, Anaya D, Artinyan A, Berger DH, Albo D. Minimally invasive surgery improves short-term outcomes in elderly colorectal cancer patients. J Surg Res. 2011;166:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | She WH, Poon JT, Fan JK, Lo OS, Law WL. Outcome of laparoscopic colectomy for cancer in elderly patients. Surg Endosc. 2013;27:308-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 31. | Scarpa M, Di Cristofaro L, Cortinovis M, Pinto E, Massa M, Alfieri R, Cagol M, Saadeh L, Costa A, Castoro C. Minimally invasive surgery for colorectal cancer: quality of life and satisfaction with care in elderly patients. Surg Endosc. 2013;27:2911-2920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Hinoi T, Kawaguchi Y, Hattori M, Okajima M, Ohdan H, Yamamoto S, Hasegawa H, Horie H, Murata K, Yamaguchi S. Laparoscopic versus open surgery for colorectal cancer in elderly patients: a multicenter matched case-control study. Ann Surg Oncol. 2015;22:2040-2050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Miyasaka Y, Mochidome N, Kobayashi K, Ryu S, Akashi Y, Miyoshi A. Efficacy of laparoscopic resection in elderly patients with colorectal cancer. Surg Today. 2014;44:1834-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Vallribera Valls F, Landi F, Espín Basany E, Sánchez García JL, Jiménez Gómez LM, Martí Gallostra M, Salgado Cruz L, Armengol Carrasco M. Laparoscopy-assisted versus open colectomy for treatment of colon cancer in the elderly: morbidity and mortality outcomes in 545 patients. Surg Endosc. 2014;28:3373-3378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Zeng WG, Zhou ZX, Hou HR, Liang JW, Zhou HT, Wang Z, Zhang XM, Hu JJ. Outcome of laparoscopic versus open resection for rectal cancer in elderly patients. J Surg Res. 2015;193:613-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Shigeta K, Baba H, Yamafuji K, Asami A, Takeshima K, Nagasaki K, Okamoto N, Murata T, Arai S, Kubochi K. Effects of laparoscopic surgery on the patterns of death in elderly colorectal cancer patients: competing risk analysis compared with open surgery. Surg Today. 2016;46:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev. 2008;CD003432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3573] [Cited by in RCA: 2738] [Article Influence: 1369.0] [Reference Citation Analysis (0)] |