Published online Jun 15, 2015. doi: 10.4251/wjgo.v7.i6.47
Peer-review started: December 31, 2014
First decision: February 7, 2015
Revised: April 2, 2015
Accepted: May 5, 2015
Article in press: May 6, 2015
Published online: June 15, 2015
AIM: To investigate efficacy and safety of second-line treatment with irinotecan-loaded drug-eluting beads (DEBIRI) and cetuximab (DEBIRITUX) of unresectable colorectal liver metastases.
METHODS: Patients with the following characteristics were included in the study: unresectable hepatic metastases from colorectal carcinoma (CRC-LM), progression after first line chemotherapy (any type of chemotherapeutic drug and combination was allowed), second line treatment (mandatory), which included for each patient (unregarding the KRas status) two cycles of DEBIRI (using 100-300 μm beads loaded with irinotecan at a total dose 200 mg) followed by 12 cycles of cetuximab that was administered weekly at a first dose of 400 mg/m2 and then 250 mg/m2; good performance status (0-2) and liver functionality (alanine aminotransferase and gamma-glutamyl transferase not exceeding three times the upper limit of normal, total bilirubin not exceeding 2.5 mg/mL). Data were collected retrospectively and included: tumor response (evaluated monthly for 6 mo then every 3 mo), overall response rate (ORR), KRas status, type and intensity of adverse events (G according to the Common Terminology Criteria for Adverse Events v3.0, CTCAE), overall survival (OS) and progression free survival (PFS).
RESULTS: Forty consecutive cases of CRC hepatic metastases were included in the study. Median duration of DEBIRITUX was 4.4 mo (range, 4.0-6.5). Sixteen patients (40%) received the planned 2 cycles of DEBIRI and an average of 10 cetuximab cycles. ORR of the whole sample was 50%, in particular 4 patients were complete responders (10%) and 16 (40%) partial responders. The most observed side effects (G2) were: post-embolization syndrome (30%), diarrhea (25%), skin rushes (38%) and asthenia (35%). The retrospective evaluation of KRas status (24 wild type, 16 mutated) showed that the group of patients with wild type KRas had ORR significantly higher than mutant KRas. Median follow-up was 29 mo (8-48 range); median PFS was 9.8 mo and OS was 20.4 mo. Future randomized trials are required in this setting to establish a role for DEBIRITUX compared with systemic chemotherapy.
CONCLUSION: DEBIRITUX seems to be efficacious after first line chemotherapy for the treatment of unresectable CRC-LM.
Core tip: Irinotecan-loaded drug-eluting beads (DEBIRI) has shown manageable toxicities and favorable response rates for unresectable colorectal liver metastasis (CRC-LM). This study is the first in the world investigating effectiveness and toxicity of the association DEBIRI and cetuximab (DEBIRITUX) as second line therapy of CRC-LM. Forty cases were enrolled. The overall response rate (ORR) was 50%. Most frequent side effects were: post-embolization syndrome, diarrhea, skin rushes and asthenia. The group of patients with wild type KRas had ORR significantly higher than mutant Kras. The median progression free survival was 9.8 mo and overall survival was 20.4 mo. DEBIRITUX regimen seems effective and safe after first line chemotherapy for CRC-LM.
- Citation: Fiorentini G, Aliberti C, Sarti D, Coschiera P, Tilli M, Mulazzani L, Giordani P, Graziano F, Gonzalez AM, Marcos RG, Mugnoz FG, Cantore M, Ricci S, Catalano V, Mambrini A. Locoregional therapy and systemic cetuximab to treat colorectal liver metastases. World J Gastrointest Oncol 2015; 7(6): 47-54
- URL: https://www.wjgnet.com/1948-5204/full/v7/i6/47.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v7.i6.47
Colorectal cancer (CRC) is the second most commonly diagnosed cancer in Europe, with an annual incidence of nearly 450000 cases and an annual mortality of more than 200000 patients[1]. Nearly 25% of patients with CRC have synchronous liver metastases, whereas recurrence occurs in almost 70% of patients after resection of the primary tumor. Although surgery remains the only option for potential cure in patients with liver metastases from CRC, many patients have unresectable disease at diagnosis. The long-term survival rate of these patients is very low[2].
In the setting of unresectable metastatic CRC, the best outcome is achieved in patients receiving fluoropyrimidines, oxaliplatin, and irinotecan in their treatment plan[3,4]. Over the last decade, several combination of these three drugs significantly increased the response rate (RR) and overall survival (OS) time, with a RR of 40%-50% and a median OS duration up to 20 mo[5-9].
In a sequential strategy, however, one third of patients are not able to receive second-line chemotherapy due to side effects or liver progression.
The majority of patients with unresectable liver metastasis from CRC receive systemic chemotherapy with one or more agents. Combination chemotherapy regimens consist of fluoropyrimidines with oxaliplatin and/or irinotecan[3,6,10]. The administration of molecular targeted agents (bevacizumab, cetuximab, panitumumab) improve response rates and significantly prolongs median survival times in patients with metastatic CRC[11-13].
Recent advances focus on targeting the pathway of epidermal growth factor receptor (EGFR). The monoclonal antibody cetuximab is an EGFR antagonist that is capable of activating internalization of the receptor and its degradation, leading to increased tumor cell apoptosis[14]. Cetuximab, initially approved for clinical use in patients with detectable EGFR who failed on irinotecan chemotherapy[15], is nowadays widely used in combination with chemotherapy, because of its different toxicity profile in respect to the classical side effects of chemotherapy.
The role of intra-arterial chemotherapy of liver metastases is constantly evolving as the technique and its practical application improve[16-20]. Recent reports show that the application of DEBIRI to the intra-arterial therapy of liver metastasis from CRC (CRC-LM) is effective, feasible and has limited side effects[21-25]. Drug-eluting bead are small particles able to carry the chemotherapeutic agents directly to arterial vessels. In this way toxic agent concentration in the liver tumors is increased, whereas the systemic exposure to drugs is decreased. The amount of cell death, moreover, is higher with DEBIRI than trans-arterial chemoembolization[26]. Since liver arteries are the main circulation of CRC-LM (90%), DEBIRI method can deliver elevate concentration of toxic agents inside the tumor, resulting in systemic low adverse events. Hence DEBIRI can be particularly useful in patients previously treated with other lines of chemotherapy.
We recently reported the data of FOLFIRI vs DEBIRI for the therapy of heavily previously treated patients with CRC-LM. The data analysis showed a statistically significant advantage of DEBIRI compared with FOLFIRI, in terms of OS, progression free survival (PFS), months to extra-hepatic progression, life quality[27]. The association of cetuximab and DEBIRI should be a further clinical research step for CRC-LM therapy, because irinotecan and cetuximab are efficacious and have acceptable, different and not cumulative toxicities.
The purpose of our analysis is to assess effectiveness and toxicity of DEBIRI in association with intravenously cetuximab as second-line chemotherapy in unresectable CRC-LM.
All patients within this study had histological confirmed non-resectable colorectal adenocarcinoma that was metastatic to the liver and were of age > 18 years. Other eligibility criteria were: good clinical conditions; tumor size evaluation with RECIST version 1.1; normal liver and renal functions; normal hematological values; one previous line of chemotherapy for metastatic disease at least 1 mo before DEBIRITUX; estimated life expectancy ≥ 3 mo. Exclusion criteria were: contraindication to angiographic and selective visceral catheterization; presence of extra hepatic disease; brain or leptomeningeal metastases; bad absorption; inflammatory intestinal disease; psychiatric severe impairment; active infection; peripheral neuropathy ≥ grade 2; pregnancy or breast feeding; previous cetuximab therapy; other severe clinical impairment.
This was a cohort study, data were collected from 40 consecutive eligible patients that had received the same second-line treatment: DEBIRITUX, notwithstanding the type of first line treatment and the KRas status.
Data collected included: blood-cells levels, biochemistry, anamnesis, objective examination, tumor size (evaluated with abdomen and pelvis computed tomography scan), carcinoembryonic antigen (CEA) levels, life quality and performance status. Positron emission tomography scan was used upon researchers’ decision to clarify disease extension. The above data were monitored before each DEBIRI, and every 4 cycles of cetuximab.
Every patient in the study received the same second line treatment (DEBIRITUX), notwithstanding the type of first line treatment and the KRas status. At the beginning of DEBIRI, an interventional radiologist performed a diagnostic angiography to assess the level of CRC-LM arterial diffusion. DEBIRI treatment consisted in the infusion of 1 mL of DC beads microspheres (100 to 300 microns diameter) charged with irinotecan (100 mg), a second DEBIRI administration was repeated after 30 d, as reported in our previous experience[28-30].
The loaded DC beads were mixed with non-ionic contrast solution and distilled water, to perform a correct infusion.
Systemic Cetuximab (maximum 12 cycles) administration was done at 400 mg/m2 and was planned one week after the first DEBIRI administration (day 1), on day 8 from study start, and continued on day 15, 22, 29. DEBIRI was repeated on day 36 and, then, cetuximab continued on day 43, 50, 57, 64, 71, 78, 85, 92 at a dose of 250 mg/m2. Cetuximab was suspended according to physician opinion or in case of progression or unbearable side effects. Cetuximab dosage could be reduced to 200 or 150 mg/m2 in cases severe side effects.
National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 was used for side effects assessment, whereas the Response Evaluation Criteria in Solid Tumors (RECIST) criteria version 1.1 was used for disease evaluation. CT scan was performed within 1, 3, 6 and 9 mo from the treatment completion according to RECIST indications[31-33].
The detections of KRas activating mutations (most frequently at codon 12 and 13) were done as previously reported[34]. Since some centers did not perform the KRas status analysis before treatment beginning, it was made retrospectively in the laboratory of the coordinating center of the study.
Kaplan-Meier analysis (MedCalc) was used for survival assessment; in particular, PFS was measured from beginning of DEBIRI to progression or death, whereas OS was computed beginning of DEBIRI to death or last follow-up date. OS analysis was performed for the whole sample, KRas wild type and KRas mutant group, to investigate differences related to KRas status.
χ2 and Student’s t test, were used to assess significativity of continous variables (P < 0.05).
From April 2011 to December 2014, 40 patients were enrolled from three centers, 24 (60%) were males and 16 (40%) females, with a median age of 61 years (range 47-74), 80% of sample had ECOG = 0. All patients had primary disease resection, 23 of them were treated with first-line chemotherapy for an average of 5 mo (range 3.5-6 mo); whereas 17 had one year of previous chemotherapy. The types of previous chemotherapy were reported in Table 1. Four patients had previous radiotherapy on the pelvis. Ninety percent of patients had increased CEA levels and 60% had values more than 10 times the upper limit range.
| DEBIRITUX | |
| Number of patients | 40 |
| Sex (M-F) | 24-16 |
| Age | 61 (range 47-74) |
| Liver involvement ( ≤ 25%- ≤ 50%) | 18-22 |
| Synchronous/metachronous disease | 0/40 |
| Number of metastases | 3.5 (range 3–9) |
| Largest diameter (cm) | 5 (range 2.5-6) |
| Performance status (0-1 and 2) | 25 and 15 |
| Extrahepatic metastases | 0 |
| Previous CHT (1 line/2 lines for ≤ 12 mo) | 23-17 |
| Types of previous CHT | 23 FOLFOX |
| 11 IFL | |
| 3 FOLFOX + bevacizumab | |
| 3 FU + cetuximab | |
| Weight loss in last 3 mo | 16 (40%) |
| CEA (ng/mL) | 90 (range 7.5-1250) |
| KRas (WT-M) | 24-16 |
| LDH (normal-high) | 32-8 |
| Albumin, g/dL (median) | 4 |
Every patient in the study received the same second line treatment (DEBIRITUX), notwithstanding the type of first line therapy. Median duration of DEBIRITUX was 4.4 mo (range, 4.0-6.5). Sixteen patients (40%) received the planned 2 cycles of DEBIRI and an average of 10 cetuximab cycles (9-12 range). Twenty-five patients (62.5%) had dose reduction of cetuximab because of toxicity, with an overall relative dosage of 85% of the planned dosage.
The most cases had several metastases (5 median; 1-8 range) and 28 (70%) had metastases in both liver lobes. Median diameter of largest lesion was 4.0 cm (range 2.0-6.5 cm), and total disease size was 8.8 cm (4-14 cm range). CRC-LM involved < 25% of liver in 28 (70%) cases, and 26%-50% in 12 (30%) patients.
Most affected lobe was the right (24 patients, 60%), whereas the left lobe was treated in 6 patients (15), and 10 patients (25%) received a bilobar treatment. The planned dosage of irinotecan was 200 mg for all patients, however 8 patients (20%) required dose reduction, due to early stasis during the arterial administration of DEBIRI. The median irinotecan dose was of 80% of the planned dosage (range, 50%-90%). Partial occlusion of blood circulation was attained in 48 DEBIRI, and was almost total in 32.
The majority of patients (90%) were hospitalized for treatment. Median hospitalization for DEBIRI was 48 h (24-72 range). Main significant effect on laboratory chemistry values was the increase in with blood-cells count from 6400 to 8700/mmc (26%). Median hemoglobin value decreased of 1%, from 12.5 to 12.0 g/dL after the treatment; median platelet count decreased of 2%, from 148 to 135 × 1000/mmc, median bilirubin value increased of 18%, from 0.8 to 1.2 mg/dL; median creatinine value decreased of 11%, from 0.9 to 0.8 mg/dL; and median albumin value decreased of 5%, from 4.1 to 4.0 g/dL. No changes were observed in INR.
Ten patients (25%) showed adverse reactions related to cetuximab. Four patients reported grade 3 adverse reactions, whereas 6 had grade 2 adverse reactions (Table 2).
| Adverse events | n (%) |
| Acne-like skin rash | 5 (50%) |
| Skin fissuring | 3 (30%) |
| Skin dryness | 3 (30%) |
| Hypersensitivity | 3 (30%) |
Post-embolic syndrome was the main side effects, and was observed as a consequence of 30% of DEBIRI. Other treatment-related events included gastritis in 6 (15%) patients, dehydration (G2) in 2 (5%) patients, cholecystitis (G3) in 1 (2.5%) case, and hypertension (G2) for 7 (17.5 %) patients. These side effects, however, were resolved without complications. These symptoms were probably related to the post embolization syndrome (PES). Elevation of liver enzymes occurred almost in every patient, probably due to the more extensive type of embolization performed.
Median follow-up was 29 mo (8-48 range). Overall response rate (ORR) was 50% after three months of therapy. Each patient, moreover, showed a > 50% reduction of CEA levels after 3 mo of treatment. This reduction was observed up to 6-mo of evaluation.
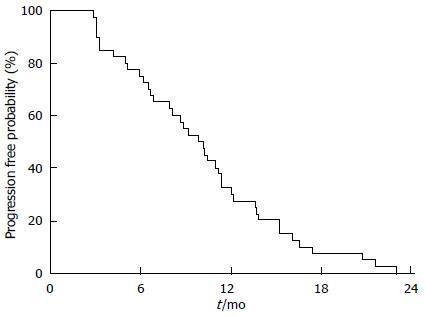
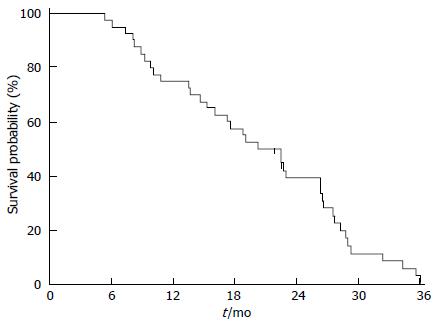
Twenty-five patients (60%) died because of disease progression. None died because of DEBIRITUX toxicity. Median PFS was 9.8 mo and OS was 20.4 mo, with 75.0% and 39.1% of patients alive at 1 and 2 years, respectively (Figures 1 and 2).
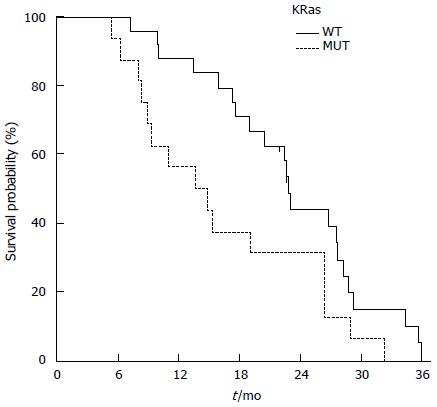
Twenty-four patients (60%) were KRas wild type (WT), whereas 16 (60%) had a mutation in KRas (MUT). Patients of the KRas wild-type group achieved a better ORR than the KRas mutated group 70.8% (95%CI: 52.6-89.0) compared to 37.5% (95%CI: 13.8-61.2) respectively. The toxicity was the same for both groups. PFS was slightly increased in KRas-WT than KRas-MUT [11.3 and 9.9 mo, respectively; HR = 1.55 (95%CI: 0.790-3.054; P = 0.148)]. OS was 14.2 mo for KRas-MUT and 22.8 mo for KRas-WT (HR = 1.97; 95%CI: 0.965-4.050; P = 0.029) (Figure 3).
Surgical resection of CRC-LM is the preferred intervention, but it can be done only 20%-35% of cases[35]. Effective chemotherapy for CRC consists in fluoropyrimidines with oxaliplatin and/or irinotecan[36]. The use of biomolecular agents, such as bevacizumab, cetuximab and panitumumab, increments RR of 50%-80% and increases survival time up to 20-24 mo in CRC-LM[10-16,36].
Many CRC-LM are not indicated for surgery after a first-line chemotherapy or progress after multiple treatment. The second line treatment in these cases is still under discussion, and their prognosis is poor[7,37,38]. New cytotoxic and biomolecular drugs have been recently introduced for CRC-LM therapy, however, the use of locoregional therapy is increasing for the improvement of RR and OS[39]. The association of systemic and locoregional treatment ia an example of the modern concept of multidisciplinary CRC metastases management, requiring more collaboration with interventional radiologists[40].
The expected advantage of trans-arterial chemoembolization (TACE) is the increase of drug concentrations inside the tumor and the decrease of systemic leakage[39]. Promising results are obtained with TACE in CRC-LM[41].
The palliative role of TACE is assessed in large case series by Vogl et al[42]. They show that 463 CRC-LM patients are treated with TACE that is repeated each 4-wk (2441 total TACE; on average 5.3 TACE/patient)[42]. They use chemotherapy with mitomycin C alone, mitomycin C/gemcitabine or mitomycin C/irinotecan, and they perform the embolization with lipiodol and microspheres for vessel occlusion[42]. They show, in particular, partial response in 12% of patients, stable disease in 51% and progressive disease in 37%, with 1-year and 2-year OS in 62% and 38% of cases respectively[42].
Many efforts have made recently to improve TACE, expecially applying new toxic agents to the liver for a longer period. New polyvinyl alcohol beads are capable of being loaded with doxorubicin and irinotecan. They continuously release the drug in the liver after injection in the arterial vessels of the liver[40]. Embolization associated with the delivery of these particles allows to decreasing the flow inside tumor-feeding arteries and, then, the washout of drugs. This procedure can increase the dwell time of anticancer drugs in proximity of tumor cells. In 2007, our group reported the first experience with DEBIRI applied to twenty CRC-LM patients, which had been previously treated with chemotherapy[23]. Most of the sample (80%) showed a response according to RECIST. As concerning toxicity, they all had G2 fever; 15 patients had G2/3 right upper quadrant pain[23]. Median hospitalization for the procedure was 3 d (1-10 range )[23].
Martin et al[43] published data from a trial with CRC-LM 55 patients, which showed a RR of 66% at 6 mo and 75% at 12 mo; OS of 19 mo and and PFS of 11 mo. Eleven (20%) patients had a significant tumor response and reduced disease intensity or stable response without progression that allowed resection, ablation or both[44].
Recently, we reported the results of a randomized trial on DEBIRI vs FOLFIRI after second or third lines of systemic chemotherapy in CRC-LM[45]. Patients treated with DEBIRI achieved a 43% OS improvement (22 vs 15 mo of FOLFIRI; P = 0.031), higher PFS (7 vs 4 mo of FOLFIRI; P = 0.006), an improved RR (68% vs 20% of FOLFIRI)[45]. DEBIRI resulted in increased toxicity (G3 pain, nausea, fever), because of post embolization syndrome[45]. Diarrhea, asthenia, leucopenia, anemia and alopecia were the most observed side effects of FOLFIRI[45]. We reported for the first time that DEBIRI improved OS, reducing cost treatments of systemic therapy. Noteworthy, the median life quality improvement was observed for 8 mo after DEBIRI and 3 after FOLFIRI (P = 0.00002).
In this paper we investigated for the first time the tumor response and toxicity of DEBIRI in association to cetuximab as second-line treatment of unresectable CRC-LM.
Our results showed that the association systemic/hepatic intra-arterial therapy was practicable and efficacious. An ORR of 50% was encouraging and comparable with previous reports[46]. The analysis of PFS and OS were also promising and further supported DEBIRITUX efficacy.
DEBIRITUX had low toxicity, showing only the known drug-related (irinotecan and cetuximab) toxicities. Diarrhea was the most observed adverse event, this was probably due to both irinotecan and cetuximab. The management of patients affected by CRC liver metastases with TACE and targeted agents was challenging, and resulted in interesting PFS and OS.
Cetuximab is a non-chemotherapeutic agent targeting the EGFR, is effective and is applied worldwide for the treatment of CRC with KRas wild type[47,48].
The aim and efficacy of loco-regional therapy of the liver is well known, however its several side effect (biliar stenosis, catheter displacement and consequent systemic leakage) may undermine its application[31-34]. DEBIRI may overcome the above disadvantages, because it is a more precise and direct method, resulting in mild intensity general side effects[21-33]. DEBIRI can also be combined with therapies involving monoclonal antibodies, such as cetuximab.
Our results do not show any severe cetuximab or irinotecan general side effects. Most common adverse events are related to the PES, including pain, nausea, and hypertension.
DEBIRI can be performed also after several previous line of therapy, provided that an adequate supportive therapy is guaranteed. This point is crucial as the duration of hospitalization may be limited and tolerability of treatment (less abdominal pain, discomfort, serum transaminases level elevation) may be improved.
KRas analysis, unfortunately, was not performed at the beginning of the treatment, but it was done retrospectively in the central laboratory of the coordinating center. This may have affected the RR, since cetuximab is not efficacious in KRas mutated cases. We show a possible correlation of KRas status and response to DEBIRITUX, this is supported also in other reports[35-39].
In conclusion, DEBIRITUX is efficacious and induces low toxicity for CRC-LM therapy, as it appears from the few side effects that was observed and the high RR, and the prolonged decrease of CEA. More clinical trials are required to address this issue, and to assess when DEBIRITUX should be applied in the therapeutic sequence of CRC-LM. This study is the first to our knowledge that confirms the efficacy of systemic plus intra-arterial therapy association in the management of CRC-LM.
Colorectal cancer (CRC) is nowadays one of the principal health care concerns and leading tumor in Europe and United States. Surgical resection is feasible only for 20% of patients with liver metastases (LM). The recently introduced loco-regional therapy with drug-eluting beads plus irinotecan (DEBIRI) shows good tumor reduction in unresectable CRC-LM that were previously treated with 2 or more chemotherapy regimens.
Cetuximab is a monoclonal antibody that antagonize the epidermal growth factor receptor and is widely used in combination with chemotherapy because of its different toxicity profile in respect to the classical side effects of chemotherapy. Cetuximab associated to irinotecan has been recently approved for CRC-LM treatment, as second line therapy. This study investigated the efficacy and safety of the association cetuximab/DEBIRI (DEBIRITUX) as second-line therapy of CRC-LM.
The aim and efficacy of loco-regional therapy of the liver is well known, however its several side effect. DEBIRI has the potential of overcome this disadvantages, since it can be a safer and more direct method, resulting in mild intensity general side effects, as reported in the literature. DEBIRI can also be combined with therapies involving monoclonal antibodies, such as cetuximab. This study is the first to the knowledge that confirms the activity of the combination of systemic plus intra-arterial therapy of CRC-LM.
DEBIRITUX is efficacious and induces low toxicity for CRC-LM therapy, as it appears from the few side effects that was observed and the high response rate, and the prolonged decrease of carcinoembryonic antigen. More clinical trials are required to address this issue, and to assess when DEBIRITUX should be applied in the therapeutic sequence of CRC-LM.
DEBIRI is an intra-arterial therapy adopting drug-eluting beads preloaded with irinotecan. Drug-eluting bead are small particles able to carry the chemotherapeutic agents directly to arterial vessels of the metastases. In this way the toxic agent concentration in the liver tumors is increased, whereas the systemic exposure to drugs is decreased. The volume of tissue necrosis, moreover, is significantly greater when using drug-eluting beads compared to a conventional trans-arterial chemoembolization. Since liver arteries are the main sustainance of CRC-LM (90%), DEBIRI method can deliver elevate concentration of toxic agents inside the tumor, resulting in systemic low adverse events.
The authors investigated the efficacy and safety of the addition of cetuximab to DEBIRI (DEBIRITUX) as second-line treatment in patients with unresectable liver metastases from CRC. It interesting that the regimen combined with DEBIRI and cetuximab appear to be effective and feasible in second line treatment.
P- Reviewer: Herszenyi L, Jimi S S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
| 1. | Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3526] [Cited by in F6Publishing: 3571] [Article Influence: 324.6] [Reference Citation Analysis (2)] |
| 2. | Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16:481-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 892] [Cited by in F6Publishing: 954] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 3. | O’Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420-1425. [PubMed] [Cited in This Article: ] |
| 4. | Adam R, Delvart V, Pascal G, Valeanu A, Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F, Ghémard O. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240:644-657; discussion 657-658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 746] [Cited by in F6Publishing: 885] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 5. | Grothey A, Sargent D, Goldberg RM, Schmoll HJ. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004;22:1209-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 800] [Cited by in F6Publishing: 858] [Article Influence: 42.9] [Reference Citation Analysis (1)] |
| 6. | Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2181] [Cited by in F6Publishing: 2147] [Article Influence: 102.2] [Reference Citation Analysis (1)] |
| 7. | Saltz LB, Cox JV, Blanke C, Rosen LS, Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta N. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2273] [Cited by in F6Publishing: 2190] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 8. | Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts SR. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1710] [Cited by in F6Publishing: 1700] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 9. | Rothenberg ML, Oza AM, Bigelow RH, Berlin JD, Marshall JL, Ramanathan RK, Hart LL, Gupta S, Garay CA, Burger BG. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003;21:2059-2069. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 504] [Cited by in F6Publishing: 527] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 10. | Falcone A, Ricci S, Brunetti I, Pfanner E, Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W, Fanchini L. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the Gruppo Oncologico Nord Ovest. J Clin Oncol. 2007;25:1670-1676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 830] [Cited by in F6Publishing: 838] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 11. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2147] [Cited by in F6Publishing: 2218] [Article Influence: 138.6] [Reference Citation Analysis (0)] |
| 12. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2901] [Cited by in F6Publishing: 3021] [Article Influence: 201.4] [Reference Citation Analysis (1)] |
| 13. | Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1218] [Cited by in F6Publishing: 1202] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 14. | Chen HX, Mooney M, Boron M, Vena D, Mosby K, Grochow L, Jaffe C, Rubinstein L, Zwiebel J, Kaplan RS. Phase II multicenter trial of bevacizumab plus fluorouracil and leucovorin in patients with advanced refractory colorectal cancer: an NCI Treatment Referral Center Trial TRC-0301. J Clin Oncol. 2006;24:3354-3360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 15. | Wu X, Fan Z, Masui H, Rosen N, Mendelsohn J. Apoptosis induced by an anti-epidermal growth factor receptor monoclonal antibody in a human colorectal carcinoma cell line and its delay by insulin. J Clin Invest. 1995;95:1897-1905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 284] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3767] [Cited by in F6Publishing: 3625] [Article Influence: 181.3] [Reference Citation Analysis (0)] |
| 17. | Brown DB, Gould JE, Gervais DA, Goldberg SN, Murthy R, Millward SF, Rilling WS, Geschwind JF, Salem R, Vedantham S. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2007;18:1469-1478. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Tang Y, Taylor RR, Gonzalez MV, Lewis AL, Stratford PW. Evaluation of irinotecan drug-eluting beads: a new drug-device combination product for the chemoembolization of hepatic metastases. J Control Release. 2006;116:e55-e56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Taylor RR, Tang Y, Gonzalez MV, Stratford PW, Lewis AL. Irinotecan drug eluting beads for use in chemoembolization: in vitro and in vivo evaluation of drug release properties. Eur J Pharm Sci. 2007;30:7-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 131] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Fiorentini G. A new tool to enhance the efficacy of chemoembolization to treat primary and metastatic hepatic tumors. Expert Opin Drug Deliv. 2011;8:409-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Lewis AL, Holden RR. DC Bead embolic drug-eluting bead: clinical application in the locoregional treatment of tumours. Expert Opin Drug Deliv. 2011;8:153-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Aliberti C, Tilli M, Benea G, Fiorentini G. Trans-arterial chemoembolization (TACE) of liver metastases from colorectal cancer using irinotecan-eluting beads: preliminary results. Anticancer Res. 2006;26:3793-3795. [PubMed] [Cited in This Article: ] |
| 23. | Fiorentini G, Aliberti C, Turrisi G, Del Conte A, Rossi S, Benea G, Giovanis P. Intraarterial hepatic chemoembolization of liver metastases from colorectal cancer adopting irinotecan-eluting beads: results of a phase II clinical study. In Vivo. 2007;21:1085-1091. [PubMed] [Cited in This Article: ] |
| 24. | Martin RC, Howard J, Tomalty D, Robbins K, Padr R, Bosnjakovic PM, Tatum C. Toxicity of irinotecan-eluting beads in the treatment of hepatic malignancies: results of a multi-institutional registry. Cardiovasc Intervent Radiol. 2010;33:960-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Aliberti C, Fiorentini G, Muzzio PC, Pomerri F, Tilli M, Dallara S, Benea G. Trans-arterial chemoembolization of metastatic colorectal carcinoma to the liver adopting DC Bead®, drug-eluting bead loaded with irinotecan: results of a phase II clinical study. Anticancer Res. 2011;31:4581-4587. [PubMed] [Cited in This Article: ] |
| 26. | Kettenbach J, Stadler A, Katzler IV, Schernthaner R, Blum M, Lammer J, Rand T. Drug-loaded microspheres for the treatment of liver cancer: review of current results. Cardiovasc Intervent Radiol. 2008;31:468-476. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 104] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Narayanan G, Barbery K, Suthar R, Guerrero G, Arora G. Transarterial chemoembolization using DEBIRI for treatment of hepatic metastases from colorectal cancer. Anticancer Res. 2013;33:2077-2083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Fiorentini G, Aliberti C, Tilli M, Mulazzani L, Graziano F, Giordani P, Mambrini A, Montagnani F, Alessandroni P, Catalano V. Intra-arterial infusion of irinotecan-loaded drug-eluting beads (DEBIRI) versus intravenous therapy (FOLFIRI) for hepatic metastases from colorectal cancer: final results of a phase III study. Anticancer Res. 2012;32:1387-1395. [PubMed] [Cited in This Article: ] |
| 29. | Gaur SK, Friese JL, Sadow CA, Ayyagari R, Binkert CA, Schenker MP, Kulke M, Baum R. Hepatic arterial chemoembolization using drug-eluting beads in gastrointestinal neuroendocrine tumor metastatic to the liver. Cardiovasc Intervent Radiol. 2011;34:566-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Fiorentini G, Aliberti C, Benea G, Montagnani F, Mambrini A, Ballardini PL, Cantore M. TACE of liver metastases from colorectal cancer adopting irinotecan-eluting beads: beneficial effect of palliative intra-arterial lidocaine and post-procedure supportive therapy on the control of side effects. Hepatogastroenterology. 2008;55:2077-2082. [PubMed] [Cited in This Article: ] |
| 31. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12751] [Cited by in F6Publishing: 12880] [Article Influence: 536.7] [Reference Citation Analysis (0)] |
| 32. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3252] [Cited by in F6Publishing: 3170] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 33. | Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753-1759. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1094] [Cited by in F6Publishing: 1067] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 34. | De Roock W, Piessevaux H, De Schutter J, Janssens M, De Hertogh G, Personeni N, Biesmans B, Van Laethem JL, Peeters M, Humblet Y. KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab. Ann Oncol. 2008;19:508-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 623] [Cited by in F6Publishing: 617] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 35. | Wieser M, Sauerland S, Arnold D, Schmiegel W, Reinacher-Schick A. Peri-operative chemotherapy for the treatment of resectable liver metastases from colorectal cancer: A systematic review and meta-analysis of randomized trials. BMC Cancer. 2010;10:309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Inoue Y, Hiro J, Toiyama Y, Tanaka K, Mohri Y, Kusunoki M. Optimal use of current chemotherapy in multimodality therapy for advanced colorectal cancer. Oncol Lett. 2012;3:363-368. [PubMed] [Cited in This Article: ] |
| 37. | Adam R. Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Ann Oncol. 2003;14 Suppl 2:ii13-ii16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 38. | Cohen AD, Kemeny NE. An update on hepatic arterial infusion chemotherapy for colorectal cancer. Oncologist. 2003;8:553-566. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Eichler K, Zangos S, Mack MG, Hammerstingl R, Gruber-Rouh T, Gallus C, Vogl TJ. First human study in treatment of unresectable liver metastases from colorectal cancer with irinotecan-loaded beads (DEBIRI). Int J Oncol. 2012;41:1213-1220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 40. | Brown DB, Geschwind JF, Soulen MC, Millward SF, Sacks D. Society of Interventional Radiology position statement on chemoembolization of hepatic malignancies. J Vasc Interv Radiol. 2006;17:217-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 41. | Herrmann KA, Waggershauser T, Sittek H, Reiser MF. Liver intraarterial chemotherapy: use of the femoral artery for percutaneous implantation of catheter-port systems. Radiology. 2000;215:294-299. [PubMed] [Cited in This Article: ] |
| 42. | Vogl TJ, Gruber T, Balzer JO, Eichler K, Hammerstingl R, Zangos S. Repeated transarterial chemoembolization in the treatment of liver metastases of colorectal cancer: prospective study. Radiology. 2009;250:281-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 43. | Martin RC, Joshi J, Robbins K, Tomalty D, Bosnjakovik P, Derner M, Padr R, Rocek M, Scupchenko A, Tatum C. Hepatic intra-arterial injection of drug-eluting bead, irinotecan (DEBIRI) in unresectable colorectal liver metastases refractory to systemic chemotherapy: results of multi-institutional study. Ann Surg Oncol. 2011;18:192-198. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 44. | Bower M, Metzger T, Robbins K, Tomalty D, Válek V, Boudný J, Andrasina T, Tatum C, Martin RC. Surgical downstaging and neo-adjuvant therapy in metastatic colorectal carcinoma with irinotecan drug-eluting beads: a multi-institutional study. HPB s. 2010;12:31-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 45. | Fiorentini G, Montagnani F, Tilli M, Mambrini A, Benea G. Trans-arterial chemoembolisation of metastatic colorectal carcinoma (mCRC) to the liver adopting polyvinylalcohol microspheres loaded with irinotecan (DEBIRI) compared to FOLFIRI (CT): evaluation at two years of a phase III clinical trial. Ann Oncol. 2010;21. [Cited in This Article: ] |
| 46. | Kemeny MM, Adak S, Gray B, Macdonald JS, Smith T, Lipsitz S, Sigurdson ER, O’Dwyer PJ, Benson AB. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy--an intergroup study. J Clin Oncol. 2002;20:1499-1505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 47. | Di Fiore F, Blanchard F, Charbonnier F, Le Pessot F, Lamy A, Galais MP, Bastit L, Killian A, Sesboüé R, Tuech JJ. Clinical relevance of KRAS mutation detection in metastatic colorectal cancer treated by Cetuximab plus chemotherapy. Br J Cancer. 2007;96:1166-1169. [PubMed] [Cited in This Article: ] |
| 48. | Normanno N, Tejpar S, Morgillo F, De Luca A, Van Cutsem E, Ciardiello F. Implications for KRAS status and EGFR-targeted therapies in metastatic CRC. Nat Rev Clin Oncol. 2009;6:519-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 331] [Article Influence: 22.1] [Reference Citation Analysis (0)] |