Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.798
Peer-review started: September 19, 2023
First decision: December 5, 2023
Revised: December 15, 2023
Accepted: January 27, 2024
Article in press: January 27, 2024
Published online: March 15, 2024
Pancreatic ductal adenocarcinoma (PDAC) is a common cancer with increasing morbidity and mortality due to changes of social environment.
To evaluate the significance of serum carbohydrate antigen 19-9 (CA19-9) and tumor size changes pre- and post-neoadjuvant therapy (NAT).
This retrospective study was conducted at the Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital. This study specifically assessed CA19-9 levels and tumor size before and after NAT.
A total of 156 patients who completed NAT and subsequently underwent tumor resection were included in this study. The average age was 65.4 ± 10.6 years and 72 (46.2%) patients were female. Before survival analysis, we defined the post-NAT serum CA19-9 level/pre-NAT serum CA19-9 level as the CA19-9 ratio (CR). The patients were divided into three groups: CR < 0.5, CR > 0.5 and < 1 and CR > 1. With regard to tumor size measured by both computed tomography and magnetic resonance imaging, we defined the post-NAT tumor size/pre-NAT tumor size as the tumor size ratio (TR). The patients were then divided into three groups: TR < 0.5, TR > 0.5 and < 1 and TR > 1. Based on these groups divided according to CR and TR, we performed both overall survival (OS) and disease-free survival (DFS) analyses. Log-rank tests showed that both OS and DFS were significantly different among the groups according to CR and TR (P < 0.05). CR and TR after NAT were associated with increased odds of achieving a complete or near-complete pathologic response. Moreover, CR (hazard ratio: 1.721, 95%CI: 1.373-3.762; P = 0.006), and TR (hazard ratio: 1.435, 95%CI: 1.275-4.363; P = 0.014) were identified as independent factors associated with OS.
This study demonstrated that post-NAT serum CA19-9 level/pre-NAT serum CA19-9 level and post-NAT tumor size/pre-NAT tumor size were independent factors associated with OS in patients with PDAC who received NAT and subsequent surgical resection.
Core Tip: Our study demonstrated that post-neoadjuvant therapy (NAT) serum carbohydrate antigen 19-9 (CA19-9) level/pre-NAT serum CA19-9 level and post-NAT tumor size/pre-NAT tumor size were independent factors associated with overall survival for patients receiving NAT and subsequently surgical resection for pancreatic ductal adenocarcinoma.
- Citation: Xia DQ, Zhou Y, Yang S, Li FF, Tian LY, Li YH, Xu HY, Xiao CZ, Wang W. Combining prognostic value of serum carbohydrate antigen 19-9 and tumor size reduction ratio in pancreatic ductal adenocarcinoma. World J Gastrointest Oncol 2024; 16(3): 798-809
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/798.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.798
Pancreatic ductal adenocarcinoma (PDAC) is a relatively common cancer with increasing morbidity and mortality due to changes of social environment. Cancer-related death was the second leading cause of death in 2019, with PDAC the third most common cause of cancer death[1,2]. At the time of diagnosis, less than 20% of patients are eligible for curative surgery. For patients with advanced pancreatic cancer (either locally advanced or metastatic disease), the mainstay of treatment is systemic chemotherapy[3]. Gemcitabine-based regimens and 5-FU-based regimens display survival benefit and have been recommended as first-line therapies. Recently researchers have demonstrated that neoadjuvant therapy (NAT), including chemotherapy and radiotherapy, is related with increased R0 surgical resection and the overall survival (OS), especially those with distant and locally advanced PDAC[4-6].
Carbohydrate antigen 19-9 (CA19-9) is a dialkylated Lewis blood group antigen and is the most widely investigated tumor marker in patients with PDAC. CA19-9 has proven useful for the diagnosis of PDAC in symptomatic patients with a sensitivity and specificity of 79%-81% and 82%-90%, respectively[7,8]. Previous studies concluded that CA19-9 was an ineffective screening tool in asymptomatic patients. Nearly 7% of patients lack this tumor antigen and may be non-secretors[9,10]. Kane et al[11], in a retrospective analysis, showed that serum CA19-9 is significantly upregulated compared with the normal range to 2 years prior to the first diagnosis of PDAC. Pre-and post-operative CA19-9 levels and the changing of CA19-9 levels after operation might even predict prognosis of patients after resection. Furthermore, studies have showed that CA19-9 levels were related with tumor size and the stage[12,13]. However, there were several limitations when CA19-9 was identified as a biomarker: The routine use of CA19-9 as a PDAC screening tool in the general population is invalid, and because the incidence rate of PDAC in the general population is relatively low, the positive predictive value is low. This is also reflected in two large-scale population studies. In addition, false positive results were observed in benign pancreatic and biliary diseases such as cholangitis, pancreatitis, and obstructive jaundice. In addition, liver cysts and pancreatic cysts may interfere with CA19-9 levels. Despite these challenges, the use of CA19-9 has shifted from screening biomarkers to prognostic biomarkers[14-17].
To address this issue, and due to the increasing utilization and importance of NAT in PDAC treatment, it is necessary to identify biomarkers of the response to guide the management of these patients. In particular, the role of serum CA19-9 and tumor size in predicting resectability, pathological response, disease recurrence, and OS has been examined. Research has shown that changes in tumor size and serum CA19-9 during NAT can capture individual differences that cannot be recognized by individual measurements at a single time point.
This study aimed to evaluate the significance of serum CA19-9 and tumor size changes pre-and post-NAT. The ratio of post-NAT serum CA19-9 level/pre-NAT serum CA19-9 level and the ratio of post-NAT tumor size/pre-NAT tumor size were used to identify the prognostic value of these factors in patients with PDAC. Moreover, we evaluated the combined prognostic value of these factors in predicting OS in patients with PDAC.
This retrospective study was conducted at the Chongqing Key Laboratory of Translational Research for Cancer Metastasis and Individualized Treatment, Chongqing University Cancer Hospital.
Inclusion criteria: Patients aged 18- 70 years; have a histopathological confirmed diagnosis of PDAC at stage IA to IIB (the 8th edition of the American Joint Committee on Cancer staging system); be deemed suitable for potentially R0 surgical resection; have an Eastern Cooperative Oncology Group performance status score of 0 or 1; and have measurable disease and adequate pulmonary and organ function.
Exclusion criteria: Multiple primary malignancies, active or history of autoimmune disease, active or suspected interstitial lung disease or moderate-to-severe pneumonia, human immunodeficiency virus or active hepatitis B or C virus infection, previous systemic antitumor therapy and chest radiation, and previous use of immunostimulants, immunosuppressants, and live vaccine within 4 wk before the first dose of study treatment.
Collected variables included age, sex, comorbidities, body mass index, National Comprehensive Cancer Network (NCCN) resectability definitions and criteria, tumor size, serum levels of CA19-9, resection status, margin status, and pathologic response to NAT following resection. Comorbidities were evaluated using the Charlson Comorbidity Index. Patients were classified as upfront resectable, borderline resectable, and locally advanced according to the NCCN resectability criteria and were evaluated by a multidisciplinary panel of experts, including hepatopancreatobiliary surgeons, medical oncologists, radiation oncologists, and radiologists using pancreatic protocol computed tomography (CT).
The study included CA19-9 levels after resolution of biliary obstruction, with total bilirubin less than 2 mg/dL. Levels of CA19-9 were compared against laboratory references. The CA19-9 response throughout surveillance was stratified on the basis of normalization. Levels of CA19-9 were assessed at diagnosis, preoperatively, after resection, and at 6-mo follow-up intervals. Patients were included in the study if they had all baseline data and a minimum of one postoperative data point. This study specifically assessed CA19-9 levels and tumor size before and after NAT. Pre-operative levels closest to the operative date but within 4 wk were recorded. Pancreatic protocol multidetector CT scans (MDCT) were obtained at these 6-mo intervals to assess recurrence. All charts with discordance between CA19-9 and MDCT scan results were re-reviewed. Recurrence was defined as radiographic evidence of disease based on radiologist, multidisciplinary tumor board review of scans, or both. Levels of CA19-9 at baseline and at follow-up assessment were correlated with disease-free survival (DFS) and OS.
The primary endpoints of this study were OS and DFS. Pathologic complete response was evaluated and defined as the absence of viable tumor cells in the resected specimen. Pathologic response to treatment was defined according to the College of American Pathologists as complete, near-complete, partial, or poor response. Overall response rate (ORR) was determined by the investigator, with ORR defined as the proportion of patients with complete response or partial response (PR) according to Response Evaluation Criteria in Solid Tumors version 1.1 (RECIST version 1.1). DFS was defined as the time from the first dose of the study drug to disease progression, local recurrence, distant metastasis, or death, whichever occurred first. Treatment-related adverse events were monitored and recorded.
The t-test was employed to evaluate the differences in microbiome species abundance between the groups. Two-sided P values were used, and the significance level was set at 0.05 for all analyses unless otherwise stated. SPSS software (version 26; IBM Corp., Armonk, NY, United States) and R software (version 4.1.2) were used for statistical analyses.
Between July 2, 2021 and May 17, 2022, a total of 156 patients with histopathologically confirmed PDAC were screened for eligibility. These patients met the inclusion criteria and were enrolled in the study. All 156 patients completed NAT and subsequently underwent tumor resection. According to the scheduled NAT protocol, 87.2% (136/156) completed NAT. The average age of the patients was 65.4 ± 10.6 years and 72 (46.2%) were female. The median follow-up period was 34.3 months (95%CI: 26.5-56.3). Most of the patients underwent pancreaticoduodenectomy (n = 117, 75%), followed by distal pancreatectomy (n = 30, 19.2%) and distal pancreatectomy with celiac axis resection (n = 9, 4.8%). The majority (n = 88, 56.4%) had open procedures, but 50 patients (32.1%) underwent laparoscopic resection and 18 (11.5%) had robotic resection. Patients were classified as resectable PDAC (n = 30, 19.2%), 76 (48.7%) as borderline resectable PDAC, and 50 (32.1%) as locally advanced PDAC, according to the NCCN resectability criteria. Detailed results of the patient cohort are presented in Table 1.
| Variables | Total patients, n = 156 | % |
| Age in yr | 65.4 ± 10.6 | |
| Sex | ||
| Female | 72 | 46.2 |
| Male | 84 | 53.8 |
| ASA-score classification | ||
| 2 | 58 | 37.2 |
| 3 | 60 | 62.8 |
| BMI | 23.3 ± 6.5 | |
| CR | ||
| < 0.5 | 45 | 28.8 |
| ≥ 0.5 or < 1 | 90 | 57.7 |
| ≥ 1 | 21 | 13.5 |
| Charlson comorbidity Index | ||
| 0 | 117 | 75 |
| 1 | 29 | 18.6 |
| 2 | 10 | 6.4 |
| Tumor and pathologic characteristics | ||
| AJCC TNM stage | ||
| Ia | 12 | 7.7 |
| Ib | 41 | 26.3 |
| Iia | 58 | 37.2 |
| Iib | 45 | 28.8 |
| Grade | ||
| 0 | 10 | 6.4 |
| G1 | 35 | 22.4 |
| G2 | 75 | 48.1 |
| G3 | 36 | 23.1 |
| Neoadjuvant Chx | ||
| Yes | 128 | 82.1 |
| No | 28 | 17.9 |
| Neoadjuvant Rtx | ||
| Yes | 37 | 33.7 |
| No | 119 | 76.3 |
| TR | ||
| < 0.5 | 4 | 2.6 |
| ≥ 0.5 or < 1 | 123 | 78.8 |
| ≥ 1 | 29 | 18.6 |
| Neural invasion | ||
| Yes | 65 | 41.7 |
| No | 91 | 58.3 |
| Vascular invasion | ||
| Yes | 76 | 48.7 |
| No | 80 | 51.3 |
| Positive lymph nodes | ||
| 0 | 50 | 32.1 |
| 1-2 | 37 | 33.7 |
| > 2 | 69 | 44.2 |
| Tumor resection | ||
| R0 | 65 | 41.7 |
| R1 | 91 | 58.3 |
| Operation type | ||
| Whipple | 117 | 75.0 |
| Distal pancreatectomy | 30 | 19.2 |
| Distal pancreatectomy with celiac axis resection | 9 | 4.8 |
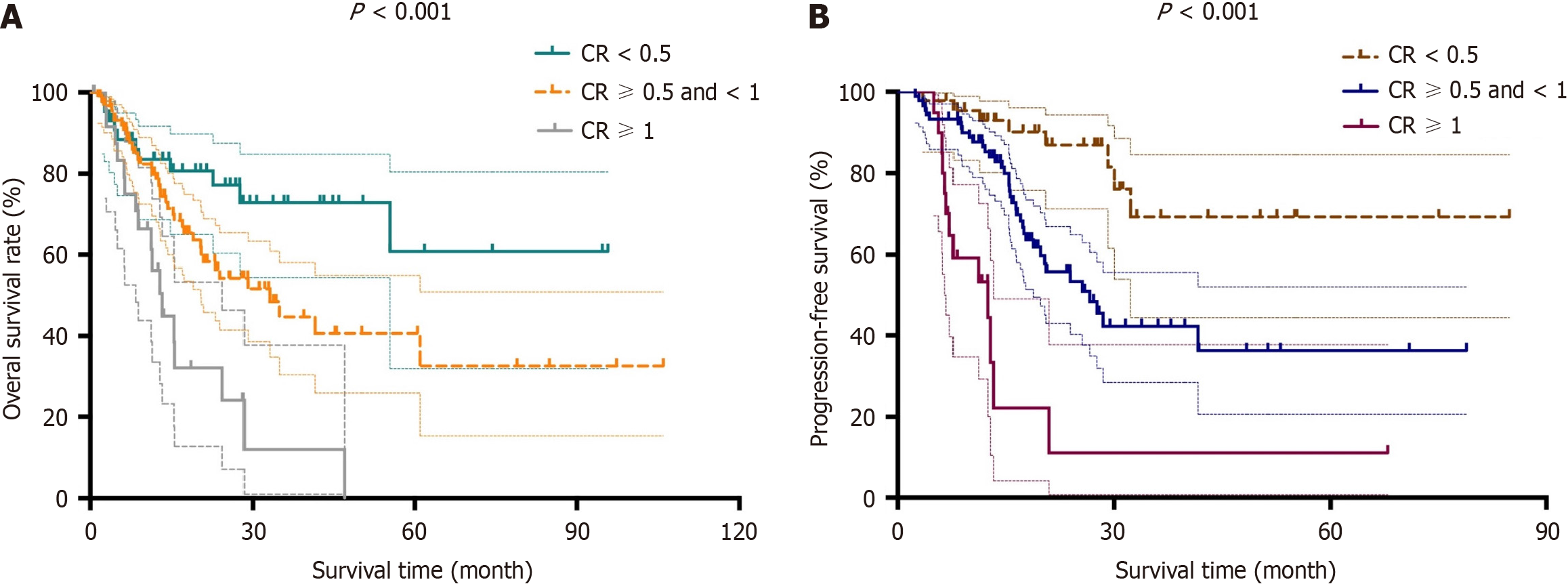
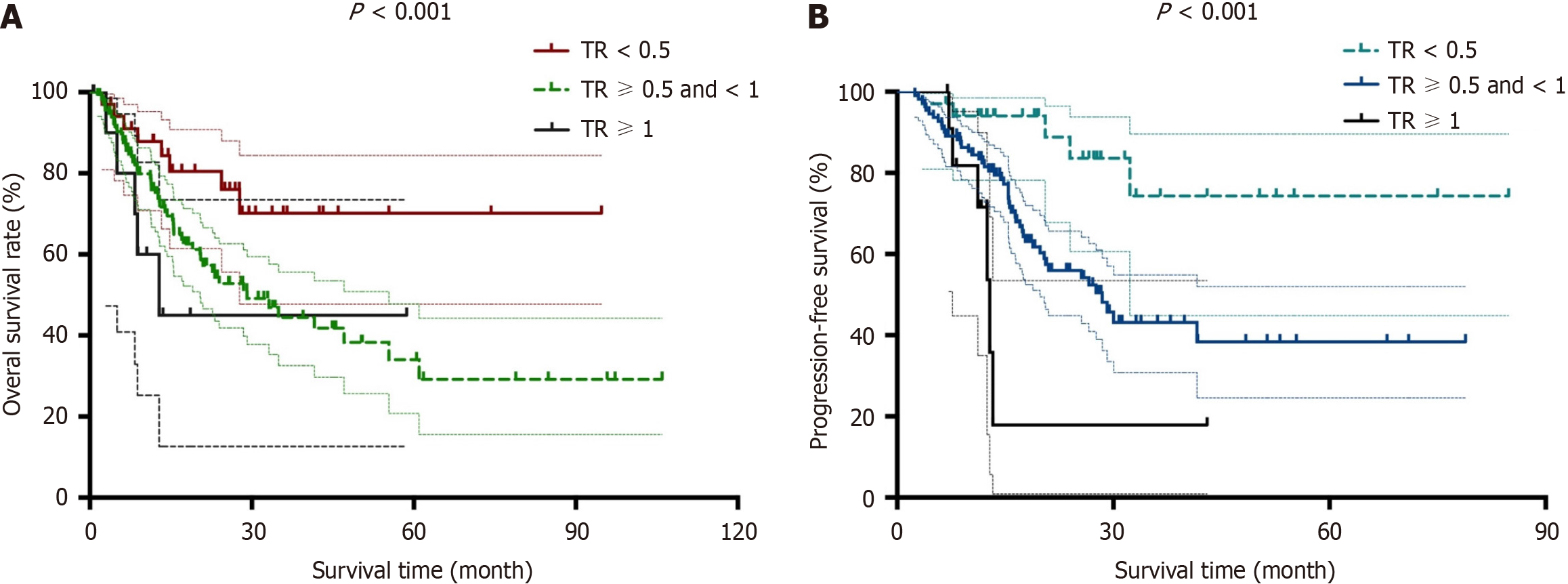
Prior to survival analysis, we defined the post-NAT serum CA19-9 level/pre-NAT serum CA19-9 level as the CA19-9 ratio (CR). The patients were then divided into the following three groups: CR < 0.5, CR > 0.5 and < 1 and CR > 1. With respect to tumor size measured by both CT and magnetic resonance imaging, we defined the post-NAT tumor size/pre-NAT tumor size as the tumor size ratio (TR). We then divided the patients into the following three groups: TR < 0.5, TR > 0.5 and < 1 and TR > 1. Based on these groups divided according to CR and TR, we determined both OS and DFS. Log-rank tests showed that both OS and DFS were significantly different among the groups divided according to CR and TR (P < 0.05). Kaplan-Meier curves for OS and DFS are presented for CR in Figure 1 and TR in Figure 2, respectively.
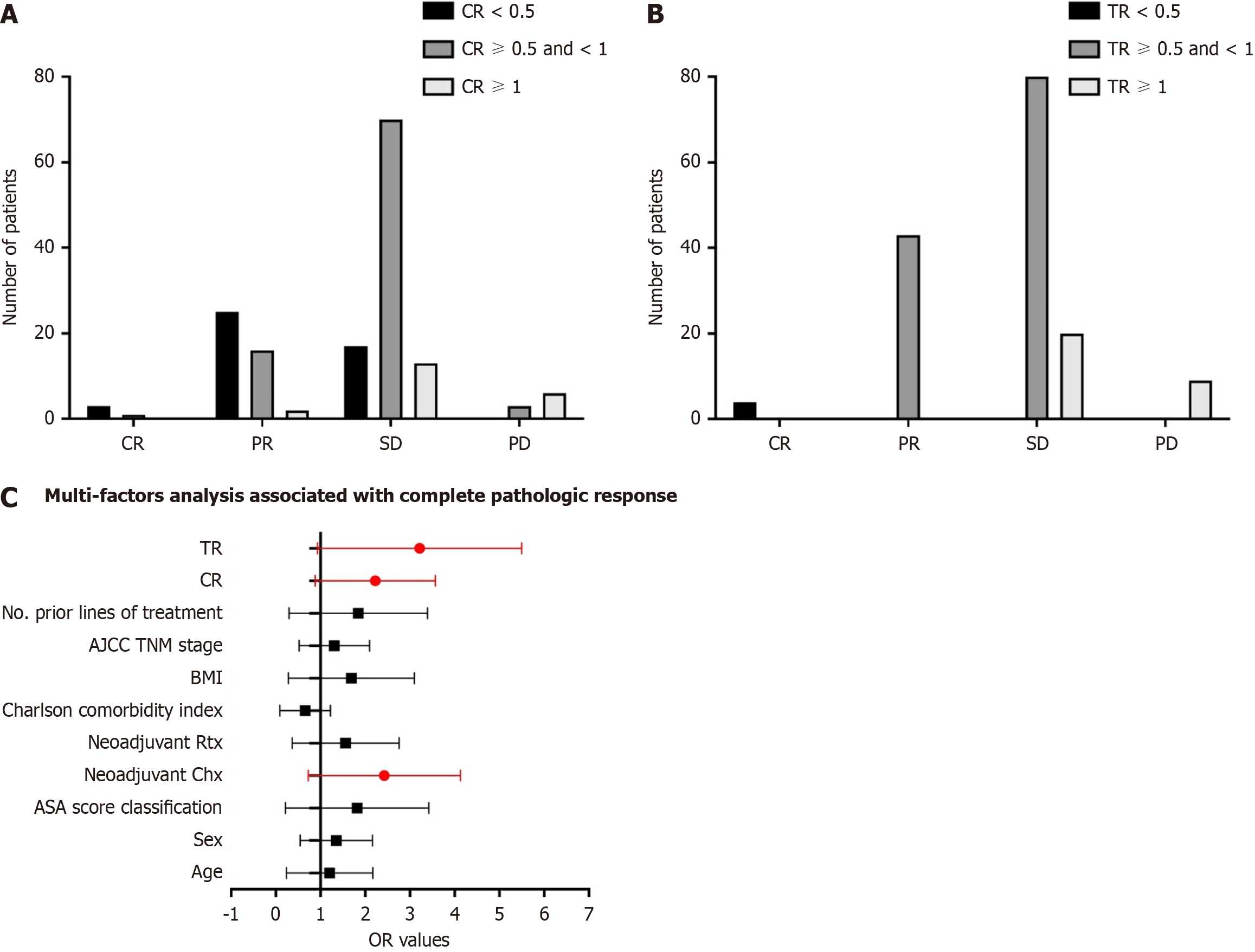
After 4-6 cycles of preoperative treatment with FOLFIRINOX or gemcitabine combined with nab-paclitaxel, 138 of 156 patients (88.6%, 95%CI: 77.5%-99.7%) had an objective response, with 10 patients (28.6%) achieving complete response, 21 patients (60%) achieving PR, and 60 patients (11.4%) achieving stable disease. Eighteen patients had progressive disease during NAT. After dividing the patients into different groups according to CR and TR, the efficacy of NAT is shown in Figure 3A and B. Furthermore, univariable analyses revealed that female sex, TNM stage, neoadjuvant chemotherapy, CR and TR were associated with complete or near-complete pathologic responses. Multivariable analyses identified that neoadjuvant chemotherapy, CR and TR were associated with increased odds of achieving a complete or near-complete pathologic response. Detailed results of the model are presented in Figure 3C.
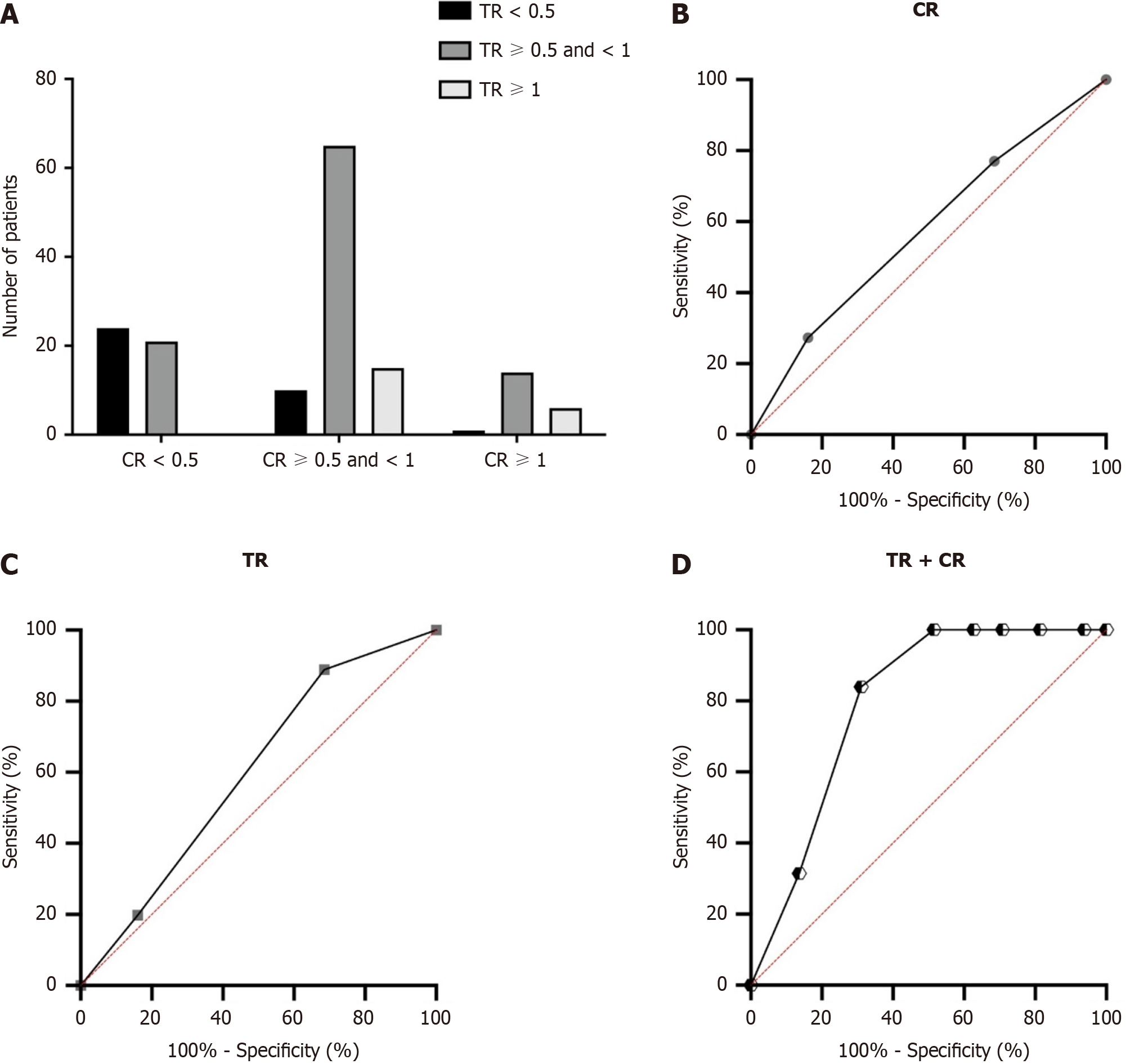
Cox proportional hazards models were used to quantify the prognostic factors associated with OS in patients with PDAC. The results of both univariable and multivariable analysis are shown in Table 2. Following univariable analysis, a multivariable analysis was performed to evaluate the factors that showed statistical significance in univariable analysis. After adjusting for competing risk factors, TNM stage [hazard ratio (HR): 1.526, 95%CI: 1.226-4.165; P = 0.007], vascular invasion (HR: 1.653, 95%CI: 1.253-3.651; P = 0.021), CR (HR: 1.721, 95%CI: 1.373-3.762; P = 0.006), and TR (HR: 1.435, 95%CI: 1.275-4.363; P = 0.014) were identified as independent factors associated with OS. Interestingly, we found that both CR and TR were independent risk factors for OS in PDAC patients. Furthermore, as shown in Figure 4A, we found that in patients with a TR < 0.5, 24 patients had CR < 0.5, 10 patients had CR ≥ 0.5 and < 1, and 1 patient had CR ≥ 1. In patients with a TR ≥ 0.5 and < 1, 21 patients had CR < 0.5, 65 patients had CR ≥ 0.5 and < 1 and 14 patients had CR ≥ 1. In patients with a TR ≥ 1, 0 patients had CR < 0.5, 15 patients had CR ≥ 0.5 and <1 and 6 patients had CR ≥ 1. Furthermore, the area under the receiver operating characteristic curves (AUROCs) were determined to compare the predictive values of CR, TR and the combined predictive value of CR and TR. The CR showed a significantly improved predictive value (AUROC: 0.674, 95%CI: 0.558-0.734) than the TR (AUROC: 0.681, 95%CI: 0.547-0.728, shown in Figure 4B and C). After combining both CR and TR, the model showed significantly improved predictive value compared with the single variables (AUROC: 0.758, 95%CI: 0.684-0.815) as shown in Figure 4D.
| Variable | Univariate | Multivariate | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age in yr, median range | 1.043 | 0.795-1.149 | 0.857 | |||
| Sex, male/female | 0.948 | 0.743-1.219 | 0.395 | |||
| ASA score classification, 0/1 or 2 | 1.103 | 0.854-1.754 | 0.194 | |||
| BMI | 0.931 | 0.803-1.140 | 0.454 | |||
| TNM stage, I or II/III | 1.436 | 1.217-2.695 | 0.015 | 1.526 | 1.226-4.165 | 0.007 |
| Neoadjuvant Chx, yes/no | 1.201 | 1.114-2.742 | 0.024 | |||
| Neoadjuvant Rtx, yes/no | 1.121 | 0.901-1.537 | 0.142 | |||
| Neural invasion, yes/no | 1.103 | 0.965-2.542 | 0.104 | |||
| Vascular invasion, yes/no | 1.439 | 1.294-3.594 | 0.003 | 1.653 | 1.253-3.651 | 0.021 |
| Positive lymph nodes, yes/no | 1.729 | 1.487-4.325 | 0.001 | |||
| Tumor resection, R0/R1 | 0.568 | 0.321-0.843 | 0.005 | |||
| Operation type, Whipple | 1.104 | 0.549-2.130 | 0.392 | |||
| CR, ≥ 1/< 1 | 1.796 | 1.429-4.104 | 0.001 | 1.721 | 1.373-3.762 | 0.006 |
| TR, ≥ 1/< 1 | 1.573 | 1.282-2.752 | 0.012 | 1.435 | 1.275-4.363 | 0.014 |
There have been several studies on the prognostic value of serum CA19-9 and tumor size changes in patients with PDAC undergoing NAT[14,18,19]. However, to our knowledge, this is the first study to evaluate the combined value of CA19-9 reduction and tumor size reduction following NAT with chemotherapy plus radiotherapy in patients with potentially resectable PDAC. NAT did not increase surgical complexity, with 43.6% of patients undergoing minimally invasive surgery[20-22].
PDAC is frequently considered as one of the worst cancers in terms of survival, with most patients dying < 2 years after diagnosis. In locally advanced pancreatic cancer, the 5-year survival rate is < 10%, making initial surgical treatment challenging[23-27]. CA19-9 has become a standard parameter in the diagnosis and monitoring of PDAC. High rates of recurrence represent significant hurdles to improving the outcome of patients with resectable disease. Elevations in CA19-9 have prognostic significance in early- and late-stage PDAC[28-30]. Studies in patients presenting with metastatic/unresectable disease showed that CA19-9 elevation is associated with worse survival, whereas CA19-9 response correlates with improved survival[31,32]. A CA19-9 decline in response to NAT can predict survival, margins, and pathologic outcome even in the absence of radiographic response[33-35]. Radiologic assessment remains a cornerstone in the decision-making process during the different stages of PDAC treatment. Currently, deep learning-based CT imaging-derived biomarkers enabled the objective and unbiased OS prediction for patients with resectable PDAC[36]. Although traditional NCCN resectability criteria have been shown to be unreliable in patients receiving NAT treatment, patients are followed up through imaging examinations during NAT to monitor disease progression[37-39]. On the other hand, since the tumor size at a single time point cannot reflect the changes observed during treatment, predicting resectability solely based on tumor size is inconsistent. Therefore, for patients undergoing NAT, the demand for new predictive strategies has not been met to improve prognostic assessment of these patients through radiological assessment using the combined effects of CA19-9 and tumor size.
In this study, based on the groups divided according to the CR and TR, we analyzed OS and DFS. Log-rank tests showed that both OS and DFS were significantly different among the groups divided according to the CR and TR (P < 0.05). In our study, the CR and TR were associated with increased odds of achieving a complete or near-complete pathologic response. More recently, serum CA19-9 has also been proposed as a marker of chemo-responsiveness. Moreover, multivariable analysis was performed to evaluate factors that demonstrated statistical significance during univariable analysis. The CR (HR: 1.721, 95%CI: 1.373-3.762; P = 0.006), and TR (HR: 1.435, 95%CI: 1.275-4.363; P = 0.014) were identified as independent factors associated with OS.
Several limitations exist in our study. Firstly, this study was retrospective, the sample size was relatively small, and it lacked a randomized control group, which could have introduced bias into the baseline histologic distribution. Secondly, the follow-up period in terms of survival data was limited at the time of data cutoff and longer-term follow-up is necessary to fully evaluate the impact of NAT on survival outcomes. Finally, while our biomarker analysis was explo
Our study demonstrated that post-NAT serum CA19-9 level/pre-NAT serum CA19-9 level and post-NAT tumor size/pre-NAT tumor size were independent factors associated with OS in patients with PDAC who received NAT and subsequent surgical resection.
Pancreatic ductal adenocarcinoma (PDAC) is a relatively common cancer with increasing morbidity and mortality due to changes of social environment. Studies have demonstrated that neoadjuvant therapy (NAT) is associated with increased resectability, negative surgical margins, and increased survival among patients with more locally advanced disease. Carbohydrate antigen 19-9 (CA19-9) is a dialkylated Lewis blood group antigen and is the most widely investigated tumor marker in patients with PDAC. However, CA19-9 as a biomarker has known limitations: Routine usage of CA19-9 as a screening tool for PDAC in the general public is ineffective and results in a low positive predictive value due to the relatively low incidence of PDAC in the general population. It has been shown that changes in tumor size and serum CA19-9 during NAT can capture differences that are not identified by individual measurements at a single point in time.
Our study demonstrated that post-NAT serum CA19-9 level/pre-NAT serum CA19-9 level and post-NAT tumor size/pre-NAT tumor size were independent factors associated with overall survival (OS) in patients with PDAC who received NAT and subsequent surgical resection.
This study aimed to evaluate the significance of serum CA19-9 and tumor size changes pre-and post-NAT. The ratio of post-NAT serum CA19-9 level/pre-NAT serum CA19-9 level and the ratio of post-NAT tumor size/pre-NAT tumor size were used to identify the prognostic value of these factors in patients with PDAC. Moreover, we evaluated the combined prognostic value of these factors in predicting OS in patients with PDAC.
The t-test was employed to evaluate the differences in microbiome species abundance between the groups. Two-sided P values were used, and the significance level was set at 0.05 for all analyses unless otherwise stated. SPSS software (version 26) and R software (version 4.1.2) were used for statistical analyses.
A total of 156 patients who completed NAT and subsequently underwent tumor resection were included in this study. The average age was 65.4 ± 10.6 years and 72 (46.2%) patients were female. Before survival analysis, we defined the post-NAT serum CA19-9 level/ pre-NAT serum CA19-9 level as the CA19-9 ratio (CR). The patients were divided into three groups: CR < 0.5, CR > 0.5 and < 1 and CR > 1. With regard to tumor size measured by both computed tomography and magnetic resonance imaging, we defined the post-NAT tumor size/pre-NAT tumor size as the tumor size ratio (TR). The patients were then divided into three groups: TR < 0.5, TR > 0.5 and < 1 and TR > 1. Based on these groups divided according to CR and TR, we performed both OS and disease-free survival (DFS) analyses. Log-rank tests showed that both OS and DFS were significantly different among the groups according to CR and TR (P < 0.05). CR and TR after NAT were associated with increased odds of achieving a complete or near-complete pathologic response. Moreover, CR (HR: 1.721, 95%CI: 1.373-3.762; P = 0.006), and TR (HR: 1.435, 95%CI: 1.275-4.363; P = 0.014) were identified as independent factors associated with OS.
Our study demonstrated that post-NAT serum CA19-9 level/pre-NAT serum CA19-9 level and post-NAT tumor size/pre-NAT tumor size were independent factors associated with OS in patients with PDAC who received NAT and subsequent surgical resection.
Serum CA19-9 level and post-NAT tumor size/pre-NAT tumor size were independent factors associated with OS in patients with PDAC who received NAT and subsequent surgical resection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Christodoulidis G, Greece; Kobayashi S, Japan S-Editor: Lin C L-Editor: Filipodia P-Editor: Zheng XM
| 1. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 906] [Cited by in F6Publishing: 968] [Article Influence: 161.3] [Reference Citation Analysis (30)] |
| 2. | Mashayekhi V, Mocellin O, Fens MHAM, Krijger GC, Brosens LAA, Oliveira S. Targeting of promising transmembrane proteins for diagnosis and treatment of pancreatic ductal adenocarcinoma. Theranostics. 2021;11:9022-9037. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Versteijne E, Suker M, Groothuis K, Akkermans-Vogelaar JM, Besselink MG, Bonsing BA, Buijsen J, Busch OR, Creemers GM, van Dam RM, Eskens FALM, Festen S, de Groot JWB, Groot Koerkamp B, de Hingh IH, Homs MYV, van Hooft JE, Kerver ED, Luelmo SAC, Neelis KJ, Nuyttens J, Paardekooper GMRM, Patijn GA, van der Sangen MJC, de Vos-Geelen J, Wilmink JW, Zwinderman AH, Punt CJ, van Eijck CH, van Tienhoven G; Dutch Pancreatic Cancer Group. Preoperative Chemoradiotherapy Versus Immediate Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Results of the Dutch Randomized Phase III PREOPANC Trial. J Clin Oncol. 2020;38:1763-1773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 574] [Article Influence: 143.5] [Reference Citation Analysis (0)] |
| 4. | Loveday BPT, Lipton L, Thomson BN. Pancreatic cancer: An update on diagnosis and management. Aust J Gen Pract. 2019;48:826-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Cloyd JM, Tsung A, Hays J, Wills CE, Bridges JF. Neoadjuvant therapy for resectable pancreatic ductal adenocarcinoma: The need for patient-centered research. World J Gastroenterol. 2020;26:375-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 10] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Springfeld C, Ferrone CR, Katz MHG, Philip PA, Hong TS, Hackert T, Büchler MW, Neoptolemos J. Neoadjuvant therapy for pancreatic cancer. Nat Rev Clin Oncol. 2023;20:318-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 52] [Reference Citation Analysis (0)] |
| 7. | Pezzilli R, Fabbri D, Imbrogno A. Pancreatic ductal adenocarcinoma screening: new perspectives. World J Gastroenterol. 2012;18:4973-4977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Scarà S, Bottoni P, Scatena R. CA 19-9: Biochemical and Clinical Aspects. Adv Exp Med Biol. 2015;867:247-260. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 9. | Rieser CJ, Zenati M, Hamad A, Al Abbas AI, Bahary N, Zureikat AH, Zeh HJ 3rd, Hogg ME. CA19-9 on Postoperative Surveillance in Pancreatic Ductal Adenocarcinoma: Predicting Recurrence and Changing Prognosis over Time. Ann Surg Oncol. 2018;25:3483-3491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Xu P, Wang X, Qian J, Li Z, Yao J, Xu A. The prognostic evaluation of CA19-9, D-dimer and TNFAIP3/A20 in patients with pancreatic ductal adenocarcinoma. Medicine (Baltimore). 2021;100:e24651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Kane LE, Mellotte GS, Mylod E, O'Brien RM, O'Connell F, Buckley CE, Arlow J, Nguyen K, Mockler D, Meade AD, Ryan BM, Maher SG. Diagnostic Accuracy of Blood-based Biomarkers for Pancreatic Cancer: A Systematic Review and Meta-analysis. Cancer Res Commun. 2022;2:1229-1243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 11] [Reference Citation Analysis (0)] |
| 12. | Azizian A, Rühlmann F, Krause T, Bernhardt M, Jo P, König A, Kleiß M, Leha A, Ghadimi M, Gaedcke J. CA19-9 for detecting recurrence of pancreatic cancer. Sci Rep. 2020;10:1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 13. | Itoh S, Tsujita E, Fukuzawa K, Sugimachi K, Iguchi T, Ninomiya M, Maeda T, Kajiyama K, Adachi E, Uchiyama H, Utsunomiya T, Ikeda Y, Maekawa S, Toshima T, Harada N, Yoshizumi T, Mori M. Prognostic significance of preoperative PNI and CA19-9 for pancreatic ductal adenocarcinoma: A multi-institutional retrospective study. Pancreatology. 2021;21:1356-1363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Imaoka H, Shimizu Y, Senda Y, Natsume S, Mizuno N, Hara K, Hijioka S, Hieda N, Tajika M, Tanaka T, Ishihara M, Niwa Y, Yamao K. Post-adjuvant chemotherapy CA19-9 levels predict prognosis in patients with pancreatic ductal adenocarcinoma: A retrospective cohort study. Pancreatology. 2016;16:658-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Capello M, Bantis LE, Scelo G, Zhao Y, Li P, Dhillon DS, Patel NJ, Kundnani DL, Wang H, Abbruzzese JL, Maitra A, Tempero MA, Brand R, Firpo MA, Mulvihill SJ, Katz MH, Brennan P, Feng Z, Taguchi A, Hanash SM. Sequential Validation of Blood-Based Protein Biomarker Candidates for Early-Stage Pancreatic Cancer. J Natl Cancer Inst. 2017;109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 16. | Dittmar RL, Liu S, Tai MC, Rajapakshe K, Huang Y, Longton G, DeCapite C, Hurd MW, Paris PL, Kirkwood KS, Coarfa C, Maitra A, Brand RE, Killary AM, Sen S. Plasma miRNA Biomarkers in Limited Volume Samples for Detection of Early-stage Pancreatic Cancer. Cancer Prev Res (Phila). 2021;14:729-740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Kinny-Köster B, Habib JR, Wolfgang CL, He J, Javed AA. Favorable tumor biology in locally advanced pancreatic cancer-beyond CA19-9. J Gastrointest Oncol. 2021;12:2484-2494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 18. | Aoki S, Motoi F, Murakami Y, Sho M, Satoi S, Honda G, Uemura K, Okada KI, Matsumoto I, Nagai M, Yanagimoto H, Kurata M, Fukumoto T, Mizuma M, Yamaue H, Unno M; Multicenter Study Group of Pancreatobiliary Surgery (MSG-PBS). Decreased serum carbohydrate antigen 19-9 levels after neoadjuvant therapy predict a better prognosis for patients with pancreatic adenocarcinoma: a multicenter case-control study of 240 patients. BMC Cancer. 2019;19:252. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 19. | Al Abbas AI, Zenati M, Reiser CJ, Hamad A, Jung JP, Zureikat AH, Zeh HJ 3rd, Hogg ME. Serum CA19-9 Response to Neoadjuvant Therapy Predicts Tumor Size Reduction and Survival in Pancreatic Adenocarcinoma. Ann Surg Oncol. 2020;27:2007-2014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Torres C, Grippo PJ. Pancreatic cancer subtypes: a roadmap for precision medicine. Ann Med. 2018;50:277-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Wang L, Xie D, Wei D. Pancreatic Acinar-to-Ductal Metaplasia and Pancreatic Cancer. Methods Mol Biol. 2019;1882:299-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Grossberg AJ, Chu LC, Deig CR, Fishman EK, Hwang WL, Maitra A, Marks DL, Mehta A, Nabavizadeh N, Simeone DM, Weekes CD, Thomas CR Jr. Multidisciplinary standards of care and recent progress in pancreatic ductal adenocarcinoma. CA Cancer J Clin. 2020;70:375-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 209] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 23. | Hamad A, Brown ZJ, Ejaz AM, Dillhoff M, Cloyd JM. Neoadjuvant therapy for pancreatic ductal adenocarcinoma: Opportunities for personalized cancer care. World J Gastroenterol. 2021;27:4383-4394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 7] [Cited by in F6Publishing: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Truty MJ, Kendrick ML, Nagorney DM, Smoot RL, Cleary SP, Graham RP, Goenka AH, Hallemeier CL, Haddock MG, Harmsen WS, Mahipal A, McWilliams RR, Halfdanarson TR, Grothey AF. Factors Predicting Response, Perioperative Outcomes, and Survival Following Total Neoadjuvant Therapy for Borderline/Locally Advanced Pancreatic Cancer. Ann Surg. 2021;273:341-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 222] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 25. | Zhou Y, Liao S, You J, Wu H. Conversion surgery for initially unresectable pancreatic ductal adenocarcinoma following induction therapy: a systematic review of the published literature. Updates Surg. 2022;74:43-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Brown ZJ, Heh V, Labiner HE, Brock GN, Ejaz A, Dillhoff M, Tsung A, Pawlik TM, Cloyd JM. Surgical resection rates after neoadjuvant therapy for localized pancreatic ductal adenocarcinoma: meta-analysis. Br J Surg. 2022;110:34-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 21] [Reference Citation Analysis (0)] |
| 27. | Hamad A, Crossnohere N, Ejaz A, Tsung A, Pawlik TM, Sarna A, Santry H, Wills C, Cloyd JM. Patient Preferences for Neoadjuvant Therapy in Pancreatic Ductal Adenocarcinoma. Pancreas. 2022;51:657-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Balaban DV, Marin FS, Manucu G, Zoican A, Ciochina M, Mina V, Patoni C, Vladut C, Bucurica S, Costache RS, Ionita-Radu F, Jinga M. Clinical characteristics and outcomes in carbohydrate antigen 19-9 negative pancreatic cancer. World J Clin Oncol. 2022;13:630-640. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Moon D, Kim H, Han Y, Byun Y, Choi Y, Kang J, Kwon W, Jang JY. Preoperative carbohydrate antigen 19-9 and standard uptake value of positron emission tomography-computed tomography as prognostic markers in patients with pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci. 2022;29:1133-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Chiu YF, Liu TW, Shan YS, Chen JS, Li CP, Ho CL, Hsieh RK, Hwang TL, Chen LT, Ch'ang HJ; Taiwan Cooperative Oncology Group pancreatic cancer study group. Carbohydrate Antigen 19-9 Response to Initial Adjuvant Chemotherapy Predicts Survival and Failure Pattern of Resected Pancreatic Adenocarcinoma but Not Which Patients Are Suited for Additional Adjuvant Chemoradiation Therapy: From a Prospective Randomized Study. Int J Radiat Oncol Biol Phys. 2023;117:74-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 31. | Bergquist JR, Puig CA, Shubert CR, Groeschl RT, Habermann EB, Kendrick ML, Nagorney DM, Smoot RL, Farnell MB, Truty MJ. Carbohydrate Antigen 19-9 Elevation in Anatomically Resectable, Early Stage Pancreatic Cancer Is Independently Associated with Decreased Overall Survival and an Indication for Neoadjuvant Therapy: A National Cancer Database Study. J Am Coll Surg. 2016;223:52-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 32. | van Manen L, Groen JV, Putter H, Pichler M, Vahrmeijer AL, Bonsing BA, Mieog JSD. Stage-Specific Value of Carbohydrate Antigen 19-9 and Carcinoembryonic Antigen Serum Levels on Survival and Recurrence in Pancreatic Cancer: A Single Center Study and Meta-Analysis. Cancers (Basel). 2020;12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Chung KH, Ryu JK, Lee BS, Jang DK, Lee SH, Kim YT. Early decrement of serum carbohydrate antigen 19-9 predicts favorable outcome in advanced pancreatic cancer. J Gastroenterol Hepatol. 2016;31:506-512. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Gu X, Zhou R, Li C, Liu R, Zhao Z, Gao Y, Xu Y. Preoperative maximum standardized uptake value and carbohydrate antigen 19-9 were independent predictors of pathological stages and overall survival in Chinese patients with pancreatic duct adenocarcinoma. BMC Cancer. 2019;19:456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Heger U, Sun H, Hinz U, Klaiber U, Tanaka M, Liu B, Sachsenmaier M, Springfeld C, Michalski CW, Büchler MW, Hackert T. Induction chemotherapy in pancreatic cancer: CA 19-9 may predict resectability and survival. HPB (Oxford). 2020;22:224-232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 36. | Yao J, Cao K, Hou Y, Zhou J, Xia Y, Nogues I, Song Q, Jiang H, Ye X, Lu J, Jin G, Lu H, Xie C, Zhang R, Xiao J, Liu Z, Gao F, Qi Y, Li X, Zheng Y, Lu L, Shi Y, Zhang L. Deep Learning for Fully Automated Prediction of Overall Survival in Patients Undergoing Resection for Pancreatic Cancer: A Retrospective Multicenter Study. Ann Surg. 2023;278:e68-e79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, Hruban RH, Weiss MJ, Zheng L, Wolfgang CL, He J. Patterns, Timing, and Predictors of Recurrence Following Pancreatectomy for Pancreatic Ductal Adenocarcinoma. Ann Surg. 2018;267:936-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 244] [Cited by in F6Publishing: 369] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 38. | Abdelrahman AM, Goenka AH, Alva-Ruiz R, Yonkus JA, Leiting JL, Graham RP, Merrell KW, Thiels CA, Hallemeier CL, Warner SG, Haddock MG, Grotz TE, Tran NH, Smoot RL, Ma WW, Cleary SP, McWilliams RR, Nagorney DM, Halfdanarson TR, Kendrick ML, Truty MJ. FDG-PET Predicts Neoadjuvant Therapy Response and Survival in Borderline Resectable/Locally Advanced Pancreatic Adenocarcinoma. J Natl Compr Canc Netw. 2022;20:1023-1032.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 39. | Soloff EV, Al-Hawary MM, Desser TS, Fishman EK, Minter RM, Zins M. Imaging Assessment of Pancreatic Cancer Resectability After Neoadjuvant Therapy: AJR Expert Panel Narrative Review. AJR Am J Roentgenol. 2022;218:570-581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |