Published online Dec 15, 2015. doi: 10.4251/wjgo.v7.i12.411
Peer-review started: June 1, 2015
First decision: August 25, 2015
Revised: September 8, 2015
Accepted: October 23, 2015
Article in press: October 27, 2015
Published online: December 15, 2015
Processing time: 200 Days and 19.1 Hours
Pancreatic surgery for malignancy is recognized as challenging for the surgeons and risky for the patients due to consistent perioperative morbidity and mortality. Furthermore, the oncological long-term results are largely disappointing, even for those patients who experience an uneventfully hospital stay. Nevertheless, surgery still remains the cornerstone of a multidisciplinary treatment for pancreatic cancer. In order to maximize the benefits of surgery, the advent of both laparoscopy and robotics has led many surgeons to treat pancreatic cancers with these new methodologies. The reduction of postoperative complications, length of hospital stay and pain, together with a shorter interval between surgery and the beginning of adjuvant chemotherapy, represent the potential advantages over conventional surgery. Lastly, a better cosmetic result, although not crucial in any cancerous patient, could also play a role by improving overall well-being and patient self-perception. The laparoscopic approach to pancreatic surgery is, however, difficult in inexperienced hands and requires a dedicated training in both advanced laparoscopy and pancreatic surgery. The recent large diffusion of the da Vinci® robotic platform seems to facilitate many of the technical maneuvers, such as anastomotic biliary and pancreatic reconstructions, accurate lymphadenectomy, and vascular sutures. The two main pancreatic operations, distal pancreatectomy and pancreaticoduodenectomy, are approachable by a minimally invasive path, but more limited interventions such as enucleation are also feasible. Nevertheless, a word of caution should be taken into account when considering the increasing costs of these newest technologies because the main concerns regarding these are the maintenance of all oncological standards and the lack of long-term follow-up. The purpose of this review is to examine the evidence for the use of minimally invasive surgery in pancreatic cancer (and less aggressive tumors), with particular attention to the oncological results and widespread reproducibility of each technique.
Core tip: Laparoscopic and robotic surgeries for pancreatic cancer are very promising for reducing the frequent complications that occur after open surgery. Nevertheless, the oncologic long-term results remain the cornerstone of any procedure. Most of the studies revealed a lack of evidence for long-term benefits and few comparisons with alternative options.
- Citation: Bencini L, Annecchiarico M, Farsi M, Bartolini I, Mirasolo V, Guerra F, Coratti A. Minimally invasive surgical approach to pancreatic malignancies. World J Gastrointest Oncol 2015; 7(12): 411-421
- URL: https://www.wjgnet.com/1948-5204/full/v7/i12/411.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v7.i12.411
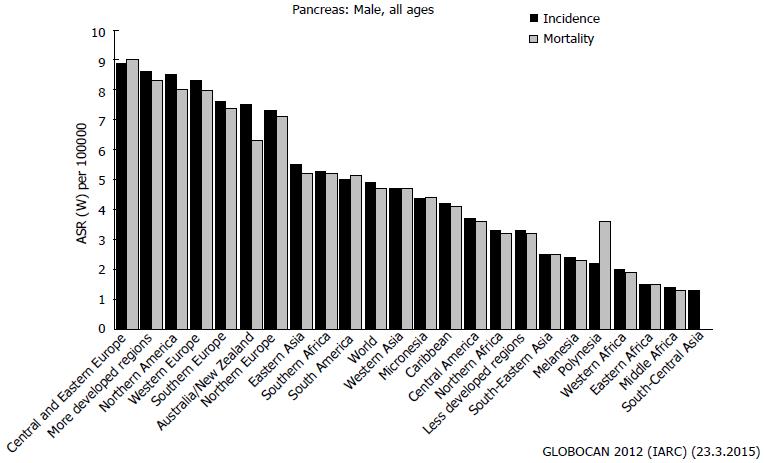
The actual incidence of pancreatic cancer [(pancreatic ductal adenocarcinoma (PDAC)] is not high worldwide, ranging from 1 to 9 new cases per 100000 inhabitants. Unfortunately, the reported mortality is almost equivalent to the incidence, illuminating the high number of affected patients who will die within a few months of the diagnosis[1] (Figure 1).
The best chance for a cure is still represented by a curative surgical resection[2]. Other pre-neoplastic lesions [i.e., intraductal papillary mucinous neoplasm - (IPMN)] and borderline neuroendocrine tumors often require a resection via a surgical approach[3,4].
Despite the histologic subtype, neoplasms growing in the pancreas can be managed through a minimally invasive approach, but the widespread adoption of such techniques is still limited. According to a large, nationwide, American database, only less than 5% of hepato-bilio-pancreatic procedures were reported to be carried out by a minimally invasive approach[5].
There is a myriad of possible explanations for the limited use of minimally invasive surgery (MIS) compared to other approaches. Firstly, the major pancreatic surgeries [i.e., pancreaticoduodenectomy (PD)] require multiple complex reconstructions, with a high incidence of severe post-operative complications. Simpler resections [i.e., distal pancreatectomy (DP) or enucleation] can bring with them the development of life-threatening fistulas or postoperative severe pancreatitis.
Moreover, an important group of published studies demonstrated a subspecialized training among surgeons as well as a caseload centralization, drastically reduced mortality and failure to rescue after a life-threatening complication occurred[6-8]. Similarly, the need for advanced laparoscopic or robotic skills requirements and expensive technical facilities required for minimally invasive pancreatic surgery is reserved to only a few subspecialized centers[9]. Lastly, the oncologic accuracy, rather than the feasibility, remains the cornerstone of pancreatic surgery for cancer[10].
However, the poor oncologic prognosis of patients affected by PDAC represents an important incentive to adopt some minimally invasive operation that is able to minimize the perioperative morbidity and mortality. Indeed, the traditional benefits of MIS over open surgery are the reduction of pulmonary complications, infections, pain, length of stay and cosmetic result. Many researchers confirmed the utility of MIS in decreasing the pro-inflammatory and immunologic response to surgical trauma that is associated with a superior oncologic result[11,12].
Interestingly, a survey within patients and medical personnel found some preference towards laparoscopic procedures when dealing with pancreatic benign disease and a preference towards open surgery in cases of cancer[13]. Nevertheless, most of the minimally invasive pancreatic procedures failed to reach a sufficient level of evidence-based efficacy to enable a routine application.
The aim of this review was to focus on the MIS (laparoscopy, robotic, hybrid) to manage malignancies and borderline neoplasms arising from the pancreas. Endoscopic and percutaneous maneuvers, although recognized as a great help when dealing with pancreatic neoplasms, did not represent the core of the article and were treated marginally.
A web-based search of MEDLINE (through PubMed and Ovid) and Cochrane databases was updated to April 2015. Many cross-matched manual references were also included. Randomized controlled clinical trials (RCTs) or meta-analyses were considered a priority. Data arising from more recent, English-written, multi-centric, international studies and those with long-term follow-up and oncologic results were also considered of major interest.
PD is a highly demanding surgical operation, even in the hands of skilled surgeons with specific training. The most challenging steps include pancreatic, biliary and gastroenteric reconstructions that can lead to leakages, perioperative complications and mortality. Most of these operations are carried out to treat malignancy, although more restrictive indications could be IPMN, neuroendocrine tumors or borderline lesions.
As in other gastrointestinal districts, many efforts have been made to limit the destructive impact of this kind of surgery through a minimally invasive approach.
Gagner et al[14] described the first laparoscopic PD (LPD) in 1994, but less than 500 operations have been reported in the literature since then[15-18] due to many unresolved issues.
First of all, the limited incidence of pancreatic tumors compared to colorectal cancer reduced the number of the centers with sufficient caseload. Second, the reconstructive steps and the vascular dissection are very complex and difficult to be achieved by the laparoscopic route whereas the benefits are still under discussion.
Croome et al[19] in a recent paper comparing 108 LPD and 214 open PD (OPD) cases well matched for pathologic parameters, reported a shorter length of hospital stay in the LPD group (6 d vs 9 d, P < 0.001). The other perioperative outcomes, including leakages, were similar. Interestingly, the authors found an earlier starting of adjuvant therapy and a longer progression-free survival in the LPD patients, although the overall survival was similar between the two groups. From a speculative point of view, the prolonged interval between surgery and the beginning of adjuvant chemotherapy may affect the overall survival.
Conversely, Dokmak et al[20] retrospectively compared 46 LPD to 46 OPD cases, matched for demographic data, associated comorbidities, and underlying disease. Patients in the laparoscopic group had a lower BMI, a softer pancreas, longer operating time (342 min vs 264 min; P < 0.001), more grade C pancreatic fistula (PF) (24% vs 6%; P = 0.007), bleedings (24% vs 7%; P = 0.02), and revision surgery (24% vs 11%; P = 0.09). According to these results, the authors concluded that LPD was not to be recommended on a routine basis.
In one updated review by Boggi et al[21] including 25 selected articles, a total of 746 minimally invasive PD cases were collected. Of these, pure LPD was used in only 386 patients (51.7%), robotic assistance in 234 (31.3%), laparoscopic assistance in 121 (16.2%), and hand assistance in five (0.6%). LPD was associated with some better perioperative parameters (i.e., blood loss and operative times) compared to robotics and hybrid approaches. Conversion to open surgery was required in 64 LPD (9.1%). No differences were noted in conversion rate, incidence of PF, morbidity, and mortality when comparing results from larger (≥ 30 LPD) and smaller (≤ 29 LPD) series. Interestingly, pure laparoscopy was employed in half of the whole cohort, while PDAC amounted only to 30% of the entire specimen. These two findings suggested how the laparoscopic approach was indicated in selected cases in the hands of skilled surgeons with wide technologic facilities available, including robotics.
In recent years, the use of robotic systems is gaining momentum as a valuable operative option in the field of pancreatic surgery. Indeed, robotics has emerged as a most interesting and promising innovation, improving the high-demanding surgical procedures, such as PD, with encouraging results[22-25].
With PD in particular, several limitations of standard laparoscopy have been partially overcome by robot-assisted surgery. The major benefits of the surgical robot are a magnified intraoperative imaging, an increased range of motion within narrow and deep spaces, and the enhanced surgical dexterity, affording optimal control during surgical dissections and reconstructions.
It is now more than 10 years since the first large series of robotic general surgical procedures was reported[26], including eight robotic PDs (RPD). The intervening years have seen RPD gaining relatively large distribution worldwide, and more than 350 robotic PDs have been made available in the literature in the last five years[22-32].
Despite the lack of evidence, based solely on retrospective analyses, the use of the robotic platform has already shown several potential advantages over both open surgery[22,33,34] and standard laparoscopy[24,34].
For example, Giulianotti et al[27] in 2010, published a cohort of 60 RPD with a rate of PF of 31.3% and only one reoperation. Another single-surgeon experience[32] reported 34 patients operated by RPD with a mean duration of surgery of 597 min and an extra cost of more than 6000 euros. However, the early outcomes were good, with a 0% 30-d mortality and a global 55% morbidity rate. The crucial point of the number of harvested nodes and the negative margins status were also highly comparable to that of open surgery.
In the largest series available in the current literature[24], 132 RPD were followed for postoperative complications in the first 90 d. The 30-d and 90-d mortality were 0.8% and 2.0%, respectively, with a percentage of important complications of 14% and 6% (grade C PF rate of 4%).
Several other non-randomized studies and meta-analyses[21-24,27-31] comparing laparoscopic, robotic and open resections showed comparable complication rates (including PF), mortality and adequacy of lymph nodes yield. Notably, wound infections, hospital stay, blood loss, transfusion rate and R1 resections were significantly lower in patients who underwent minimally invasive resections, with several observations supporting the potential advantages of robotics over conventional laparoscopy[32-34].
Unfortunately, data from the inherent knowledge still fail to provide definitive conclusions concerning the actual role of MIS in performing PD. Further investigations are strongly required, with a special need for randomized analyses comparing robotics and standard laparoscopy. Nonetheless, robot-assisted surgery seems to offer potential advantages in favoring the application of MIS for the treatment of pancreatic neoplasms.
DP is considered a less challenging operation for the surgeon, with a minor impacting postoperative recovery for the patient compared with PD. The reason is found in the lack of multiple anastomoses, including the potential life-threatening pancreatic remnant pancreaticojejunostomy or pancreaticogastrostomy. Therefore, minimally invasive DP has been widely accepted in the worldwide surgical community.
Gagner et al[35] published the first laparoscopic DP (LDP) in the mid-nineties, to manage neoplasms with a borderline behavior (i.e., neuroendocrine tumor). Since then, many retrospective experiences and less comparative series had been published, with the LDP becoming almost the gold-standard approach to both malignant and borderline-benign (mostly) lesions arising from the body-tail of the pancreas[10,36].
Unfortunately, there are some important discrepancies between the literature and ongoing surgical practice. For example, according to a survey conducted on a nationwide database during the period 1998-2009[37] and sampling 20% of United States hospitals, only 5% of DPs were carried out using a minimally invasive approach. However, a similar study regarding 2003-2009[9], found that LDP was utilized in 15%-27% of patients, although many postoperative parameters and the overall costs favored the laparoscopic route. A third[38], more recent (years 2005-2013) cohort study from 17 expert centers in the United States reported that LDP was superior to open distal pancreatectomy (ODP) regarding postoperative morbidity and length of hospital stay. However, only 64 (10%) patients of a total 633 had undergone LDP.
A possible explanation of these surveys could be the presence of few specialized environments with the available expertise and facilities to address pancreatic diseases, although a specific training could improve both the use and outcomes of LDP.
Obviously, the greater the experience, the lesser the patient selection, including complex patients, in maintaining the same postoperative morbidity[39]. Conversely, other authors suggested continuing a careful patient selection for laparoscopy to guarantee the reduction of blood loss and postoperative stay[40].
Nevertheless, many of the available reviews on LDPs include only retrospective case-series with short-term follow-up, different techniques and confusing data reporting[41,42]. One of the largest comparative series was that published by Jayaraman et al[43] from the Memorial Sloan Kettering Center on a total of 343 distal pancreatectomies during the 7-years study observation. One hundred seven (31%) of the 343 patients were approached laparoscopically, with a high conversion rate of 30%. However, the LDPs resulted in better outcomes (27% vs 40% of postoperative complication; P = 0.03), reduced blood loss, and shorter hospital stay (median 5 d vs 7 d; P < 0.0001), compared to standard operated controls. However, the operative times were longer (median 163 min vs 194 min; P < 0.0001), and the specific incidence of pancreatic leaks was similar in the two groups (15% vs 13%; P = NS).
Kooby et al[44] collected data from eight centers performing ODP and LDP, matching patients for age, American Society of Anesthesiologists score, tumor size, and diagnosis. The final analysis included 667 DPs, with 159 (24%) attempted laparoscopically. The conversion rate was 13%. In the final comparison (200 ODP vs 142 LDP), the authors reported no differences in the positive margin rates, operative times, or leak rates (18% vs 11%; P = 0.1). However, LDP had lower blood loss (357 mL vs 588 mL; P < 0.01), fewer complications (40% vs 57%; P < 0.01), and shorter hospital stays (5.9 d vs 9.0 d; P < 0.01).
Vijan et al[45] compared 100 matched patients undergoing LDP to an equal cohort undergoing ODP with similar demographic characteristics, but larger tumor size in the ODP group. The LDP group experienced decreased blood loss (171 mL vs 519 mL; P < 0.001) and shorter duration of hospital stay (6.1 d vs 8.6 d; P < 0.001). Conversely, they reported no differences in the operative time, pancreatic leak rate (17%), 30-d morbidity (34% vs 29%; P = 0.45), and 30-d mortality (3% vs 1%; P = 0.62).
According to an economic perspective, the cost-effectiveness of LDP vs ODP was also reported due to the cumulative reduction of hospital stay (5 d vs 7 d; P < 0.001)[46].
A recent, very impressive, review[47] of all studies comparing LDP and ODP collected data from 29 observational studies (3701 patients overall) to conduct a rigorous meta-analysis. The conclusion was that LDP was superior in terms of blood loss, time to first oral intake, and hospital stay.
Another review by Pericleous et al[48] selected only four comparative articles with an above average quality (none was a RCT) reporting LDP to have longer operative time but the reduced length of postoperative stay. Another more recent meta-analysis[49] found 18 comparative studies including more than 1800 patients. LDP was found to reduce blood loss, length of hospital stay, and overall complications.
Although the morbidity related to a distal PF is less dangerous than the morbidity that occurs after PD, the crucial issue of how to reduce its incidence is still unresolved[50]. Many systematic reviews of comparative retrospective studies conclude that the real incidence of fistula after LDP and open surgery are similar, with the stapled or anastomotic closure being the preferred methods despite the access route[51].
Interestingly, a specific analysis[52] of the prognostic factors related to pancreatic remnant leaks, conducted in a comparative matter between 439 OLP and 254 LDP, reported how patients with a body mass index (BMI) ≤ 27, without adenocarcinoma, and with a pancreatic specimen length ≤ 8.5 cm had significantly higher rates of PF after OLP than after LDP.
Unfortunately, many of the published series reported different surgical indications for LDP, including PDAC, IPMN and neuroendocrine tumors; these last two are able to be managed more conservatively or tolerate a suboptimal oncological adequacy. Nevertheless, when dealing with PDAC, the minimum prerequisite is to maintain the same oncological outcomes of open surgery, including the overall survival and the disease-free survival. Surrogate parameters, such as the number of harvested lymph nodes and the negative margins of resections, should also be taken into consideration.
A recent paper by Shin et al[53] was specifically targeted to compare LDP and ODP in 150 patients operated on for PDAC after using unmatched and propensity score-matched analyses. The oncologic adequacy was considered a primary endpoint whereas the postoperative recovery was marginal. LDP was associated significantly with a shorter median postoperative time to restarting diet and a shorter hospital stay in both matched and unmatched analysis. Interestingly, the 5-year survival rates, the length of surgery, the number of harvested lymph nodes, the resection margin status, and the incidence of PF were all similar.
Another retrospective study[54] reported no evidence of oncological detriment of patients with PDAC and operation by LDP, when cohorts were adjusted for factors affecting selection of operative technique.
A review by Fischer et al[55] included only studies reporting pancreatic laparoscopic resections for confirmed malignancies, and the author concluded that LDP (but not LPD) achieved the same rates of margin-positive resections and numbers of retrieved lymph nodes without different long-term survival. Alternatively[56], another study concluded that, due to a lack of statistically powered studies, LDP might not be advised for aggressive tumors. Another group from the United Kingdom[57] reported that LDP, although increasing, should be reserved to benign to low grade malignancies.
Spleen preservation, when indicated in the case of IPMN or less aggressive neuroendocrine tumors, should be the preferred strategy because it leads to a reduction in both blood loss and postoperative complication[58-61]. The recent advent of robotic assisted distal pancreatic resection (RDP) should, potentially, resolve many of the major issues of pure laparoscopy, including the preservation of the spleen[27,62]. Some retrospective series reported the spleen left in situ after a preoperative decision in more than 95% of cases[63,64], compared to inferior percentages (< 90%) achieved by both an open or laparoscopic approach[65].
Moreover, when dealing with PDAC, a more radical dissection and regional lymphadenectomy allowed by the robotic instrumentations should be of some help[36,66,67]. Lastly, the conversion rates seem to decrease with the robotic assistance (0%-18.3%)[24,68] with respect to laparoscopy; this represents an indirect proof of better feasibility or a superior control of bleeding.
One of the first large statistical studies was published by Giulianotti et al[27] and colleagues in 2010, with 134 robot-assisted pancreatic operations, including 46 RDPs. Conversion, morbidity and mortality rates for the whole series were 10.4%, 26% and 2.2 %, respectively. The rate of PF was 20.9% after RDP. Only one patient was re-operated on.
The largest series was published by Zureikat et al[24] on 250 robotic pancreatic resections, 83 of which were RDP. The 30-d mortality was 0.8%, the rate of Clavien-Dindo grade 3 (or more) complications were 6%, and the type C PF was only 4%. The mean operative time was 257 for RDP.
A very intriguing paper by Daouadi et al[69] retrospectively compared 94 LDP with 30 RDP patients well matched for age, sex, race, ASA score, and tumor size. Postoperative length of hospital stay and rates of PF, blood transfusion, and readmission were not significantly different. However, patients in the RDP group had less conversions than the LDP group (16%, P < 0.05) and reduced risk of blood loss. Moreover, the percentage of PDAC that was approached robotically was higher (43%) than laparoscopically (15%) (P < 0.05), but the oncological outcomes were superior for the RDP, with higher rates of margin negative resection and improved lymph-node clearance (P < 0.0001).
A meta-analysis by Zhang et al[63], which included seven trials, merged the data of 137 robotic and 203 open pancreatectomies. Many of the analyzed parameters, including morbidity, redo surgery, resection margins, blood loss and length of hospital stay, had a trend favoring the robotic procedures, but none of them reached statistical significance. Conversely, the length of surgery was demonstrated to be significantly shorter in the open group, whereas fistula formation and mortality rates were similar.
Traditionally, total pancreatectomy (TP) is a rarely performed procedure due to its high mortality and morbidity[70]. Nevertheless, the number of TP has been increasing over the years due to the higher number of multifocal pathologies discovered during advanced imaging[71]. In high volume centers, TP makes up 6.7% of all pancreatic procedures[72].
The surgical indications include multifocal neuroendocrine tumors, diffuse IPMN, renal cell metastasis, and MEN-1 syndrome[73-75]. In approximately 20% of cases, the decision to perform a TP is made intraoperatively for PDAC with persistent positive margins in frozen sections[70,74,75] or in the case of fragile pancreatic stump with unacceptable anastomotic risk[76]. TP for chronic pancreatitis has been abandoned with the advent of more efficacious medical management[71].
Post-operative endocrine insufficiency is the most concerning sequel, despite the great improvements in insulin regimen management. Other improvements have been made for the treatment of exocrine deficiency[71].
Similar to PD and DP, a minimally invasive (laparoscopic and robotic) approach to TP has been proposed in recent years. Obviously, the indications for minimally invasive TP are the same as for open surgery.
However, only a few small case-series reporting laparoscopic TP are available in current literature. Nevertheless, preliminary anecdotal papers report laparoscopic TP (LTP) to be safe and feasible, although technically demanding. Morbidity and mortality rates were low after LTP[77,78].
The robotic technique may overcome the intrinsic limits of pure laparoscopy and may provide some advantages compared to open surgery, including spleen preservation[75]. Giulianotti et al[74] reported safety and feasibility of this procedure allowing acceptable peri-operative morbidity and shorter hospital stays. Globally, morbidity and mortality rate ranges up to 70% and 16%, respectively, with consistent differences according to the surgical indication[75].
Boggi et al[79] published a case-matched study comparing 11 robotic TP (RTP) and 11 open TP (OTP). There was no conversion to open surgery in the RTP group. The operative time was longer and the blood loss was lower for RTP, whereas morbidity was similar (lower severity in the RTP group). The length of stay was similar between the two groups, but the robotic patients experienced faster recovery and lower pain. Interestingly, lymph-node collection was higher for the robotic group (45 vs 36, not statistically significant).
In the series of 10 RTPs published by Zureikat et al[75], there was one conversion to laparotomy, no 90-d mortality, a 20% Clavien III-IV complication rate and only one readmission within 90 d.
Due to the routine use of high resolution imaging techniques, diagnosis of small benign or low-grade malignant lesions of the pancreas has increased in the last years, leading to a higher number of proposed resections. Nevertheless, major pancreatic resections are still at risk of potentially life-threatening complications, even if performed through a minimally invasive approach.
From this perspective, much effort should be attempted to spare healthy tissue and to avoid unnecessary pancreatic anastomosis in non-frankly malignant tumors. Pancreatic enucleation (PE) and central pancreatectomy (CP) are the most frequent proposed operations, whereas duodenum-preserving pancreatic head resection (DPPHR), pancreatic head resection with segmental duodenectomy, inferior head resection, dorsal pancreatectomy, pancreatic head excavation, middle-preserving pancreatectomy[80] and resection of the uncinate process[81,82] are less popular, very rare alternatives. All of these procedures had been proven to be safe and feasible with low mortality and recurrence rates[83].
The selective indications for these conservative operations include cystoadenomas, pseudopapillary neoplasms, non-invasive branch-type IPMN, endocrine tumors[84-87] and isolated metastasis from renal cancer[82].
One major drawback is the high complication rates mostly related to PF[80] that were mostly grade A or B (slightly higher rate of severe grades after CP compared to PE) and managed conservatively[83].
Parenchyma-sparing resections performed in a minimally invasive fashion would be the ideal procedure for those patients. The introduction of new instruments and growing experience make laparoscopic techniques broadly used even in conservative pancreatic laparoscopic resections. For example, the laparoscopic ultrasonography probe is a powerful tool to accurately find the lesion and its correlation with vessels and the pancreatic duct, thus overcoming the absence of any tactile sense[87-89].
Moreover, the robotic assistance may overcome some limitations of laparoscopy itself with a dedicated flexible probe that has been developed to replace standard laparoscopic ones. This integrated robotic probe is moved by the console surgeon and allows reproducing all of the movements of open surgery. Lastly, the ultrasound screen is seen in picture-in-picture mode.
In his systematic review, Beger et al[83] reported the results of PE in 838 patients (22.5% of them underwent minimal-invasive surgery) demonstrating an overall morbidity rate of 41.3% (with a 9.6% of severe complications), a PF rate of 36.7%, a reoperation rate of 4.7% and a mortality rate lower than 1%. Zhang et al[90] collected data from 119 patients, which showed 0% exocrine insufficiency, no worsening of diabetes after surgery and 2.8% new-onset endocrine insufficiency. Cardiac impairment and operative time longer than 180 min were found to be independent risk factors for PF.
Unfortunately, no RCTs comparing open PE (OPE) and laparoscopic pancreatic enucleation (LPE) are available in the current literature, although many case series and retrospective comparative studies reported feasibility, safety and effectiveness of the minimally invasive approaches, with lower blood loss and length of hospital stay[85,88,91-93]. The conversion rate ranges from 20% to 33%[88]. Overall, the morbidity is similar between the OPE and LPE groups, but major complications are more frequent in the open group[89]. The incidence of PF after LPE ranged from 13% to 38%[92], which is lower than the rate after OPE. The long-term results of LPE are still lacking.
In his systematic review including 101 patients treated with a LPE, Briggs et al[94] reported a conversion rate ranging from 10% to 44% and a morbidity rate ranging from 22% to 67% without significant differences in morbidity and mortality rates compared to open pancreatic surgery.
Interestingly, pancreatic robotic enucleation seems to be both safe and feasible with lower intraoperative blood loss, better perioperative outcome, mortality rates less than 1% and shorter hospital stays compared with open surgery. However, rigorous trials matching robotics, laparoscopy and open surgery are still lacking[24].
CP is performed more rarely (less than 3% of pancreatic resections in high volume centers)[95,96]. Indications for CP include tumors up to 5-6 cm in size arising from the pancreatic neck or body, which are in proximity to the pancreatic duct and are not suitable for PE. Many options for proximal stump are possible, including staple or suturing techniques, but none have proven a real superiority over another[96-99].
The indications for minimally invasive CP are equivalent to that for open CP. However, laparoscopic CP (LCP) remains controversial due to the difficulties in pancreatic reconstruction. Preliminary results show its safety and feasibility[82,100,101].
Again, the robotic platform was reported to overcome some of the limitations of a pure laparoscopic approach. Nevertheless, only a few small case-series of robotic central pancreatectomies (RCP) reported the same high rate of PF and longer operative times, but faster recovery compared to open surgery[98,102,103].
Zureikat et al[24] reported the results of 13 cases of RCP with a conversion rate of 15%, no perioperative mortality, but a 92% PF rate. Abood et al[104] reported a PF rate of 22.2%, and R0 surgery in all nine patients with no endocrine or exocrine impairment. Kang et al[102], in his retrospective match-compared study of five patients treated robotically and ten patients treated with open CP, demonstrated no significant differences in overall complication rate, perioperative mortality and length of hospital stay. The intraoperative blood loss was significantly lower in the robotic group and operation time was longer compared to the open procedure.
Interestingly, Machado et al[105] performed a review on 22 cases of LCP versus 27 RCP cases. The study showed low blood loss, PF rate of 46%, no mortality, no recurrence at a mean follow-up of 19.6 mo and no exocrine or endocrine deficiency. Chen et al[101] reported the results of LCP performed in 10 cases. The incidence of PF was 20% (grade A), and there was no recurrence (median follow-up of 22.9 mo) of either exocrine or endocrine pancreatic insufficiency.
Resection of the uncinate process and DPPHR are very rarely performed procedures, and only a few cases reports describing any laparoscopic approach are available in the literature. Most of these cases had a high rate of PF[82]. In a very inclusive review, Beger et al[83] reported the results of 431 DPPHR cases, demonstrating a rate of severe complications of 11.5%, a PF rate of 20%, a reoperations rate of 1.8% and mortality lower than 1%.
Patients affected by PDAC are still expected to die a few years after surgery (if indicated), with the overall survival slightly increased by a regimen of perioperative adjuvant radio-chemotherapy. Borderline neoplasm and even pre-cancerous lesions require complex management, which often includes a surgical approach with some potential life-threatening complications.
A strong effort to minimize those complications and to enhance the recovery after surgery could be a great help to those patients (Table 1).
| Type of procedure | Open surgery | Laparoscopic surgery | Robotic | Level of evidence1 |
| Distal pancreatectomy | Standard/accepted | Being standard | Pioneeristic | LE 2 |
| Pancreaticoduodenectomy | Standard | Pioneeristic | Pioneeristic | LE 2 |
| Total pancreatectomy | Standard | Pioneeristic | Pioneeristic | LE 4 |
| Enucleation | Accepted/standard | Standard/accepted | Pioneeristic | LE 3 |
In this view, the minimally invasive surgical approach (laparoscopic and robotic operations) to pancreatic neoplasm leads to many benefits, including recovery, cosmetic results, early access to adjuvant therapies and psychological implications. Unfortunately, most of the articles published and reviewed were flawed by a weak statistical power (heterozygous methods, facilities and devices employed, insufficient case load), and many reported conflicting results. A possible explanation is the extreme weight of technologic equipment and expertise needed to develop a minimally invasive pancreatic cancer program in addition to the low incidence of such pathology. However, a strategic centralization of pancreatic malignancies together with more rigorous scientific data reporting should be mandatory in future years.
P- Reviewer: Bilir C, Li YY S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray . GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer 2013; Available from: http://globocan.iarc.fr, accessed on 23/03/2015. |
| 2. | McDowell BD, Chapman CG, Smith BJ, Button AM, Chrischilles EA, Mezhir JJ. Pancreatectomy predicts improved survival for pancreatic adenocarcinoma: results of an instrumental variable analysis. Ann Surg. 2015;261:740-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 4. | Dickson PV, Behrman SW. Management of pancreatic neuroendocrine tumors. Surg Clin North Am. 2013;93:675-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Ejaz A, Sachs T, He J, Spolverato G, Hirose K, Ahuja N, Wolfgang CL, Makary MA, Weiss M, Pawlik TM. A comparison of open and minimally invasive surgery for hepatic and pancreatic resections using the Nationwide Inpatient Sample. Surgery. 2014;156:538-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Gooiker GA, van Gijn W, Wouters MW, Post PN, van de Velde CJ, Tollenaar RA. Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg. 2011;98:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 7. | Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011;49:1076-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 399] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 8. | Amini N, Spolverato G, Kim Y, Pawlik TM. Trends in Hospital Volume and Failure to Rescue for Pancreatic Surgery. J Gastrointest Surg. 2015;19:1581-1592. [PubMed] |
| 9. | Rosales-Velderrain A, Bowers SP, Goldberg RF, Clarke TM, Buchanan MA, Stauffer JA, Asbun HJ. National trends in resection of the distal pancreas. World J Gastroenterol. 2012;18:4342-4349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Bencini L, Bernini M, Farsi M. Laparoscopic approach to gastrointestinal malignancies: toward the future with caution. World J Gastroenterol. 2014;20:1777-1789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Goldfarb M, Brower S, Schwaitzberg SD. Minimally invasive surgery and cancer: controversies part 1. Surg Endosc. 2010;24:304-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Sharma B, Baxter N, Grantcharov T. Outcomes after laparoscopic techniques in major gastrointestinal surgery. Curr Opin Crit Care. 2010;16:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Kwon W, Jang JY, Park JW, Han IW, Kang MJ, Kim SW. Which method of pancreatic surgery do medical consumers prefer among open, laparoscopic, or robotic surgery? A survey. Ann Surg Treat Res. 2014;86:7-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 658] [Article Influence: 21.2] [Reference Citation Analysis (1)] |
| 15. | Gagner M, Palermo M. Laparoscopic Whipple procedure: review of the literature. J Hepatobiliary Pancreat Surg. 2009;16:726-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg. 2010;145:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 256] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 17. | Asbun HJ, Stauffer JA. Laparoscopic vs open pancreaticoduodenectomy: overall outcomes and severity of complications using the Accordion Severity Grading System. J Am Coll Surg. 2012;215:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Kim SC, Song KB, Jung YS, Kim YH, Park do H, Lee SS, Seo DW, Lee SK, Kim MH, Park KM. Short-term clinical outcomes for 100 consecutive cases of laparoscopic pylorus-preserving pancreatoduodenectomy: improvement with surgical experience. Surg Endosc. 2013;27:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 19. | Croome KP, Farnell MB, Que FG, Reid-Lombardo KM, Truty MJ, Nagorney DM, Kendrick ML. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg. 2014;260:633-638; discussion 638-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 349] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 20. | Dokmak S, Ftériche FS, Aussilhou B, Bensafta Y, Lévy P, Ruszniewski P, Belghiti J, Sauvanet A. Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg. 2015;220:831-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 21. | Boggi U, Amorese G, Vistoli F, Caniglia F, De Lio N, Perrone V, Barbarello L, Belluomini M, Signori S, Mosca F. Laparoscopic pancreaticoduodenectomy: a systematic literature review. Surg Endosc. 2015;29:9-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 136] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 22. | Zhou NX, Chen JZ, Liu Q, Zhang X, Wang Z, Ren S, Chen XF. Outcomes of pancreatoduodenectomy with robotic surgery versus open surgery. Int J Med Robot. 2011;7:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 23. | Zeh HJ, Zureikat AH, Secrest A, Dauoudi M, Bartlett D, Moser AJ. Outcomes after robot-assisted pancreaticoduodenectomy for periampullary lesions. Ann Surg Oncol. 2012;19:864-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Zureikat AH, Moser AJ, Boone BA, Bartlett DL, Zenati M, Zeh HJ. 250 robotic pancreatic resections: safety and feasibility. Ann Surg. 2013;258:554-559; discussion 554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 268] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 25. | Nguyen TK, Zenati MS, Boone BA, Steve J, Hogg ME, Bartlett DL, Zeh HJ, Zureikat AH. Robotic pancreaticoduodenectomy in the presence of aberrant or anomalous hepatic arterial anatomy: safety and oncologic outcomes. HPB (Oxford). 2015;17:594-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, Caravaglios G. Robotics in general surgery: personal experience in a large community hospital. Arch Surg. 2003;138:777-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 771] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 27. | Giulianotti PC, Sbrana F, Bianco FM, Elli EF, Shah G, Addeo P, Caravaglios G, Coratti A. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc. 2010;24:1646-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 266] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 28. | Chan OC, Tang CN, Lai EC, Yang GP, Li MK. Robotic hepatobiliary and pancreatic surgery: a cohort study. J Hepatobiliary Pancreat Sci. 2011;18:471-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Chalikonda S, Aguilar-Saavedra JR, Walsh RM. Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc. 2012;26:2397-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 30. | Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy--a comparative study. Int J Surg. 2012;10:475-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 31. | Cirocchi R, Partelli S, Trastulli S, Coratti A, Parisi A, Falconi M. A systematic review on robotic pancreaticoduodenectomy. Surg Oncol. 2013;22:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 32. | Boggi U, Signori S, De Lio N, Perrone VG, Vistoli F, Belluomini M, Cappelli C, Amorese G, Mosca F. Feasibility of robotic pancreaticoduodenectomy. Br J Surg. 2013;100:917-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 33. | Fernandes E, Giulianotti PC. Robotic-assisted pancreatic surgery. J Hepatobiliary Pancreat Sci. 2013;20:583-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Strijker M, van Santvoort HC, Besselink MG, van Hillegersberg R, Borel Rinkes IH, Vriens MR, Molenaar IQ. Robot-assisted pancreatic surgery: a systematic review of the literature. HPB (Oxford). 2013;15:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Gagner M, Pomp A, Herrera MF. Early experience with laparoscopic resections of islet cell tumors. Surgery. 1996;120:1051-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 307] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 36. | Kang CM, Chung YE, Jung MJ, Hwang HK, Choi SH, Lee WJ. Splenic vein thrombosis and pancreatic fistula after minimally invasive distal pancreatectomy. Br J Surg. 2014;101:114-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Tran Cao HS, Lopez N, Chang DC, Lowy AM, Bouvet M, Baumgartner JM, Talamini MA, Sicklick JK. Improved perioperative outcomes with minimally invasive distal pancreatectomy: results from a population-based analysis. JAMA Surg. 2014;149:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 38. | de Rooij T, Jilesen AP, Boerma D, Bonsing BA, Bosscha K, van Dam RM, van Dieren S, Dijkgraaf MG, van Eijck CH, Gerhards MF. A nationwide comparison of laparoscopic and open distal pancreatectomy for benign and malignant disease. J Am Coll Surg. 2015;220:263-270.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 39. | Kneuertz PJ, Patel SH, Chu CK, Fisher SB, Maithel SK, Sarmiento JM, Weber SM, Staley CA, Kooby DA. Laparoscopic distal pancreatectomy: trends and lessons learned through an 11-year experience. J Am Coll Surg. 2012;215:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Nakamura M, Nakashima H. Laparoscopic distal pancreatectomy and pancreatoduodenectomy: is it worthwhile? A meta-analysis of laparoscopic pancreatectomy. J Hepatobiliary Pancreat Sci. 2013;20:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 41. | Borja-Cacho D, Al-Refaie WB, Vickers SM, Tuttle TM, Jensen EH. Laparoscopic distal pancreatectomy. J Am Coll Surg. 2009;209:758-65; quiz 800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Iacobone M, Citton M, Nitti D. Laparoscopic distal pancreatectomy: up-to-date and literature review. World J Gastroenterol. 2012;18:5329-5337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 43. | Jayaraman S, Gonen M, Brennan MF, D’Angelica MI, DeMatteo RP, Fong Y, Jarnagin WR, Allen PJ. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg. 2010;211:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 44. | Kooby DA, Gillespie T, Bentrem D, Nakeeb A, Schmidt MC, Merchant NB, Parikh AA, Martin RC, Scoggins CR, Ahmad S. Left-sided pancreatectomy: a multicenter comparison of laparoscopic and open approaches. Ann Surg. 2008;248:438-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 278] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 45. | Vijan SS, Ahmed KA, Harmsen WS, Que FG, Reid-Lombardo KM, Nagorney DM, Donohue JH, Farnell MB, Kendrick ML. Laparoscopic vs open distal pancreatectomy: a single-institution comparative study. Arch Surg. 2010;145:616-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 46. | Fox AM, Pitzul K, Bhojani F, Kaplan M, Moulton CA, Wei AC, McGilvray I, Cleary S, Okrainec A. Comparison of outcomes and costs between laparoscopic distal pancreatectomy and open resection at a single center. Surg Endosc. 2012;26:1220-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 47. | Mehrabi A, Hafezi M, Arvin J, Esmaeilzadeh M, Garoussi C, Emami G, Kössler-Ebs J, Müller-Stich BP, Büchler MW, Hackert T. A systematic review and meta-analysis of laparoscopic versus open distal pancreatectomy for benign and malignant lesions of the pancreas: it’s time to randomize. Surgery. 2015;157:45-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 201] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 48. | Pericleous S, Middleton N, McKay SC, Bowers KA, Hutchins RR. Systematic review and meta-analysis of case-matched studies comparing open and laparoscopic distal pancreatectomy: is it a safe procedure? Pancreas. 2012;41:993-1000. [PubMed] |
| 49. | Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg. 2012;255:1048-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 388] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 50. | Paye F, Micelli Lupinacci R, Bachellier P, Boher JM, Delpero JR. Distal pancreatectomy for pancreatic carcinoma in the era of multimodal treatment. Br J Surg. 2015;102:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 51. | Zhang H, Zhu F, Shen M, Tian R, Shi CJ, Wang X, Jiang JX, Hu J, Wang M, Qin RY. Systematic review and meta-analysis comparing three techniques for pancreatic remnant closure following distal pancreatectomy. Br J Surg. 2015;102:4-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 52. | Cho CS, Kooby DA, Schmidt CM, Nakeeb A, Bentrem DJ, Merchant NB, Parikh AA, Martin RC, Scoggins CR, Ahmad SA. Laparoscopic versus open left pancreatectomy: can preoperative factors indicate the safer technique? Ann Surg. 2011;253:975-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Shin SH, Kim SC, Song KB, Hwang DW, Lee JH, Lee D, Lee JW, Jun E, Park KM, Lee YJ. A comparative study of laparoscopic vs. open distal pancreatectomy for left-sided ductal adenocarcinoma: a propensity score-matched analysis. J Am Coll Surg. 2015;220:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 54. | Magge D, Gooding W, Choudry H, Steve J, Steel J, Zureikat A, Krasinskas A, Daouadi M, Lee KK, Hughes SJ. Comparative effectiveness of minimally invasive and open distal pancreatectomy for ductal adenocarcinoma. JAMA Surg. 2013;148:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 55. | Fisher SB, Kooby DA. Laparoscopic pancreatectomy for malignancy. J Surg Oncol. 2013;107:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 56. | Kudsi OY, Gagner M, Jones DB. Laparoscopic distal pancreatectomy. Surg Oncol Clin N Am. 2013;22:59-73, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 57. | Ammori BJ, Ayiomamitis GD. Laparoscopic pancreaticoduodenectomy and distal pancreatectomy: a UK experience and a systematic review of the literature. Surg Endosc. 2011;25:2084-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 58. | Salky BA, Edye M. Laparoscopic pancreatectomy. Surg Clin North Am. 1996;76:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Mabrut JY, Fernandez-Cruz L, Azagra JS, Bassi C, Delvaux G, Weerts J, Fabre JM, Boulez J, Baulieux J, Peix JL. Laparoscopic pancreatic resection: results of a multicenter European study of 127 patients. Surgery. 2005;137:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 313] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 60. | Jean-Philippe Adam B, Fernández-Cruz L, Sa-Cunha A. Laparoscopic spleen-preserving distal pancreatectomy: splenic vessel preservation compared with the Warshaw technique. JAMA Surg. 2013;148:246-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 61. | Kang CM, Lee SH, Lee WJ. Minimally invasive radical pancreatectomy for left-sided pancreatic cancer: current status and future perspectives. World J Gastroenterol. 2014;20:2343-2351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 62. | Choi SH, Kang CM, Lee WJ, Chi HS. Robot-assisted spleen-preserving laparoscopic distal pancreatectomy. Ann Surg Oncol. 2011;18:3623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 63. | Zhang J, Wu WM, You L, Zhao YP. Robotic versus open pancreatectomy: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20:1774-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 64. | Hwang HK, Kang CM, Chung YE, Kim KA, Choi SH, Lee WJ. Robot-assisted spleen-preserving distal pancreatectomy: a single surgeon’s experiences and proposal of clinical application. Surg Endosc. 2013;27:774-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 65. | Jain G, Chakravartty S, Patel AG. Spleen-preserving distal pancreatectomy with and without splenic vessel ligation: a systematic review. HPB (Oxford). 2013;15:403-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 66. | Parisi A, Coratti F, Cirocchi R, Grassi V, Desiderio J, Farinacci F, Ricci F, Adamenko O, Economou AI, Cacurri A. Robotic distal pancreatectomy with or without preservation of spleen: a technical note. World J Surg Oncol. 2014;12:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 67. | Lee SH, Kang CM, Hwang HK, Choi SH, Lee WJ, Chi HS. Minimally invasive RAMPS in well-selected left-sided pancreatic cancer within Yonsei criteria: long-term (& gt; median 3 years) oncologic outcomes. Surg Endosc. 2014;28:2848-2855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 68. | Coratti A, Annecchiarico M. Robot-assisted pancreatic surgery. Br J Surg. 2014;101:593-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Daouadi M, Zureikat AH, Zenati MS, Choudry H, Tsung A, Bartlett DL, Hughes SJ, Lee KK, Moser AJ, Zeh HJ. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg. 2013;257:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 70. | Crippa S, Tamburrino D, Partelli S, Salvia R, Germenia S, Bassi C, Pederzoli P, Falconi M. Total pancreatectomy: indications, different timing, and perioperative and long-term outcomes. Surgery. 2011;149:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 71. | Almond M, Roberts KJ, Hodson J, Sutcliffe R, Marudanayagam R, Isaac J, Muiesan P, Mirza D. Changing indications for a total pancreatectomy: perspectives over a quarter of a century. HPB (Oxford). 2015;17:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Janot MS, Belyaev O, Kersting S, Chromik AM, Seelig MH, Sülberg D, Mittelkötter U, Uhl WH. Indications and early outcomes for total pancreatectomy at a high-volume pancreas center. HPB Surg. 2010;2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Casadei R, Monari F, Buscemi S, Laterza M, Ricci C, Rega D, D’Ambra M, Pezzilli R, Calculli L, Santini D. Total pancreatectomy: indications, operative technique, and results: a single centre experience and review of literature. Updates Surg. 2010;62:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 74. | Giulianotti PC, Addeo P, Buchs NC, Bianco FM, Ayloo SM. Early experience with robotic total pancreatectomy. Pancreas. 2011;40:311-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 75. | Zureikat AH, Nguyen T, Boone BA, Wijkstrom M, Hogg ME, Humar A, Zeh H. Robotic total pancreatectomy with or without autologous islet cell transplantation: replication of an open technique through a minimal access approach. Surg Endosc. 2015;29:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 76. | Hartwig W, Gluth A, Hinz U, Bergmann F, Spronk PE, Hackert T, Werner J, Büchler MW. Total pancreatectomy for primary pancreatic neoplasms: renaissance of an unpopular operation. Ann Surg. 2015;261:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 77. | Choi SH, Hwang HK, Kang CM, Yoon CI, Lee WJ. Pylorus- and spleen-preserving total pancreatoduodenectomy with resection of both whole splenic vessels: feasibility and laparoscopic application to intraductal papillary mucin-producing tumors of the pancreas. Surg Endosc. 2012;26:2072-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 78. | Dallemagne B, de Oliveira AT, Lacerda CF, D’Agostino J, Mercoli H, Marescaux J. Full laparoscopic total pancreatectomy with and without spleen and pylorus preservation: a feasibility report. J Hepatobiliary Pancreat Sci. 2013;20:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 79. | Boggi U, Palladino S, Massimetti G, Vistoli F, Caniglia F, De Lio N, Perrone V, Barbarello L, Belluomini M, Signori S. Laparoscopic robot-assisted versus open total pancreatectomy: a case-matched study. Surg Endosc. 2015;29:1425-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 80. | Sperti C, Beltrame V, Milanetto AC, Moro M, Pedrazzoli S. Parenchyma-sparing pancreatectomies for benign or border-line tumors of the pancreas. World J Gastrointest Oncol. 2010;2:272-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 81. | Rotellar F, Pardo F, Benito A, Martí-Cruchaga P, Zozaya G, Cienfuegos JA. Laparoscopic resection of the uncinate process of the pancreas: the inframesocolic approach and hanging maneuver of the mesenteric root. Surg Endosc. 2011;25:3426-3427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Kuroki T, Eguchi S. Laparoscopic parenchyma-sparing pancreatectomy. J Hepatobiliary Pancreat Sci. 2014;21:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Beger HG, Siech M, Poch B, Mayer B, Schoenberg MH. Limited surgery for benign tumours of the pancreas: a systematic review. World J Surg. 2015;39:1557-1566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 84. | Fernández-Cruz L, Blanco L, Cosa R, Rendón H. Is laparoscopic resection adequate in patients with neuroendocrine pancreatic tumors? World J Surg. 2008;32:904-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 85. | Crippa S, Boninsegna L, Partelli S, Falconi M. Parenchyma-sparing resections for pancreatic neoplasms. J Hepatobiliary Pancreat Sci. 2010;17:782-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 86. | Ge C, Luo X, Chen X, Guo K. Enucleation of pancreatic cystadenomas. J Gastrointest Surg. 2010;14:141-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 87. | Choi KS, Chung JC, Kim HC. Feasibility and outcomes of laparoscopic enucleation for pancreatic neoplasms. Ann Surg Treat Res. 2014;87:285-289. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 88. | Fernández-Cruz L, Molina V, Vallejos R, Jiménez Chavarria E, López-Boado MA, Ferrer J. Outcome after laparoscopic enucleation for non-functional neuroendocrine pancreatic tumours. HPB (Oxford). 2012;14:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 89. | Al-Kurd A, Chapchay K, Grozinsky-Glasberg S, Mazeh H. Laparoscopic resection of pancreatic neuroendocrine tumors. World J Gastroenterol. 2014;20:4908-4916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 90. | Zhang T, Xu J, Wang T, Liao Q, Dai M, Zhao Y. Enucleation of pancreatic lesions: indications, outcomes, and risk factors for clinical pancreatic fistula. J Gastrointest Surg. 2013;17:2099-2104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | DiNorcia J, Ahmed L, Lee MK, Reavey PL, Yakaitis EA, Lee JA, Schrope BA, Chabot JA, Allendorf JD. Better preservation of endocrine function after central versus distal pancreatectomy for mid-gland lesions. Surgery. 2010;148:1247-1254; discussion 1254-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 92. | Dedieu A, Rault A, Collet D, Masson B, Sa Cunha A. Laparoscopic enucleation of pancreatic neoplasm. Surg Endosc. 2011;25:572-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 93. | Song KB, Kim SC, Hwang DW, Lee JH, Lee DJ, Lee JW, Jun ES, Sin SH, Kim HE, Park KM. Enucleation for benign or low-grade malignant lesions of the pancreas: Single-center experience with 65 consecutive patients. Surgery. 2015;158:1203-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 94. | Briggs CD, Mann CD, Irving GR, Neal CP, Peterson M, Cameron IC, Berry DP. Systematic review of minimally invasive pancreatic resection. J Gastrointest Surg. 2009;13:1129-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 95. | Lavu H, Knuth JL, Baker MS, Shen C, Zyromski NJ, Schmidt M, Nakeeb A, Howard TJ. Middle segment pancreatectomy can be safely incorporated into a pancreatic surgeon’s clinical practice. HPB (Oxford). 2008;10:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Iacono C, Verlato G, Ruzzenente A, Campagnaro T, Bacchelli C, Valdegamberi A, Bortolasi L, Guglielmi A. Systematic review of central pancreatectomy and meta-analysis of central versus distal pancreatectomy. Br J Surg. 2013;100:873-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 97. | Sauvanet A, Partensky C, Sastre B, Gigot JF, Fagniez PL, Tuech JJ, Millat B, Berdah S, Dousset B, Jaeck D. Medial pancreatectomy: a multi-institutional retrospective study of 53 patients by the French Pancreas Club. Surgery. 2002;132:836-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 114] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 98. | Cheng K, Shen B, Peng C, Deng X, Hu S. Initial experiences in robot-assisted middle pancreatectomy. HPB (Oxford). 2013;15:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 99. | Du ZY, Chen S, Han BS, Shen BY, Liu YB, Peng CH. Middle segmental pancreatectomy: a safe and organ-preserving option for benign and low-grade malignant lesions. World J Gastroenterol. 2013;19:1458-1465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 100. | Rotellar F, Pardo F, Montiel C, Benito A, Regueira FM, Poveda I, Martí-Cruchaga P, Cienfuegos JA. Totally laparoscopic Roux-en-Y duct-to-mucosa pancreaticojejunostomy after middle pancreatectomy: a consecutive nine-case series at a single institution. Ann Surg. 2008;247:938-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 101. | Chen XM, Zhang Y, Sun DL. Laparoscopic central pancreatectomy for solid pseudopapillary tumors of the pancreas: our experience with ten cases. World J Surg Oncol. 2014;12:312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 102. | Kang CM, Kim DH, Lee WJ, Chi HS. Initial experiences using robot-assisted central pancreatectomy with pancreaticogastrostomy: a potential way to advanced laparoscopic pancreatectomy. Surg Endosc. 2011;25:1101-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 103. | Milone L, Daskalaki D, Wang X, Giulianotti PC. State of the art of robotic pancreatic surgery. World J Surg. 2013;37:2761-2770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 104. | Abood GJ, Can MF, Daouadi M, Huss HT, Steve JY, Ramalingam L, Stang M, Bartlett DL, Zeh HJ, Moser AJ. Robotic-assisted minimally invasive central pancreatectomy: technique and outcomes. J Gastrointest Surg. 2013;17:1002-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 105. | Machado MA, Surjan RC, Epstein MG, Makdissi FF. Laparoscopic central pancreatectomy: a review of 51 cases. Surg Laparosc Endosc Percutan Tech. 2013;23:486-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |