Copyright
©The Author(s) 2024.
World J Gastrointest Oncol. Apr 15, 2024; 16(4): 1384-1420
Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1384
Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1384
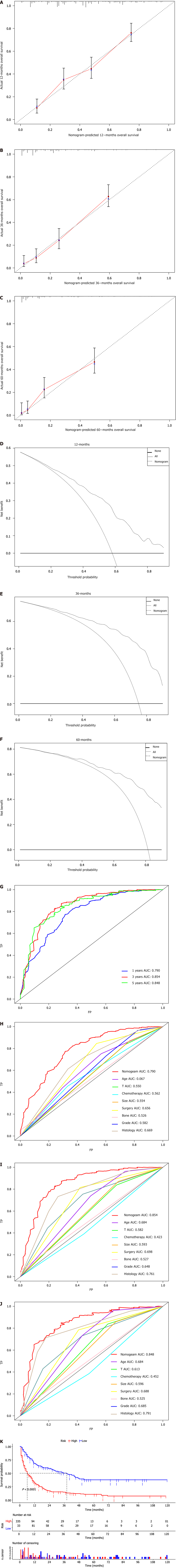
Figure 9 Validating the prognostic nomogram in the expanded testing set.
A: The calibration curves of the nomogram for the 12 months in the expanded testing set; B: The calibration curves of the nomogram for the 36 months in the expanded testing set; C: The calibration curves of the nomogram for the 60 months in the expanded testing set; D: The decision curve analysis of the nomogram for the 12 months in the expanded testing set; E: The decision curve analysis of the nomogram for the 36 months in the expanded testing set; F: The decision curve analysis of the nomogram for the 60 months in the expanded testing set; G: Comparison of area under the receiver operating characteristic curves between nomogram and all independent factors for the 12 months in the expanded testing set; H: Comparison of area under the receiver operating characteristic curves between nomogram and all independent factors for the 36 months in the expanded testing set; I: Comparison of area under the receiver operating characteristic curves between nomogram and all independent factors for the 60 months in the expanded testing set; J: Time-dependent receiver operating characteristic curve analysis of the nomogram for the 12, 36, and 60 months in the expanded testing set; K: The Kapla-Meier survival curve of the patients in the expanded testing set. AUC: Area under the curve.
- Citation: Shang JR, Xu CY, Zhai XX, Xu Z, Qian J. Risk factors, prognostic factors, and nomograms for distant metastasis in patients with diagnosed duodenal cancer: A population-based study. World J Gastrointest Oncol 2024; 16(4): 1384-1420
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1384.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1384