Copyright
©The Author(s) 2025.
World J Gastrointest Oncol. Jun 15, 2025; 17(6): 106161
Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.106161
Published online Jun 15, 2025. doi: 10.4251/wjgo.v17.i6.106161
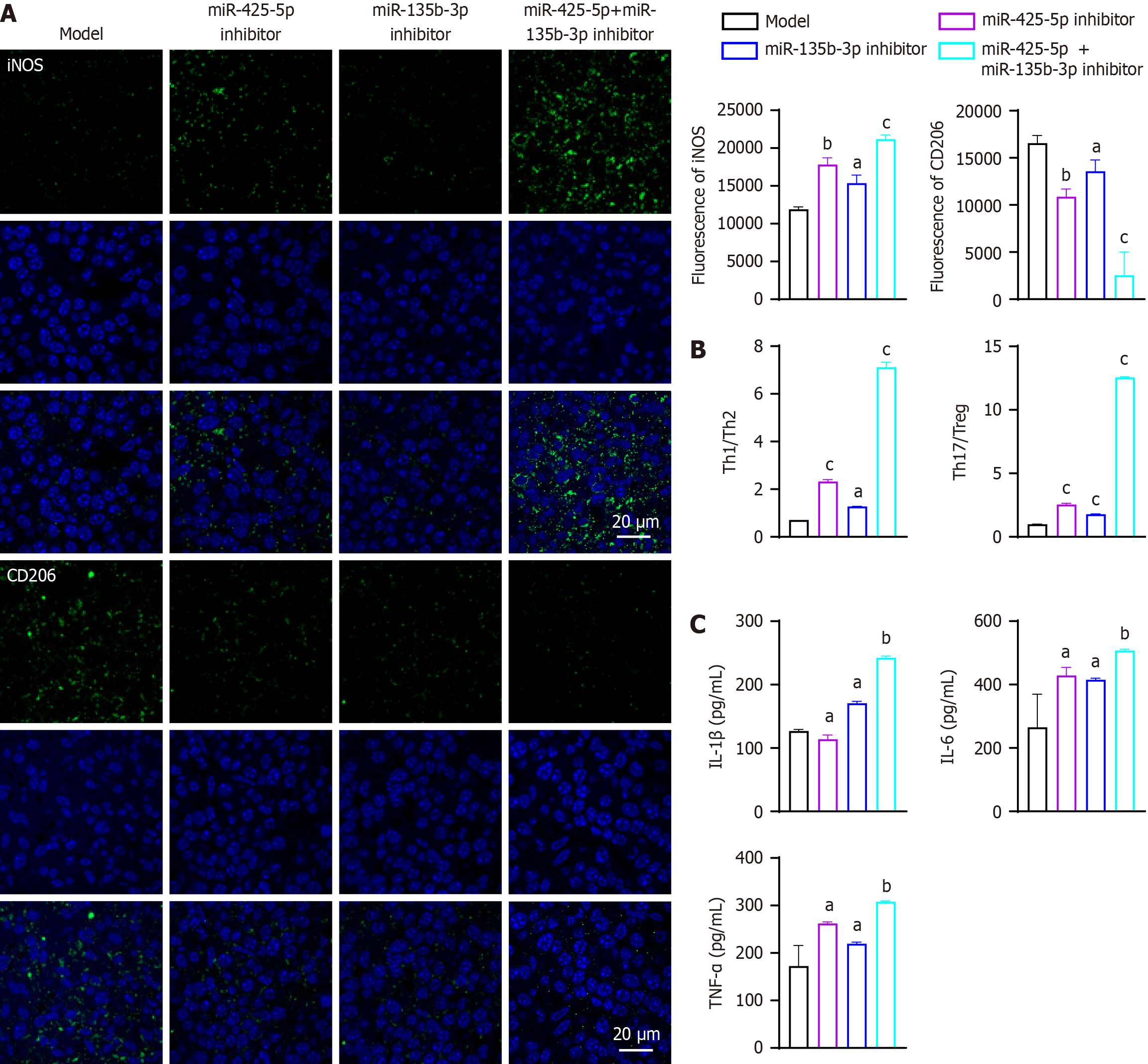
Figure 5 Exosomal miR-425-5p and mir-135b-3p inhibition modulates the immune microenvironment in tumor tissues.
A: Immunofluore
- Citation: Feng CZ, Zhong SQ, Ye SW, Zheng Z, Sun H, Zhou SH. Tumor-derived exosomal miR-425-5p and miR-135b-3p enhance colorectal cancer progression through immune suppression and vascular permeability promotion. World J Gastrointest Oncol 2025; 17(6): 106161
- URL: https://www.wjgnet.com/1948-5204/full/v17/i6/106161.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i6.106161