Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.583
Peer-review started: November 25, 2023
First decision: December 6, 2023
Revised: December 17, 2023
Accepted: January 11, 2024
Article in press: January 11, 2024
Published online: March 15, 2024
During the last few years, epidemiological data from many countries suggest that the incidence and prevalence of many cancers of the digestive system are shifting from the older to the younger ages, the so-called “early-onset cancer”. This is particularly evident in colorectal cancer and secondarily in other malignant digestive neoplasms, mainly stomach and in a lesser degree pancreas, and biliary tract. It should be emphasized that data concerning digestive neoplasms, except for those referring to the colon and stomach, could be characterized as rather insufficient. The exact magnitude of the shift in younger ages is expected to become clearer shortly, as long as relevant epidemiological data from many parts of the world would be available. The most important question concerns the etiology of this phenomenon, since its magnitude cannot be explained solely by the better diagnostic methodology and the preventive programs applied in many countries. The existing data support the assumption that a number of environmental factors may play a primary role in influencing carcinogenesis, sometimes from childhood. Changes that have appeared in the last decades related mainly to eating habits, consistency of gut microbiome and an increase of obese people interacting with genetic factors, ultimately favor the process of carcinogenesis. Even these factors however, are not entirely sufficient to explain the age-related changes in the frequency of digestive neoplasms. Studies of the individual effect of each of the already known factors or factors likely to be involved in the etiology of this phenomenon and studies using state-of-the-art technologies to accurately determine the degree of the population exposure to these factors are required. In this article, we attempt to describe the epidemiological data supporting the age-shifting of digestive malignancies and their possible pathogenesis. Finally, we propose some measures regarding the attitude of the scientific community to this alarming phenomenon.
Core Tip: Incidence and prevalence of many cancers of the digestive system are shifting from the older to the younger ages, the so-called “early-onset cancer”. We propose some measures regarding the attitude of the scientific community to this alarming phenomenon.
- Citation: Triantafillidis JK, Georgiou K, Konstadoulakis MM, Papalois AE. Early-onset gastrointestinal cancer: An epidemiological reality with great significance and implications. World J Gastrointest Oncol 2024; 16(3): 583-597
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/583.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.583
The term early cancer is defined as the appearance of cancer in any organ of the body at the age of less than 50 years[1]. During the last decades, the incidence of early cancer has increased significantly in many organs of the digestive system including primarily the colon and stomach and secondarily the esophagus, bile ducts, and pancreas, as well as other organs and systems. For the interpretation of this phenomenon, it is argued that there was probably an exposure during the early life to factors that favor the development of benign or malignant neoplasms. It is believed that changes related to lifestyle, eating habits, gaining excess body weight, as well as changes in the gut microbiome began to occur decades ago and continue to exist in many countries around the world. These changes interacting with genetic factors may be responsible for the appearance of this epidemiological phenomenon[2]. However, the carcinogenic effects of each of the factors remain largely unknown.
In this review, we attempt to describe the epidemiological data supporting the age-shifting of digestive malignancies and the possible underlying pathogenetic mechanisms. Also, we propose some measures regarding the attitude of the scientific community to this alarming phenomenon. A future goal of the scientific community should be the reduction of the treatment-related morbidity of the underlying carcinoma, as well as to prevent the occurrence of psychosocial sequelae and to treat them accordingly. Epidemiological studies should still be carried out with an emphasis on the investigation of etiological mechanisms and the development of methods of prevention and early diagnosis.
Cancer today is one of the most important causes of morbidity and mortality. According to Global Cancer Statistics 2020, breast (11.7%), lung (11.4%), and colorectal cancer (CRC) (10.0%) are the most common malignancies, while lung cancer is the leading cause of cancer death (18%), followed by colon (9.4%), and liver cancer (8.3%). We know that the incidence of cancer is higher in adults over 50 years of age, but the incidence of early-onset cancer has increased worldwide, resulting in significant individual and societal impacts. Since the beginning of the 20th century, changes in diet, lifestyle, and the environment have resulted in an increase in cancer rates around the world. Increasing obesity rates, lack of physical exercise and environmental pollution have also contributed to the incidence of early cancer. In addition, alcohol, smoking and exposure to harmful agents during pregnancy are likely to have influenced the incidence of early-onset cancer.
In a recent study, the epidemiological parameters of early-onset cancers (incidence, deaths, disability-adjusted life years and risk factors) were investigated based on the Global Burden of Disease 2019 study with reference to 29 types of cancer worldwide[3]. It was found that between the years 1990 and 2019 the incidence of early-onset cancer increased by 79.1% (1.82 million cases of early-onset cancer in 1990 and 3.26 million cases worldwide in 2019). The number of early cancer deaths increased also by 27.7% (0.83 million cancer deaths in 1990 and 1.06 million deaths in 2019). The shift towards younger age of breast, respiratory system, stomach and colon cancers showed the highest mortality and disability-adjusted life years in 2019. Globally, early liver cancer showed the steepest decline. Early colon cancers exhibited high disability-adjusted life years for both, men and women. Areas with a high and medium socio-demographic index showed the highest incidence of early cancer. Projective indicators suggest that the global number of cases and deaths from early-onset cancer will increase by 31% and 21% in 2030, respectively. However, for the acceptance of these data, the possible influence of various other factors should also be taken into account. For example, in the above study, the authors did not take into account population growth and the age of individuals. The world population between the years 1990 and 2019 has increased by 46%, and the authors’ calculations were based on absolute numbers rather than standardized age percentages. Similarly, the authors calculated the change in deaths using absolute, rather than population- or age-adjusted, numbers. In the discussion of the results the authors acknowledge that the study has several limitations that could affect the results, such as variations in the quality and availability of data in different countries. Moreover, some details regarding the accuracy of the data are the data concerning certain countries. The authors, for example, compared the Solomon Islands with the other 203 nations and concluded that the inhabitants of these islands had the highest standardized rate of death from early-onset cancer (82.9 per 100000). However, this tiny South Pacific nation, whose population is spread across 350 islands, began collecting cancer data after 2008 and established its first oncology unit in 2019. The authors also reported sharp increases in cases of cancers diagnosed between the years 1990 and 2019 in the United Arab Emirates (1127.6%), Qatar (1089.5%) and Saudi Arabia (896.0%). It is estimated that by 2030 the incidence of cancer in the Middle East countries will increase by 1.8 times. The highest number of cases has been observed in Egypt and Lebanon (159.4 and 165.8 cases/100000 population respectively), while the lowest number of cases has been observed in Saudi Arabia and Sudan (96.4 and 95.7 cases/100000 population respectively)[4]. Despite this, the prevalence of malignant diseases is still lower than that of Western countries.
Therefore, if population growth is taken into account, the data can be expected to change significantly. Thus, if the analysis is population-adjusted, the global incidence of early-onset cancers is estimated to have increased by 6% over the past 30 years, while cancer deaths actually decreased by 25% based on additional data from the above study. Despite these remarks, and based on the available data, we can accept that the morbidity from early-onset cancer continues to increase, showing variations in mortality and disability-adjusted life years depending on the country, sex, and type of cancer. Encouraging a healthy lifestyle in combination with other modifications could reduce the burden of early cancer.
Early-onset esophageal adenocarcinoma: Concerning the epidemiological changes and clinical characteristics of early-onset esophageal adenocarcinoma (early-EAC) recent data suggests that the incidence of this malignancy is increasing in many countries of the world. It is worrying that the majority of patients have end-stage disease and therefore a poor prognosis. The available data also suggests that despite the implementation of screening and surveillance programs for patients with Barrett’s esophagus, early esophageal cancer occurs at advanced stages and is associated with a poorer survival as compared to the elderly. The consequences of the increase in the frequency of early esophageal cancer are expected to be significant concerning early diagnosis, therapeutic treatment, preventive strategy, as well as the etiological approach to this epidemiological phenomenon[5].
The existing data regarding the epidemiological and clinical parameters of early-EAC are considered rather insufficient, probably due to the relative rarity of the disease. In a population-based cohort study involving the residents of Sweden for the period 1993 to 2019 and aiming to find differences in the incidence and survival between early and late-onset esophageal cancer in a population of 470 patients, it was found that the prevalence of the disease in men was greater in those with early-EAC. Even patients with early-EAC had better survival mainly in stages 0 to II. No differences were observed in the incidence of the disease in the groups of women with early and late-onset, respectively[6].
Lai et al[7] studied a total of 18278 patients with gastroesophageal junction adenocarcinoma who were divided into three age groups: Less than 50, 50-69, and equal to or greater than 70 years. The clinical and histological characteristics as well as the prognosis of the three groups in three periods (1975-1989, 1990-2004, and 2005-2017) were examined. It was found that patients with early-EAC had higher tumor grades, advanced nodal and distant metastatic disease at diagnosis, compared to the other groups. Early-onset patients also received chemo-radiotherapy at a higher rate compared to older patients, with better overall survival. Finally, patients with early-EAC were more likely than other age groups to present with advanced disease at diagnosis and with a better prognosis[7].
In a study from the United States[8], the authors found that for the period 2000-2002 to 2015-2017, the incidence of esophageal adenocarcinoma increased in all age groups from 2.9 to 3.3 per 100000 population. Specifically, they analyzed 114123 patients diagnosed with esophageal adenocarcinoma. Most patients were diagnosed at an advanced stage (53.6% stage III/IV). At diagnosis, the percentage of patients with early-EAC and stage IV disease was significantly higher compared to the late-onset group, while the percentage of patients with stage I early-EAC was lower compared to late-onset patients. However, median survival was higher and overall survival was better in the early-EAC compared to the late-onset group. These data support that in the United States the increasing incidence of esophageal adenocarcinoma is largely due to the onset of the disease in older adults. Contrary to the data concerning early-CRC in Western countries, the results show consistent rates of early-EAC.
Early-onset squamous esophageal cancer: In relation to esophageal squamous cell cancer, the existing data are also insufficient, mainly coming from countries with an increased incidence and prevalence of the disease. East African countries have the highest percentages of patients with esophageal squamous cell cancer.
An interesting epidemiological feature of this type of carcinoma is its high incidence at young ages with 1/3 of cases being diagnosed under the age of 45. In a study from Tanzania, the authors compared 471 patients with esophageal squamous cell cancer younger than 45 (100 patients, 21%) and a group of esophageal cancer patients older than 45 years, and with a group of 471 sex- and age-matched controls with benign diseases. They found that patients with early onset of the disease reported infrequent tooth cleaning, exposure to secondhand smoke, and increased exposure to factors such as pest infestation of grain and/or nuts. In the group of elderly patients, it appeared that low socioeconomic status, family history of esophageal cancer, smoking, drinking alcohol, storing grains or nuts at home, and using firewood when cooking food were risk factors for the development of carcinoma. Finally, the intake of hot drinks was associated with an increased risk in both age groups[9]. It appears, therefore, that risk factors for the development of esophageal cancer in Tanzania differ between age groups and that environmental and behavioral factors play an important role in the high incidence of early-EAC.
The diagnosis of early-onset gastric cancer (early-GC) represents an important individual and societal challenge. In general, those who are younger than 45-years-old when the carcinoma is diagnosed are considered to be suffering from early-GC. Epidemiological data support that early-GC constitutes about 5% of the total cases of stomach cancer with a percentage of 3% accompanying hereditary syndromes and the rest constituting sporadic cases. Interest in early-GC has recently been rekindled due to the increase in reported cases internationally. The clinical and epidemiological characteristics of this form of GC differ from sporadic and familial GC suggesting that it constitutes a largely distinct clinical entity that requires an adapted preventive, diagnostic, and therapeutic strategy[10]. Disease progression is rapid; with distant metastases being diagnosed early after the disease is confirmed. The degree of differentiation of the neoplasm is low, and the molecular and genetic mechanisms involved in the processes of carcinogenesis are largely different from those of sporadic GC. The prognosis of the disease is generally poor with survival rates being particularly low[11]. These data are confirmed by data from a German study of 46110 patients with early-GC. The results showed that the incidence of signet ring cell carcinoma was increased in the cases of patients with early-GC. Patients with early-GC were in a greater proportion male while the disease stage and tumor differentiations were more advanced compared to cases of late-onset GC. Interestingly, the survival of patients with early-GC despite the advanced stage, the aggressiveness of the tumor, and the less treatment they received, their survival was significantly better than that of patients with advanced age (5-year survival: 44% vs 31%) confirming the results of other studies showing that age is an independent predictor of better survival[12].
In the study by Zhou et al[13] the survival curves of patients with late-onset signet ring cell carcinoma showed that these patients had worse survival than patients with early onset. This study also confirms that patients with early signet ring cell GC had a higher chance of distant metastases compared to patients in the late-onset group and that age younger than 45 years, is indeed an independent risk factor for distant metastases[13].
In the United States, while the incidence of GC is decreasing, the incidence of early-GC is steadily increasing. Early-GC currently exceeds 30% of all GC cases in the United States, being a genetically and clinically distinct type from traditional GC. Also, early-GC cases were more likely to be Epstein-Barr virus-related or genomically stable, while late-GC was more likely to be a microsatellite instability subtype[14]. Giryes et al[15] also investigated the clinical, epidemiological, and genetic differences between early-GC and traditional GC in 95323 patients between the years 2000 and 2014. They found that while the incidence of traditional GC was decreasing during the study period, the incidence of early-GC remained constant. Early-GC was less common in men and whites while presenting more aggressive histological subtypes. They found no differences in risk factors between the two groups[15]. All authors of the above mentioned studies agree that further studies are needed to investigate the possible causal basis of the clinical effects of these two types of GC.
The existing epidemiological data worldwide suggest that despite the implementation of screening, public information about risk factors, and the implementation of improved diagnostic and therapeutic strategies, CRC continues to be a major health problem representing the second cause of cancer death worldwide. This is proven in the study by Hu et al[16], who described the epidemiological data of CRC in the period 1990 to 2019 in 204 countries using linear and join point regression models. Globally, a slight decrease in the age-standardized rate of disability-adjusted life-year was found, which was more evident in the female sex and the countries of Western Europe and Australia. During the next 20 years, a small increase in morbidity is predicted, a faster decrease in mortality, and a shift in the age of onset of the disease towards younger ages[16]. However, it is generally accepted that the epidemiological data of CRC in countries of low socio-economic level is lacking, and this concerns those particularly in African countries for which CRC is diagnosed late, resulting in increased mortality[17].
Regarding the epidemiological data of early-onset CRC (early-CRC), it appears that this type of CRC in the year 2030 will be represented in many countries with about 11% of the total number of CRC and 30% of rectal cancers in both men and women[18]. The increase in early-CRC cases is greater in middle and high-socio-demographic index countries as well as in East Asian countries in which appropriate adaptation of screening guidelines needs to be done with the adoption and introduction of more effective preventive and diagnostic interventions[19].
Pan et al[20] studied the global incidence, prevalence, mortality, and disability-adjusted life years of this type from 1990 to 2019. The results showed that the global incidence of early-CRC cases more than doubled, increasing their number from 95737 cases per 100000 population in 1990 to 226782 cases per 100000 population in the year 2019. The incidence of deaths increased from 50997 per 100000 in 1990 to 87014 per 100000 population in 2019. Finally, disability-adjusted life years increased significantly mainly in middle socio-demographic index countries in which aging and population growth play an important role[20].
In general, early-CRC follows a birth-cohort effect due to long exposure to risk factors for the development of cancer. Of concern is the phenomenon of diagnosis in advanced stages with histological features associated with a poor prognosis. Early-CRC presents some special features such as the frequent presence of microsatellite instability. So far there are no relevant treatment protocols for early-CRC cases depending on the patient’s age. The finding of high rates of germline pathological mutations in patients with early-CRC makes it necessary to evaluate the existence of genetic risk[21].
According to recent data from the American Cancer Society, it is estimated that in the United States in 2023, 153020 people will be diagnosed with CRC and 52550 people will die from the disease. These numbers also include 19550 cases of early-CRC from which 3750 people will die. A decrease in CRC incidence of 1% per year during 2011-2019 was observed compared to 3%-4% per year during the 2000s, possibly due to an increase in the proportion of people under 50 years of age. The incidence of early-CRC since 2010 has increased by 2%-3% per year for locoregional disease and by 0.5%-3% for disease with distant metastases, per year. A shift towards left-sided colon tumors was also seen with the rate of rectal cancer increasing from 27% in 1995 to 31% in 2019. Mortality decreased by 2% per year from 2011-2020 but increased by 0.5%-3% per year for people under 50-years-old. Collectively, and despite continued overall declines, CRC is rapidly shifting toward younger ages, more advanced stages, and the left colon[22]. In a recent study, it was also confirmed that the majority of early-CRC cases are located in the left colon, but patients with localization in the right colon have a higher mortality[23].
Zaki et al[24] determined 5-year survival and differences in survival by race and ethnicity in 33777 patients with early-CRC. Five-year survival ranged from 57.6% (black patients) to 69.1% (white patients). Survival improved from 2003 to 2013 but only for whites, while there was no improvement in black, Asian, and Hispanic patients. The study demonstrates racial-ethnic disparities in survival and mortality in patients with early-CRC[24].
It is well-known that racial/ethnic disparities of the epidemiological data regarding the early-CRC become more pronounced over time. People belonging to the black race have a higher incidence of early-CRC and a shorter survival compared to white people. Recent data demonstrated that early-CRC rates have increased significantly among American Indians/Alaska Natives, Hispanics, and Whites. Also, compared to whites, the rate of increase among American Indians/Alaska Natives is close to 21% while it is 6% higher among blacks. In contrast, although the rates of late-onset CRC are decreasing, they remain 29% higher in blacks and 15% higher in American Indian/Alaska Natives compared to whites. The overall incidence of CRC is higher in males except for CRC of the left colon which is higher in females[25]. These epidemiologic data suggest that access to CRC screening should be ensured in Black and American Indian/Alaska Native people, conditional on their acceptance of the screening recommendation. Also, recent comparative data on the prevalence of early-CRC among Africans in Nigeria and African Americans in the United States show that among 5019 black individuals, 379 were Nigerian and 4640 were African American. Black Nigerians with early-CRC were eight times more likely to have rectal cancer compared with African Americans with early-CRC. Of interest is the fact that early-CRC patients from Nigeria present particular clinical features compared to the corresponding African-American patients[26]. Recent data also suggest that the incidence rates of rectal adenocarcinoma are 39% lower in black individuals with the gap widening between white and black women[27].
Likely, the rigorous study of the pathology data and the biological heterogeneity of black patients with early-CRC will help to better understand the racial differences observed, as well as to design more effective strategies for the prevention, detection, and treatment of patients. Even though the age of screening for CRC has shifted to 45 instead of 50 years in all racial groups, this age shifting could succeed after appropriate publicity to increase the number of young people undergoing screening for CRC.
Li et al[28] studied the epidemiologic and clinical parameters and risk factors of Chinese patients with early-CRC and compared the results with counterparts from the G20 countries. The results showed that between 1990 and 2019, the age-standardized incidence rate and age-standardized prevalence rate of early-CRC in China increased (estimated annual percentage change 4.61 and 5.82). Compared to G20 countries, China ranked 13th in terms of age-standardized incidence rate in 1990 to reach 2nd in 2019 (after Japan). Age-standardized prevalence rates increased in all G20 countries, while they were highest in Saudi Arabia, followed by China and Mexico. In addition, China had the highest age-standardized mortality rate and the highest age-standardized disability-adjusted life years in 2019. The main risk factors for both sexes in Chinese patients in 2019 were a diet low in milk and calcium, drinking alcohol, smoking, and a diet high in red meat. It is estimated that during the next 10 years the age-standardized incidence rate, the age-standardized mortality rate, and the age-standardized rate of disability-adjusted life years will continuously increase in both sexes[28].
Pancreatic cancer (PC) represents the most lethal malignant disease in humans. It is claimed that by the year 2040, PC will be the second leading cancer-related cause of death in the United States. Pancreatic adenocarcinoma is the most common histological type with the lowest 5-year survival rate. The available epidemiological data [Surveillance, Epidemiology, and End Results data (SEER)] demonstrate a significant increase in the incidence of pancreatic and biliary tract cancers in younger populations during the last three decades. In the majority of cases of early-onset pancreatic cancer (early-PC) which represents 5%-12% of all PC cases no hereditary or familial background is observed, suggesting that behavioral, lifestyle, and dietary factors, in combination with microbial, environmental, and host factors, participate in its pathogenesis[29].
Huang et al[30] using data from the United States North American Association of Central Cancer Registries compared the incidence of early-PC with the incidence of late-onset PC (LOPC) by sex, race, and ethnicity in the entire United States population. The authors analyzed 713622 (80908 early-PC and 632714 LOPC) cases of invasive pancreatic adenocarcinoma from 1995 to 2018. They found that patients with early-PC were more often male, less likely to be non-Hispanic white, and more likely to have advanced-stage cancer compared with the group of patients with LOPC. They also found that the incidence of early-PC and LOPC increased steadily over the study period, with the mean annual percentage change being twice as high for the LOPC compared to the early-PC group. The incidence was also greater in males compared to females for both early-PC and LOPC. However, only women showed a significant increasing trend for early-PC while both men and women showed a similar increasing trend for LOPC[30]. The increased incidence of early-PC in women could be due to histological subtypes of the disease more commonly seen in women (e.g., cystic adenocarcinomas) which was also observed in this study. The faster increase in the incidence of early-PC in non-Hispanic white and Hispanic United States women suggests that these racial groups would likely benefit from more targeted surveillance practices and other treatment interventions.
Ren et al[31] studied 42414 patients with PC of whom 2916 (6.9%) had early-PC using the SEER database. Patients with early-PC were more often male and had a larger tumor size, a higher rate of vascular infiltration, and a higher rate of distant metastases compared to the LOPC group. However, surgical resection rates were comparable, and histological features were similar in patients who underwent surgical resection of the tumor. The early-PC group had a longer 5-year overall survival and specific 5-year survival with a similar prognosis[31].
In a study from the Czech Republic involving 18888 patients of whom 1324 (7.0%) had early-PC for the period 1985 to 2015, the authors observed a mean annual percentage change in the frequency of patients with early-PC of -1.0%. The average annual percentage change for male patients with early-PC was -2.0% while for females it was +0.6%. The incidence of average annual percentage changes for LOPC was +1.3%. There were no differences in tumor stage, grade, or location between early-PC and LOPC. Younger patients were more often male and had better overall survival, which means that continuous efforts should be made for the early diagnosis and treatment of patients with early-PC[32].
Regarding the epidemiological characteristics of early pancreatic neuroendocrine neoplasms (early-EPNs), although they are of substantial scientific interest, they have not been sufficiently investigated. A recent study using data from the SEER database and between the years 2000 and 2018, investigated the incidence, clinical and histological characteristics, treatment, and survival of 5172 patients with pancreatic endocrine neoplasms, of which 1267 cases (24.5%) involved early-EPNs and 3905 cases (75.5%) involved late-onset EPNs. It was found that the age-adjusted incidence increased significantly in patients with late-onset EPNs in contrast to the incidence of early-EPNs which remained relatively stable. Early-EPNs were more common in women, had better tumor differentiation, and were treated surgically in a higher proportion compared to late-onset ERNs. For localized disease at presentation, surgery has been the most commonly used regimen over the past two decades. Overall survival and cancer-specific survival were significantly better in the early-EPNs group. This study found that early-EPNs are a rare clinical entity distinct from the late-onset EPNs group[33]. Certainly, further studies are needed to better understand the characteristics of this subgroup of pancreatic endocrine neoplasms.
Data originating in developed Western countries suggest that early-onset biliary cancer (early-BC) - a rare malignant disease of the biliary tract and gallbladder - shows continuously increasing trends. In a recent study from Sweden, the authors analyzed data from all patients aged 20-84 years diagnosed with biliary tract cancer between 1993 to 2019 (14083 patients). The authors divided the study period into three parts, 1993-2001, 2002-2010, and 2011-2019. The 14083 patients were divided into two groups: The early-BC group (1377 patients, 9.8%) and the late-BC group (12706 patients, 90.2%). The results showed that the incidence of gallbladder cancer decreased, while intrahepatic cholangiocarcinoma increased in the early-BC patient group. Also, both intrahepatic and extrahepatic cholangiocarcinoma increased in both age groups with the increase being more prominent in the early-BC group[34]. Further studies need to be conducted in order to interpret the causes of these epidemiological changes and trends.
Since the 1950s, major lifestyle changes, such as antibiotic use, low physical activity, and obesity, may all be significant risk factors for developing early digestive tract cancer. Although the etiology of this phenomenon is not fully understood, several factors seem to contribute to the emergence of this epidemiological change. These factors are analyzed below.
Smoking has long been known to participate in the carcinogenesis processes of many human organs and systems, including the digestive one. The effect of smoking on the occurrence of early gastrointestinal cancer has not been adequately studied. The existing data are mainly referred to CRC.
In a recent study, Li et al[35] investigated the smoking habits of 724 patients with early-CRC and 5540 cases of patients with late-CRC. They found that smoking exposure was much higher in patients with early-CRC compared to patients in the late cardiopulmonary resuscitation group. Adjusted odds ratios for the two groups were 1.57 and 1.46 respectively, and in the quitters: 1.39 and 1.24 respectively. These data did not differ in patients with disease located in the rectum or the rest of the large intestine[35].
In a systematic review and meta-analysis, Li et al[36] evaluated the relationship of smoking habits with the occurrence of early- or late-onset CRC. They included six studies in their analysis. They found that current smokers are at a high risk of developing early-CRC compared to nonsmokers in contrast to quitters for whom they found no significant differences compared to non-smokers. These data suggest that smoking is an important risk factor for both early-CRC and late-CRC, and that quitters are at the same risk as nonsmokers. Therefore, smoking can with sufficient certainty be one of the factors responsible for the increase in the incidence of early-CRC[36].
It is estimated that worldwide nutritional risks for disability-adjusted life years account for 34.4%, due to the low consumption of milk, whole grains, and calcium. A diet high in sodium is a risk factor for early-GC. Diet-related environmental factors also participate in the pathogenesis of early-CRC and the increase of its incidence. Especially for early-CRC, it is argued that the increase in the incidence of metabolic diseases such as diabetes mellitus, seen in young ages, contributes to the increase in early-CRC cases diagnosed in the last decades.
In a recently published systematic review and meta-analysis, Khoa Ta et al[37] analyzed 33359 early-CRC cases and 14259289 controls included in 12 studies. A significant positive correlation was found between diabetes mellitus and an increased risk of early-CRC which suggests that diabetes mellitus is a risk factor for the development of early-CRC. The higher prevalence of diabetes mellitus among younger adults observed may contribute to the increasing incidence of early-CRC[37]. It is therefore concluded that decisive interventions to reduce this two-way risk should be studied more extensively as this will contribute to the development of effective prevention and treatment strategies.
Chen et al[38] investigated the possible associations of a group of dietary factors and the incidence of early-CRC in people aged 25-49 years during the period 1977-2016 in the United States, using negative binomial regression models. They found that the incidence of early-CRC was positively associated with smoking and alcohol consumption for both men and women. No correlations were found with other dietary factors such as obesity and fiber intake. Alcohol consumption in particular, which has increased significantly among young adults since the 1980s, may have contributed to the increased incidence of early-CRC showing its epidemiological impact since the 1990s. It is argued that increased alcohol consumption may have contributed to the recent increases in the incidence of early-CRC[38].
The role of serum 25-hydroxyvitamin D levels in early-CRC cancer prevention has not been adequately studied. Kim et al[39] studied serum 25(OH)D levels in 236382 Korean adults. Vitamin 25(OH)D levels were categorized as < 10, 10-20 and ≥ 20 ng/mL. The authors correlated serum 25(OH)D levels with the risk of CRC. The results showed that during 1393741 person-years of follow-up, 341 people developed CRC. Among the early-CRC subjects, serum 25(OH)D levels were inversely associated with risk of CRC [hazard ratio (HR) = 0.61 and 0.41 for 25(OH)D 10-19 ng/mL and ≥ 20 ng/mL, respectively], concerning the reference (< 10 ng/mL). For late-CRC subjects the associations were similar compared to younger subjects. The results of this study suggest that serum vitamin 25(OH)D levels are favorably associated with the risk of developing both early-CRC and late-CRC[39].
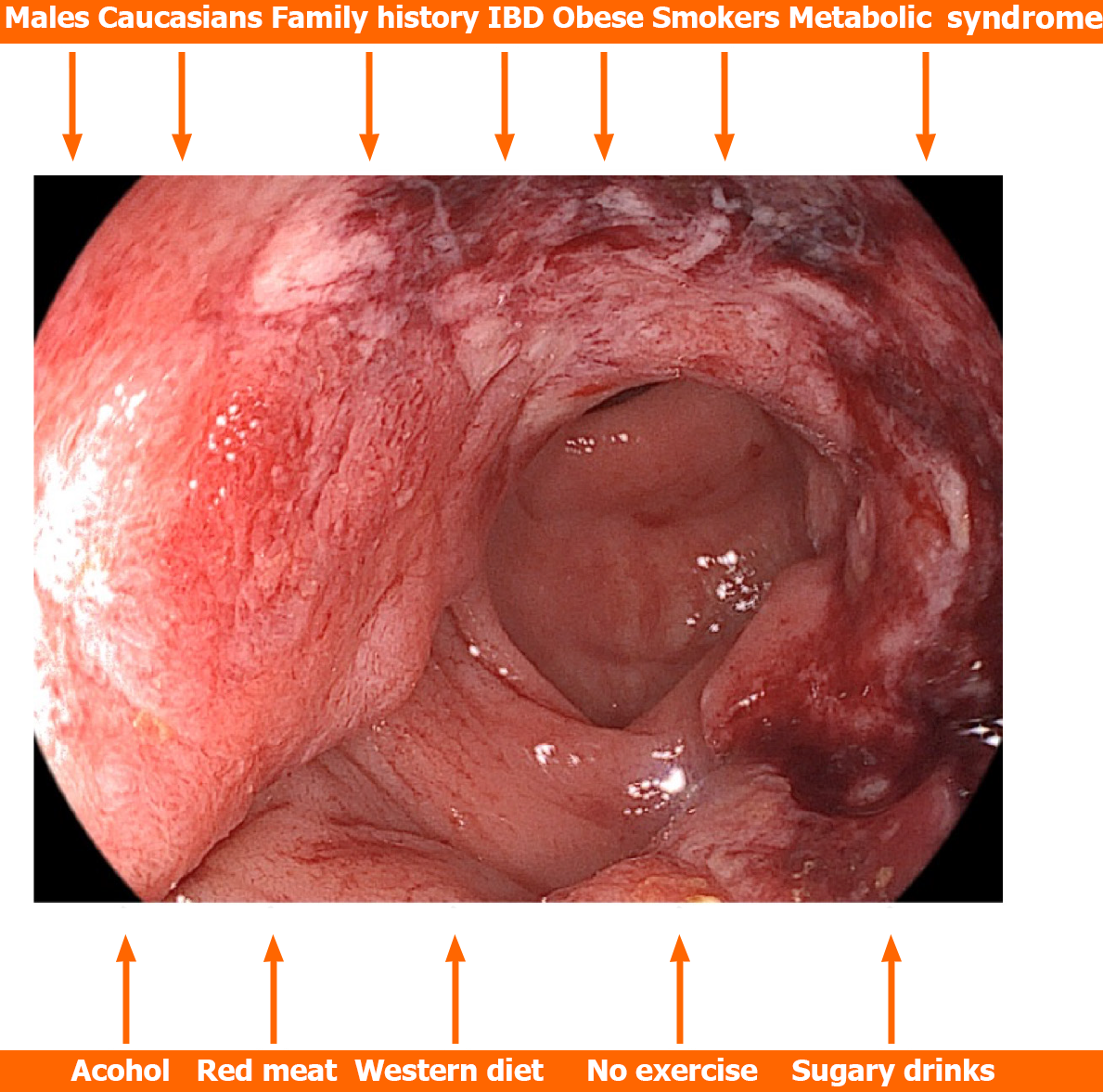
In a systematic review and meta-analysis involving 36 and 30 studies respectively, Hua et al[40] investigated risk factors for early-CRC occurrence. They found that significant risk factors were male sex, people of Caucasian race, people with a positive family history of CRC, patients with inflammatory bowel disease, obese and overweight people, people with hypertriglyceridemia, hypertension, and metabolic syndrome, smokers, people with a history of alcohol consumption, processed or red meat, as well as people with Western dietary patterns, people who do not exercise and people who consume sugary drinks. They found no significant differences between hyperlipidemia and hyperglycemia (Figure 1). Vitamin D was a protective factor[40]. Based on these data, risk prediction models specific to early-CRC as well as tailored screening strategies should be created.
Regarding obesity, although it is known to be a risk factor for digestive system cancer, its relationship with the development of early gastrointestinal cancer has not been sufficiently studied. In a 2004-2008 study from the United States, the authors found that obesity [body mass index (BMI) > 30] was significantly associated with a lower age of diagnosis in all four types of cancer studied, namely 3.25 ± 0.53 years for GC, 4.56 ± 0.18 years for CRC, 4.73 ± 0.73 years for esophageal cancer and 5.35 ± 0.72 for PC. People with morbid obesity (BMI > 40) had an even younger age of cancer onset namely 5.48 ± 0.96 years for GC, 7.75 ± 0.30 years for CRC, 7.67 ± 1.26 years for esophageal cancer, and 8.19 ± 1.25 years for PC. The association of obesity, particularly morbid obesity, with early onset of all 4 of these malignancies remained positive even after adjustment for other predisposing risk factors[41]. Therefore, the causal relationship between obesity and early-GC should be further investigated. Furthermore, data from a recent study from Norway confirmed that increased BMI in young adults (males and females) increases the risk of early-PC. Survival was lower in overweight and obese subjects in both sexes. The analysis, adjusted for sex, age, and period of diagnosis, confirmed the increased risk of death from cancer in obese individuals. Overweight and obese individuals were less likely to undergo curative surgery while obese individuals had a higher risk of death from cancer[42].
Regarding the metabolic syndrome, it appears that male patients present more often early-CRC than late-CRC. Even patients over 50 years of age present more often advanced serrated lesions than conventional adenomas[43]. Prevention of metabolic syndrome is expected to have a favorable effect on the incidence of colon adenomas especially in people over 50 years of age in the following years.
Although a significant percentage of early-onset digestive system cancers are related to germline genetic variants, in the majority of early-onset digestive system other factors such as diet, lifestyle, environmental factors and the gut microbiome participate in the processes of carcinogenesis exerting their influence from young to mature age. However, the exact contribution of each of these factors remains unclear.
Evidence so far suggests that different types of diet, lifestyle, and environmental exposures alter the oral and gut microbiome[44]. The animal-based diet, the so-called Western diet, causes a shift in the dominant microflora and their metabolic activity, which can disturb the homeostasis of hydrogen sulfide concentration. It is reasonably hypothesized that bacterial sulfur metabolism is a critical mechanism involved in the pathogenesis of early-CRC. The microbial sulfur diet facilitates the induction of inflammatory changes and mucosal damage, changes that contribute to the development of CRC[45].
It is argued that individuals with primary immunodeficiencies as well as individuals with innate immune deficiencies present an increased risk of developing early digestive system cancer. This assumption is supported by the data of a recent systematic review. In this study, the authors included all patients with inborn errors of immunity and primary immunodeficiencies who, during the disease, developed a malignant neoplasm of the digestive tract (stomach, large and small intestine). They found that the common variable of hypogammaglobulinemia was the most common immunodeficiency situation linked with the development of malignancy of the gastrointestinal tract. They also observed that the age of onset of the neoplasm was younger compared to the general population. Another 12% of patients had molecular genetic diseases, in which the three most frequently involved genes were ATM, CARMIL2, and CTLA4. Decreased humoral immunity and Epstein-Barr virus infection were the most likely underlying etiological factors[46]. These data support that patients with primary immunodeficiencies and other congenital immunodeficiencies should be considered as patients at increased risk for early-GC and therefore screening should be started at an earlier age than recommended.
Regarding the effect of the therapeutic use of antibiotics during childhood, use which has been etiopathogenetically associated with a multitude of morbid conditions in later adult life (e.g., inflammatory bowel disease). Jiang et al[47] found that long-term or recurrent antibiotic use by early life span is associated with an increased risk of early-CRC and adenomas and that the association with adenomas was significantly greater among individuals with the rs281377 TT/CT genotype. However, further studies are needed to investigate how long-term or recurrent antibiotic use contributes (together with genetic factors) to the modification of early-CRC risk, especially regarding the microbiome-related pathway that governs colon carcinogenesis.
Genetic factors and complex molecular mechanisms participate to varying degrees and proportions in the pathogenesis of early digestive system carcinomas. Good quality data exist for early-CRC and early-PC which are analyzed below.
In early-CRC: The existing histological and molecular characteristics of early-CRC cases suggest that these tumors differ from their counterparts in late-CRC patients. The observed DNA, RNA, and protein-level changes appear to be unique to early-CRC and highlight the need for biomarkers and new therapeutic targets and strategies. The majority of molecular studies related to early-CRC have been performed in Western and Asian countries. Genetic and molecular features that have been studied in late-CRC such as high-throughput analyses of histone modifications, mRNA splicing, and proteomics have not yet been investigated in early-CRC. As highlighted by Marx et al[48], the complex relationship between cancer and the aging processes of the organism should be taken into account when studying the molecular substrates of early-CRC. Approximately 10% of CRC cases are associated with pathogenic variants. These pathogenic variants were detected in 15%-33% of individuals with early-CRC regardless of family history of CRC. It is noteworthy that next-generation sequencing has improved the accessibility of genetic tests to detect the existence of cancer susceptibility genes. As a result, genetic testing is expected to have a significant impact on the early-CRC shortly.
It is argued that because the colon genome differs in its different segments the prevalence of certain molecular features of CRC may vary gradually in the different segments of the colon. Understanding the molecular differences of cancer in different segments of the colon at different ages may contribute to individualized patient management. Ugai et al[49] studied microsatellite instability, CpG island methylator phenotype, and KRAS and BRAF mutations in 14004 cases with CRC including 3089 cases of early-CRC, in different locations of the cancer (caecum, anion, transverse, cation, sigmoid colon and rectum). The comparison of the early- and late-CRC groups demonstrated that the rates of early-CRC with high microsatellite instability, high CpG island methylator phenotype, and mutated BRAF were lower in the rectum and higher in the ascending or transverse colon. Compared to late-CRC, early-CRC tumors showed a higher prevalence of microsatellite instability (MSI)-high status and a lower prevalence of CpG island methylator phenotype-high status and BRAF mutations in most subregions. The prevalence of KRAS mutation was higher in the cecum compared with the other carcinoma sites in both early- and late-CRCs. Of note, MSI-high late-CRCs showed a continuous decrease in the prevalence of KRAS mutations from rectal to anion followed by an increase in the cecum, whereas MSI-high early-CRCs showed no such trend[49]. These findings support the biogeographic and pathogenetic heterogeneity of colon carcinomas in its various parts.
In conclusion, there is a need to identify critical biomarkers with the aim of early diagnosis and improvement of effective therapeutic strategies. In addition, the understanding of dysregulated pathways such as PI3K/Akt, Notch, and Wnt related to the progression and metastasis of CRC will contribute decisively to the design of a more effective treatment regimen[50].
In early-PC: Emerging evidence suggests that early-PC exhibits a distinct genetic substrate and that tumor biology is distinct from that of late-PC. However, the role of genetic factors in the development of early-PC needs further investigation as the studies were conducted in a small number of patients and the conclusions are relatively ambiguous.
Existing data support that early-PC patients display distinct genomic features involving several tumor suppressor and oncogenic genes, including SMAD4, RAS wild type, CDKN2A BRCA1, BRCA2, and FOXC2 suggesting that early-PC is a dynamic evolving entity. Furthermore, the high incidence of pathogenic germline variants appears to be involved in the etiology of early-PC. The association with sex found in many studies also suggests the involvement of genetic or socio-environmental factors in the development of LOPC[51]. Additional PI3KCA mutations are more frequently observed in early-PC compared to LOPC. However, KRAS mutations are relatively rare in early-PC. Therefore, it appears that the adverse outcomes noted in the treatment of early-PC are related both to the more advanced stage of the tumor at diagnosis and to the particular biology of the tumor[52].
Between 2008 and 2018, 450 patients with early-PC were diagnosed at Memorial Sloan Kettering, of which 132 (29.3%) underwent somatic testing. Of these, 15.9% had RAS wild-type cancers with actionable alterations including ETV6-NTRK3, TPR-NTRK1, SCA5-NRG1 and ATP1B1-NRG1 fusions, IDH1 R132C mutation, and mismatch repair deficiency. A total of 30.7% of patients underwent germline testing, with 31.9% of them showing pathogenic germline variants and 27.5% showing changes in cancer susceptibility genes. Interestingly, patients with a pathogenic germline variant had reduced mortality compared to patients without a pathogenic germline variant. It appears that pathogenic germline variants are present in a predictable proportion of patients with early-PC[53]. All these observations support recent guidelines recommending universal germline testing and somatic profiling in PC patients.
In the study by Nodari et al[54], 912 early-PC cases and 10222 control cases were analyzed. The associations between polygenic risk score, smoking, alcohol consumption, type 2 diabetes, and the risk of PC were assessed. The researchers found that the polygenic risk score, smoking, and diabetes mellitus adversely affected the risk of developing early-PC. In this study, no new genetic variants were found to be associated with the development of early-PC, but they found that smoking and diabetes mellitus plays an important role in the carcinogenesis of early-PC[54].
The GNA15 gene is known to be expressed in human pancreatic ductal adenocarcinoma cancer cells. The encoded Gα15 protein redirects GPCR signaling to pathways with oncogenic potential. Innamorati et al[55] detected the expression and distribution of this protein in preneoplastic lesions of the pancreas. They found that only increased expression of GNA15 and not any other GNA gene was associated with poor prognosis. The de novo expression of wild-type GNA15 characterizes the mutant pancreatic cells, an expression that persists throughout the progression of the carcinoma and is associated with a poor prognosis. Ectopic Gα15 signaling constitutes a different mechanism activated in the early stages of pancreatic carcinogenesis.
The prevalence of mismatch repair deficiency (a hallmark of Lynch syndrome) in early-onset carcinomas of the pancreas, duodenum, and ampulla of Vater, as well as the underlying molecular mechanisms, were retrospectively investigated in 90 cases of early-onset pancreatic, duodenal, and ampulla of Vater carcinomas. It was found that all cases of mismatch repair deficiency were observed in patients with Lynch syndrome[56]. The agreement of mismatch repair deficiency with the underlying hereditary condition justifies the application of detecting its presence in all cases of early pancreatic and duodenal cancer.
Castet et al[57] retrospectively analyzed the clinical and genomic characteristics of 139 patients with early-PC compared with a group of 197 late-PC patients. The early-PC group had a lower rate of diabetes mellitus at diagnosis, better performance status, higher carbohydrate antigen 19-9 and serum albumin levels. In the multivariate analysis, no differences were observed in the overall survival rate. Age was associated with an increased incidence of KRASMUT. The group of patients with early-PC showed longer progression-free survival and overall survival, as well as better results regarding metastases. The future now concerns precision oncology-based approaches.
Concerning the Arab world, factors considered responsible for changes in the epidemiology of cancer seem to be partly related to the increase in the life expectancy of citizens and also to the adoption of a Western way of living. The modernization of life caused significant changes in the way of life of the Arab people. Even the increasing incidence of obesity, lack of physical exercise, and stressful stimuli may contribute to the increasing incidence of malignancies. The increase in migration flows could theoretically contribute to the appearance of these changes, but no relevant data are available. On the other hand, the lower rates of smoking, alcohol consumption, and genetic predisposition compared to Western countries seem to be protective prognostic factors. Low levels of medical services and late diagnosis are responsible for low survival intervention. Social perceptions of health issues being largely incorrect result in a low level of public consent for cancer screening. Especially for CRC, population screening is particularly low because it is not accepted by local communities due to religious and cultural barriers and a high rate of mistrust regarding the effectiveness of screening. This as mentioned above interprets the advanced stage of CRC at diagnosis which implies an unfavorable outcome[58]. Some countries, such as Qatar, have begun to implement national healthcare transformation programs with proposals that might be adopted by other Middle Eastern countries. According to Brown et al[59], the countries of the Gulf region (Iran, Iraq, Kuwait, Saudi Arabia, Bahrain, Qatar, UAE, and Oman) should make a significant effort towards reforming their health system to provide the population with frontline cancer research and care. Large clinical trials should be encouraged to further clarify the effect of religious fasting and type of diet in Middle Eastern countries, as well as to identify hereditary cancer genes in the Arab population.
The actions that have been adopted in most countries regarding the prevention of early-CRC concern the education and enlightenment of the normal population regarding the magnitude of this threat to public health, through lectures, informative broadcasts via public and private mass media as well as organization and execution of preventive control programs with the cooperation of state and private agencies. This information should also include health workers, especially primary care physicians. The education will concern not only the factors predisposing to the appearance of the disease but also the seeking of medical attention as soon as symptoms or signs of the disease appear.
Public awareness about the role of colonoscopy as a preventive method, especially among people belonging to high-risk groups, is necessary. The detection of fecal hemoglobin with the immunochemical test is almost equally effective in identifying benign and malignant lesions of the colon. People with a family history of CRC or certain genetic conditions may benefit from genetic counseling and genetic testing to assess risk and determine appropriate screening measures. Furthermore, the importance of taking a family history to identify cases of non-polyposis CRC syndrome should not be underestimated. The genetic control of the members of these families is necessary. In the future, the identification of a specific genetic profile could contribute significantly to the improvement of risk stratification[60]. This could in the future help implement a more personalized screening strategy by allocating resources directed at addressing the emerging threat of early-CRC. Finally, current endoscopic techniques for submucosal resection have been applied in the treatment of early-CRC. The indications, advantages, and risks of applying these endoscopic methods are beyond the scope of this editorial. In general, the treatment of early-CRC does not differ from that of CRC occurring in older age.
Primary prevention of GC is achieved by adopting measures aiming to avoid the known risk factors for the development of carcinoma. The development of vaccines against Helicobacter pylori (H. pylori) represents a prospect. Other factors including chemopreventive agents (e.g., vitamins) do not appear to offer significant help. Screening for H. pylori infection in the asymptomatic population is not recommended. Regarding the effects of H. pylori eradication, the results of a recent meta-analysis of seven randomized controlled trials showed that among 4206 subjects who received eradication therapy, 1.6% developed GC, compared with 3% of 4117 subjects who received placebo or no treatment. According to the authors’ calculations, the implementation of screening and subsequent treatment of H. pylori-positive individuals would result in a gain of 8.8 million disability-adjusted life years globally[61]. Dietary recommendations to avoid foods that favor the appearance of GC should be implemented from a young age. Vitamin C found in vegetables and fruits, is associated with a reduced risk of GC.
The most effective way of early-GC detection is the endoscopic monitoring at regular intervals of patients with precancerous conditions of the upper digestive tract by taking multiple biopsies. More effective targeted biopsies can be obtained by applying the new advanced endoscopic techniques with high-resolution endoscopes. For the diagnosis of early-GC, the help provided by current tests such as photofluorography is important. Screening-indicated GC cases have a better 5-year survival compared to cases diagnosed based only on the existence of symptoms. In Japan, preventive endoscopic screening reduces GC mortality by 30%[62].
For the detection of early-GC, new molecular markers related to DNA and RNA, are continuously emerging and applied. These markers can help in improving prognosis, estimating the oncological burden, predicting the emergence of resistance to treatment, and estimating the degree of residual disease. In the initial stages, the so-called epigenetic alterations such as pathological DNA methylation, and histone modifications, which do not involve permanent changes in the DNA sequence, appear very often, representing key elements of GC development and evolution. The most well-studied epigenetic alterations are aberrant DNA methylation, histone modifications, and dysregulated expression of non-coding RNAs[63]. Surgical mini-invasive approaches are applied to patients with early-GC in whom endoscopic submuscular dissection (ESD) application is impossible[64].
Regarding the diffuse GC type, it is known that 25%-30% of affected families meet the criteria for the presence of hereditary diffuse GC germline mutations of the CDH1 (E-cadherin) gene. The recently revised recommendations include broadening the criteria for performing the mutation detection test. Based on these recommendations, mutation-positive individuals should undergo total gastrectomy. However, regular endoscopic surveillance is recommended for patients who do not wish to undergo gastrectomy.
For the treatment of early-GC, ESD is the gold standard for patients having a low risk of lymph node metastases. The application of endoscopic mucosal resection or ESD depends on the possibility of lymph node metastases as well as the possibility of achieving radical endoscopic resection of the tumor. Intramucosal adenocarcinomas (pT1a) have a 2%-5% chance of lymph node metastases while submucosally invasive adenocarcinomas (pT1b) have a 10%-25% chance[65]. Surgical mini-invasive approaches are applied to patients with early-GC in whom ESD application is impossible or not indicated. Furthermore, H. pylori-positive patients who have undergone ESD should be submitted to eradication therapy to avoid the risk of late-GC[66].
In conclusion, screening programs for early-GC should be applied only in high-risk countries, although lifestyle changes and the ever-increasing exposure to risk factors as mentioned above may make population screening necessary also in medium-risk countries as well. Knowledge and adequate understanding by the gastroenterologists of the data and significance of gastric precancerous conditions will also help to understand the implications of secondary screening in these patients. Although the data concerning the application of biomarkers are insufficient, they represent an exciting and promising future research field.
The implications of the epidemiologic data described could be related to programs and strategies to promote screening of large population groups. This promotion of screening strategies can increase the incidence of early cancer detection. For example, increasing the screening rate of 40-year-old and 49-year-old women for cervical cancer, as well as recom
To better understand the underlying mechanism and define the relationships between environmental factors and the development of early-CRC, long-term prospective studies with lifestyle data from childhood and the time limit for data collection are needed[68]. Furthermore, progress against CRC could be accelerated by unraveling the etiology of the increasing incidence in generations born since 1950 and increasing access to high-quality screening and treatment among all populations, particularly Native Americans[22].
Finally, the burden of early-CRC incidence in China and other G20 countries is alarming, indicating that concerted efforts are needed to conduct high-quality research, allocate medical resources, adapt screening guidelines, and develop effective treatment and prevention strategies in the group of G20 countries.
The data described raise problems and challenges that should be addressed in the near future. A future goal should be to significantly reduce the treatment-related morbidity of the underlying carcinoma, as well as prevent the occurrence of psychosocial sequelae. Epidemiological studies should be conducted with an emphasis on the investigation of etiological mechanisms and the development of prevention and early diagnosis mechanisms. These studies should be based on large numbers of patients through dedicated biobanking and data collection technologies. It is also necessary to conduct prospective clinical studies on a large number of patients to investigate the factors involved in carcinogenesis processes, as well as interdisciplinary research approaches, including environmental sciences and various technologies. Regarding early-EAC, future studies should focus on age-related differences in survival as well as why esophageal adenocarcinoma in young people presents at a more advanced stage.
Future early-PC-related studies should seek to clarify whether the differences observed between races and ethnicities
The awareness of the public and of doctors to the various parameters of the phenomenon of cancer moving to younger ages is necessary. Whether early screening and prevention programs for early-onset cancer should be expanded to include people aged 40-44 years and 45-49 years is worth investigating. Finally, encouraging people to adopt healthier lifestyles, including healthy eating, limiting tobacco and alcohol consumption, and appropriate outdoor activity are factors that will help reduce the number of new cancer patients.
During the last years, several studies confirmed the fact that several environmental factors including lack of physical exercise, obesity, high intake of processed carbohydrates, and adoption of the so-called “Western diet” are responsible, among others, for the increase in the early-CRC incidence. Because cancer is a long-term, multistage process, it is understandable that these factors have an impact from the first years of a person’s life, and therefore informing the public about the risks of these habits should be the main task of the authorities of each country. In addition, changes in the intestinal microflora that occur from early life (e.g., delivery method, mother’s eating habits) result in the appearance of dysbiosis which participates in the processes of carcinogenesis[69]. In the future, an effort should be made to modify the intestinal microbiome long-term through probiotics or other drugs, a process that may contribute to reducing the occurrence of early-CRC. Also, a greater effort should be made to improve public compliance with the screening procedures and programs for the asymptomatic population, which should be started earlier than the existing recommendations[70].
Regarding GC, despite the enormous advances made in understanding the mechanisms by which H. pylori infection promotes gastrointestinal carcinogenesis by affecting immune responses, as well as the physiology and histology of the stomach and colon, a full understanding of the mechanisms involved in the various stages of gastric carcinogenesis, has not yet been adequately achieved. Elucidation and understanding of the sequence of genetic and epigenetic events leading to the appearance of precancerous lesions and later-GC remains incompletely understood. Furthermore, H. pylori infection is known to be associated with the appearance of distinct microbial population structures in the digestive tract that contribute to the pathogenesis of GC[71]. These data could contribute to the development of new preventive and therapeutic strategies for GC through modification of the gastric and colon microbiota as well as the immune system. Better and more efficient identification of biomarkers will enable further improvement of the existing screening strategies for GC. It is possible that prevention and treatment strategies could become personalized if interactions between environmental factors (e.g., nutrition, microbiome) and genetic factors could be studied in depth.
According to the prevailing opinion, there is a real increase in the shift in the appearance of many neoplasms of the digestive tract and other systems that are occurring at younger ages. It is possible that the adoption and use of screening programs by a large part of the asymptomatic population contributed to this epidemiological change. The existing data also support the assumption that a number of environmental factors may play a primary role in influencing carcinogenesis from childhood. Changes that have appeared in the last decades related mainly to eating habits, consistency of gut microbiome and increase of obese people, interacting with genetic factors ultimately favor the process of carcinogenesis. Even these factors, however, are not sufficient to explain the age-related changes in the frequency of digestive neoplasms. Patients with early digestive tract cancer face unique challenges and unmet needs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Brisinda G, Italy; Majumdar APN, United States S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Zhang XD
| 1. | Gu WJ, Pei JP, Lyu J, Akimoto N, Haruki K, Ogino S, Zhang CD. The Burden of Early-Onset Colorectal Cancer and Its Risk Factors from 1990 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Cancers (Basel). 2022;14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Ugai T, Sasamoto N, Lee HY, Ando M, Song M, Tamimi RM, Kawachi I, Campbell PT, Giovannucci EL, Weiderpass E, Rebbeck TR, Ogino S. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat Rev Clin Oncol. 2022;19:656-673. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 109] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 3. | Zhao J, Xu L, Sun J, Song M, Wang L, Yuan S, Zhu Y, Wan Z, Larsson SC, Tsilidis K, Dunlop M, Campbell H, Rudan I, Song P, Theodoratou E, Ding K, Li X. Global trends in incidence, death, burden and risk factors of early-onset cancer from 1990 to 2019. BMJ Oncol. 2023;2:e000049. [DOI] [Cited in This Article: ] |
| 4. | Arafa MA, Farhat KH. Why cancer incidence in the Arab counties is much lower than other parts of the world? J Egypt Natl Canc Inst. 2022;34:41. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 5. | Lumish MA, Walch H, Maron SB, Chatila W, Kemel Y, Maio A, Ku GY, Ilson DH, Won E, Li J, Joshi SS, Gu P, Schattner MA, Laszkowska M, Gerdes H, Jones DR, Sihag S, Coit DG, Tang LH, Strong VE, Molena D, Stadler ZK, Schultz N, Janjigian YY, Cercek A. Clinical and Molecular Characteristics of Early-Onset versus Average-Onset Esophagogastric Cancer. J Natl Cancer Inst. 2023;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 6. | Radkiewicz C, Asplund J, Lagergren J. Incidence Trends and Survival in Early-Onset Esophagogastric Adenocarcinoma: A Swedish Population-Based Cohort Study. Cancer Epidemiol Biomarkers Prev. 2023;OF1-OF8. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 7. | Lai H, Zheng J, Zhou G, Li Y. Clinical characteristics and prognostic outcomes for adenocarcinoma of esophagogastric junction in early-onset patients: a population-based appraisal. J Cancer Res Clin Oncol. 2023;149:14941-14952. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 8. | Kolb JM, Han S, Scott FI, Murphy CC, Hosokawa P, Wani S; Early Onset Esophageal Adenocarcinoma Study Group. Early-Onset Esophageal Adenocarcinoma Presents With Advanced-Stage Disease But Has Improved Survival Compared With Older Individuals. Gastroenterology. 2020;159:2238-2240.e4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Buckle GC, Mmbaga EJ, Paciorek A, Akoko L, Deardorff K, Mgisha W, Mushi BP, Mwaiselage J, Hiatt RA, Zhang L, Van Loon K. Risk Factors Associated With Early-Onset Esophageal Cancer in Tanzania. JCO Glob Oncol. 2022;8:e2100256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Petrillo A, Federico P, Marte G, Liguori C, Seeber A, Ottaviano M, Tufo A, Daniele B. Non-hereditary early onset gastric cancer: An unmet medical need. Curr Opin Pharmacol. 2023;68:102344. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 11. | Ma Z, Liu X, Paul ME, Chen M, Zheng P, Chen H. Comparative investigation of early-onset gastric cancer. Oncol Lett. 2021;21:374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Kist M, Thomaschewski M, Keck Y, Abdalla TSA, Zeissig SR, Kleihues-van Tol K, Wellner UF, Keck T, Hoeppner J, Hummel R. Specifics of Young Gastric Cancer Patients: A Population-Based Analysis of 46,110 Patients with Gastric Cancer from the German Clinical Cancer Registry Group. Cancers (Basel). 2022;14. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 13. | Zhou QP, Ge YH, Liu CY. Comparison of metastasis between early-onset and late-onset gastric signet ring cell carcinoma. BMC Gastroenterol. 2020;20:380. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Bergquist JR, Leiting JL, Habermann EB, Cleary SP, Kendrick ML, Smoot RL, Nagorney DM, Truty MJ, Grotz TE. Early-onset gastric cancer is a distinct disease with worrisome trends and oncogenic features. Surgery. 2019;166:547-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Giryes A, Oweira H, Mannhart M, Decker M, Abdel-Rahman O. Exploring the differences between early-onset gastric cancer and traditional-onset gastric cancer. J Gastrointest Oncol. 2018;9:1157-1163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Hu S, Li Y, Zhu W, Liu J, Wei S. Global, region and national trends and age-period-cohort effects in colorectal cancer burden from 1990 to 2019, with predictions to 2039. Environ Sci Pollut Res Int. 2023;30:83245-83259. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 17. | Anugwom C, Braimoh G, Sultan A, Johnson WM, Debes JD, Mohammed A. Epidemiology and genetics of early onset colorectal cancer-African overview with a focus on Ethiopia. Semin Oncol. 2023;50:28-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 18. | Spaander MCW, Zauber AG, Syngal S, Blaser MJ, Sung JJ, You YN, Kuipers EJ. Young-onset colorectal cancer. Nat Rev Dis Primers. 2023;9:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 15] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 19. | Pardamean CI, Sudigyo D, Budiarto A, Mahesworo B, Hidayat AA, Baurley JW, Pardamean B. Changing Colorectal Cancer Trends in Asians: Epidemiology and Risk Factors. Oncol Rev. 2023;17:10576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 20. | Pan H, Zhao Z, Deng Y, Zheng Z, Huang Y, Huang S, Chi P. The global, regional, and national early-onset colorectal cancer burden and trends from 1990 to 2019: results from the Global Burden of Disease Study 2019. BMC Public Health. 2022;22:1896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 19] [Reference Citation Analysis (0)] |
| 21. | Saraiva MR, Rosa I, Claro I. Early-onset colorectal cancer: A review of current knowledge. World J Gastroenterol. 2023;29:1289-1303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 6] [Cited by in F6Publishing: 28] [Article Influence: 28.0] [Reference Citation Analysis (3)] |
| 22. | Siegel RL, Wagle NS, Cercek A, Smith RA, Jemal A. Colorectal cancer statistics, 2023. CA Cancer J Clin. 2023;73:233-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 359] [Reference Citation Analysis (1)] |
| 23. | Tom CM, Mankarious MM, Jeganathan NA, Deutsch M, Koltun WA, Berg AS, Scow JS. Characteristics and Outcomes of Right- Versus Left-Sided Early-Onset Colorectal Cancer. Dis Colon Rectum. 2023;66:498-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 6] [Reference Citation Analysis (0)] |
| 24. | Zaki TA, Liang PS, May FP, Murphy CC. Racial and Ethnic Disparities in Early-Onset Colorectal Cancer Survival. Clin Gastroenterol Hepatol. 2023;21:497-506.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 12] [Reference Citation Analysis (0)] |
| 25. | Petrick JL, Barber LE, Warren Andersen S, Florio AA, Palmer JR, Rosenberg L. Racial Disparities and Sex Differences in Early- and Late-Onset Colorectal Cancer Incidence, 2001-2018. Front Oncol. 2021;11:734998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Holowatyj AN, Maude AS, Musa HS, Adamu A, Ibrahim S, Abdullahi A, Manko M, Aminu SM, Mohammed A, Idoko J, Ukwenya Y, Carpten J, Chandler PD, Hampel H, Faruk M. Patterns of Early-Onset Colorectal Cancer Among Nigerians and African Americans. JCO Glob Oncol. 2020;6:1647-1655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Montminy EM, Zhou M, Maniscalco L, Penrose H, Yen T, Patel SG, Wu XC, Karlitz JJ. Trends in the Incidence of Early-Onset Colorectal Adenocarcinoma Among Black and White US Residents Aged 40 to 49 Years, 2000-2017. JAMA Netw Open. 2021;4:e2130433. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Li Q, Yu M, Lv H, Zhang L, Deng Y, Yu H. Burden of early-onset colorectal cancer along with attributable risk factors from 1990 to 2019: a comparative study between China and other G20 countries. BMC Public Health. 2023;23:1463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 29. | Ben-Aharon I, van Laarhoven HWM, Fontana E, Obermannova R, Nilsson M, Lordick F. Early-Onset Cancer in the Gastrointestinal Tract Is on the Rise-Evidence and Implications. Cancer Discov. 2023;13:538-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 22] [Reference Citation Analysis (0)] |
| 30. | Huang BZ, Liu L, Zhang J; USC Pancreas Research Team, Pandol SJ, Grossman SR, Setiawan VW. Rising Incidence and Racial Disparities of Early-Onset Pancreatic Cancer in the United States, 1995-2018. Gastroenterology. 2022;163:310-312.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Ren S, Sadula A, Ye C, Chen Q, Yuan M, Meng M, Lei J, Li G, Yuan C. Clinical characteristics, treatment patterns and survival outcomes of early-onset pancreatic adenocarcinoma: a population-based study. Am J Transl Res. 2023;15:407-421. [PubMed] [Cited in This Article: ] |
| 32. | Whitley A, Kocián P, Nikov A, Krejčí D, Pehalová L, Blaha M, Dušek L, Gürlich R. Early-onset pancreatic cancer: A national cancer registry study from the Czech Republic and review of the literature. J Hepatobiliary Pancreat Sci. 2023;30:1324-1333. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 33. | Yang Z, Liu C, Leng K, Liu L, Shi G. Early-onset pancreatic neuroendocrine neoplasms: A distinct disease with improved survival compared with old individuals. Front Endocrinol (Lausanne). 2023;14:1025485. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 34. | Rahman R, Ludvigsson JF, von Seth E, Lagergren J, Bergquist A, Radkiewicz C. Age trends in biliary tract cancer incidence by anatomical subtype: A Swedish cohort study. Eur J Cancer. 2022;175:291-298. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 35. | Li H, Chen X, Hoffmeister M, Brenner H. Associations of smoking with early- and late-onset colorectal cancer. JNCI Cancer Spectr. 2023;7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 36. | Li Q, Weitz J, Li C, Schardey J, Weiss L, Wirth U, Zimmermann P, Bazhin AV, Werner J, Kühn F. Smoking as a risk factor for colorectal neoplasms in young individuals? A systematic meta-analysis. Int J Colorectal Dis. 2023;38:114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 37. | Khoa Ta HD, Nguyen NN, Ho DKN, Nguyen HD, Ni YC, Yee KX, Pan SR, Nguyen HS, Thai Hoang Phuoc T, Chen MJ, Lee KH. Association of diabetes mellitus with early-onset colorectal cancer: A systematic review and meta-analysis of 19 studies including 10 million individuals and 30,000 events. Diabetes Metab Syndr. 2023;17:102828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 38. | Chen J, Zhang IL, Terry MB, Yang W. Dietary Factors and Early-Onset Colorectal Cancer in the United States-an Ecologic Analysis. Cancer Epidemiol Biomarkers Prev. 2023;32:217-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 39. | Kim Y, Chang Y, Cho Y, Chang J, Kim K, Park DI, Park SK, Joh HK, Kim MK, Kim C, Wild SH, Byrne CD, Ryu S. Serum 25-Hydroxyvitamin D Levels and Risk of Colorectal Cancer: An Age-Stratified Analysis. Gastroenterology. 2023;165:920-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 40. | Hua H, Jiang Q, Sun P, Xu X. Risk factors for early-onset colorectal cancer: systematic review and meta-analysis. Front Oncol. 2023;13:1132306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 41. | Juo YY, Gibbons MAM, Dutson E, Lin AY, Yanagawa J, Hines OJ, Eibl G, Chen Y. Obesity Is Associated with Early Onset of Gastrointestinal Cancers in California. J Obes. 2018;2018:7014073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Saeed U, Myklebust TÅ, Robsahm TE, Møller B, Mala T, Skålhegg BS, Yaqub S. Body mass index and pancreatic adenocarcinoma: A nationwide registry-based cohort study. Scand J Surg. 2023;112:11-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 43. | Zhang C, Zhang L, Tian Y, Guan B, Li S. Association between metabolic syndrome and early-stage colorectal cancer. BMC Cancer. 2023;23:1020. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 44. | Mima K, Hamada T, Inamura K, Baba H, Ugai T, Ogino S. The microbiome and rise of early-onset cancers: knowledge gaps and research opportunities. Gut Microbes. 2023;15:2269623. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 45. | Moon JY, Kye BH, Ko SH, Yoo RN. Sulfur Metabolism of the Gut Microbiome and Colorectal Cancer: The Threat to the Younger Generation. Nutrients. 2023;15. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 46. | Zheng B, Artin MG, Chung H, Chen B, Sun S, May BL, Hur C, Green PHR, Wang TC, Park J, Kong XF. Immunogenetics of gastrointestinal cancers: A systematic review and retrospective survey of inborn errors of immunity in humans. J Gastroenterol Hepatol. 2022;37:973-982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Jiang F, Boakye D, Sun J, Wang L, Yu L, Zhou X, Zhao J, Bian Z, Song P, He Y, Zhu Y, Chen J, Yuan S, Song M, Larsson SC, Giovannucci EL, Theodoratou E, Ding K, Li X. Association between antibiotic use during early life and early-onset colorectal cancer risk overall and according to polygenic risk and FUT2 genotypes. Int J Cancer. 2023;153:1602-1611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Marx O, Mankarious M, Yochum G. Molecular genetics of early-onset colorectal cancer. World J Biol Chem. 2023;14:13-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 3] [Cited by in F6Publishing: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 49. | Ugai T, Haruki K, Harrison TA, Cao Y, Qu C, Chan AT, Campbell PT, Akimoto N, Berndt S, Brenner H, Buchanan DD, Chang-Claude J, Fujiyoshi K, Gallinger SJ, Gunter MJ, Hidaka A, Hoffmeister M, Hsu L, Jenkins MA, Milne RL, Moreno V, Newcomb PA, Nishihara R, Pai RK, Sakoda LC, Slattery ML, Sun W, Amitay EL, Alwers E, Thibodeau SN, Toland AE, Van Guelpen B, Woods MO, Zaidi SH, Potter JD, Giannakis M, Song M, Nowak JA, Phipps AI, Peters U, Ogino S. Molecular Characteristics of Early-Onset Colorectal Cancer According to Detailed Anatomical Locations: Comparison With Later-Onset Cases. Am J Gastroenterol. 2023;118:712-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Housini M, Dariya B, Ahmed N, Stevens A, Fiadjoe H, Nagaraju GP, Basha R. Colorectal cancer: Genetic alterations, novel biomarkers, current therapeutic strategies and clinical trials. Gene. 2024;892:147857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 51. | Li Y, Zhang X. Pancreatic cancer in young adults - an evolving entity? Am J Cancer Res. 2023;13:2763-2772. [PubMed] [Cited in This Article: ] |
| 52. | Ulanja MB, Moody AE, Beutler BD, Antwi-Amoabeng D, Rahman GA, Alese OB. Early-onset pancreatic cancer: a review of molecular mechanisms, management, and survival. Oncotarget. 2022;13:828-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 53. | Varghese AM, Singh I, Singh R, Kunte S, Chou JF, Capanu M, Wong W, Lowery MA, Stadler ZK, Salo-Mullen E, Saadat LV, Wei AC, Reyngold M, Basturk O, Benayed R, Mandelker D, Iacobuzio-Donahue CA, Kelsen DP, Park W, Yu KH, O'Reilly EM. Early-Onset Pancreas Cancer: Clinical Descriptors, Genomics, and Outcomes. J Natl Cancer Inst. 2021;113:1194-1202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 54. | Nodari Y, Gentiluomo M, Mohelnikova-Duchonova B, Kreivenaite E, Milanetto AC, Skieceviciene J, Landi S, Lawlor RT, Petrone MC, Arcidiacono PG, Lovecek M, Gazouli M, Bijlsma MF, Morelli L, Kiudelis V, Tacelli M, Zanette DL, Soucek P, Uzunoglu F, Kaaks R, Izbicki J, Boggi U, Pezzilli R, Mambrini A, Pasquali C, van Laarhoven HW, Katzke V, Cavestro GM, Sperti C, Loos M, Latiano A, Erőss B, Oliverius M, Johnson T, Basso D, Neoptolemos JP, Aoki MN, Greenhalf W, Vodicka P, Archibugi L, Vanella G, Lucchesi M, Talar-Wojnarowska R, Jamroziak K, Saeedi MA, van Eijck CHJ, Kupcinskas J, Hussein T, Puzzono M, Bunduc S, Götz M, Carrara S, Szentesi A, Tavano F, Moz S, Hegyi P, Luchini C, Capurso G, Perri F, Ermini S, Theodoropoulos G, Capretti G, Palmieri O, Ginocchi L, Furbetta N, Canzian F, Campa D. Genetic and non-genetic risk factors for early-onset pancreatic cancer. Dig Liver Dis. 2023;55:1417-1425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 55. | Innamorati G, Wilkie TM, Malpeli G, Paiella S, Grasso S, Rusev B, Leone BE, Valenti MT, Carbonare LD, Cheri S, Giacomazzi A, Zanotto M, Guardini V, Deiana M, Zipeto D, Serena M, Parenti M, Guzzi F, Lawlor RT, Malerba G, Mori A, Malleo G, Giacomello L, Salvia R, Bassi C. Gα15 in early onset of pancreatic ductal adenocarcinoma. Sci Rep. 2021;11:14922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 56. | Kryklyva V, Brosens LA, Marijnissen-van Zanten MA, Ligtenberg MJ, Nagtegaal ID. Mismatch repair deficiency in early-onset duodenal, ampullary, and pancreatic carcinomas is a strong indicator for a hereditary defect. J Pathol Clin Res. 2022;8:181-190. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 57. | Castet F, Fabregat-Franco C, Castillo G, Navarro V, Sierra A, Acosta DA, López-Valbuena D, Dienstmann R, Tabernero J, Vivancos A, Tian TV, Macarulla T. Clinical and genomic characterisation of early-onset pancreatic cancer. Eur J Cancer. 2023;194:113338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 58. | AlZaabi A. Colorectal Cancer in the Arab World. In: Al-Shamsi, H.O., Abu-Gheida, I.H., Iqbal, F., Al-Awadhi, A. (eds) Cancer in the Arab World. Springer, Singapore. [DOI] [Cited in This Article: ] |
| 59. | Brown R, Kerr K, Haoudi A, Darzi A. Tackling cancer burden in the Middle East: Qatar as an example. Lancet Oncol. 2012;13:e501-e508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Valle L, de Voer RM, Goldberg Y, Sjursen W, Försti A, Ruiz-Ponte C, Caldés T, Garré P, Olsen MF, Nordling M, Castellvi-Bel S, Hemminki K. Update on genetic predisposition to colorectal cancer and polyposis. Mol Aspects Med. 2019;69:10-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 61. | Ford AC, Yuan Y, Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: systematic review and meta-analysis. Gut. 2020;69:2113-2121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 197] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 62. | Hamashima C, Ogoshi K, Okamoto M, Shabana M, Kishimoto T, Fukao A. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS One. 2013;8:e79088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 63. | Shigeyasu K, Toden S, Zumwalt TJ, Okugawa Y, Goel A. Emerging Role of MicroRNAs as Liquid Biopsy Biomarkers in Gastrointestinal Cancers. Clin Cancer Res. 2017;23:2391-2399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 64. | Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M, Matsui T. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 370] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 65. | Conti CB, Agnesi S, Scaravaglio M, Masseria P, Dinelli ME, Oldani M, Uggeri F. Early Gastric Cancer: Update on Prevention, Diagnosis and Treatment. Int J Environ Res Public Health. 2023;20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 66. | Choi J, Kim SG, Yoon H, Im JP, Kim JS, Kim WH, Jung HC. Eradication of Helicobacter pylori after endoscopic resection of gastric tumors does not reduce incidence of metachronous gastric carcinoma. Clin Gastroenterol Hepatol. 2014;12:793-800.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 67. | Yeh JH, Tseng CH, Wang WL, Chen CI, Liu YP, Lee YC, Wang JY, Lin YC. Performance of the Fecal Immunochemical Test in Detecting Advanced Colorectal Neoplasms and Colorectal Cancers in People Aged 40-49 Years: A Systematic Review and Meta-Analysis. Cancers (Basel). 2023;15. [PubMed] [DOI] [Cited in This Article: ] [Reference Citation Analysis (0)] |
| 68. | Akimoto N, Ugai T, Zhong R, Hamada T, Fujiyoshi K, Giannakis M, Wu K, Cao Y, Ng K, Ogino S. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol. 2021;18:230-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 241] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 69. | Kosumi K, Mima K, Baba H, Ogino S. Dysbiosis of the gut microbiota and colorectal cancer: the key target of molecular pathological epidemiology. J Lab Precis Med. 2018;3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (1)] |
| 70. | Hogan NM, Hanley M, Hogan AM, Sheehan M, McAnena OJ, Regan MP, Kerin MJ, Joyce MR. Awareness and uptake of family screening in patients diagnosed with colorectal cancer at a young age. Gastroenterol Res Pract. 2015;2015:194931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Kienesberger S, Cox LM, Livanos A, Zhang XS, Chung J, Perez-Perez GI, Gorkiewicz G, Zechner EL, Blaser MJ. Gastric Helicobacter pylori Infection Affects Local and Distant Microbial Populations and Host Responses. Cell Rep. 2016;14:1395-1407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 106] [Article Influence: 13.3] [Reference Citation Analysis (1)] |