Published online Feb 15, 2024. doi: 10.4251/wjgo.v16.i2.557
Peer-review started: October 25, 2023
First decision: November 30, 2023
Revised: December 13, 2023
Accepted: January 11, 2024
Article in press: January 11, 2024
Published online: February 15, 2024
Hyperbilirubinemia with hepatic metastases is a common complication and a poor prognostic factor for colorectal cancer (CRC). Effective drainage is often im
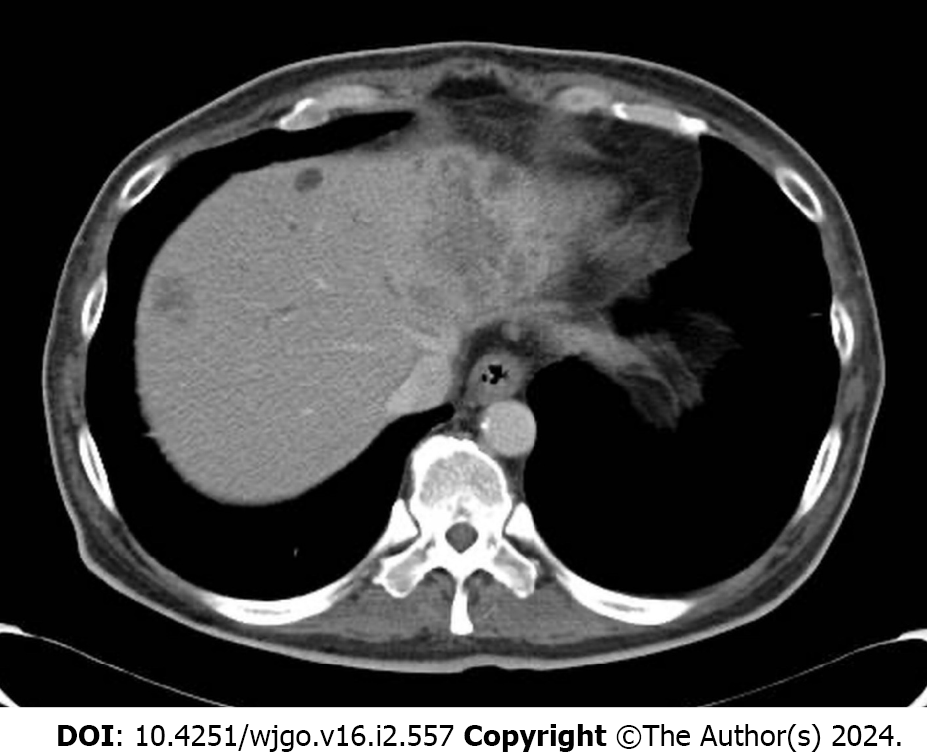
The patient, a man in his 50s, presented with progressive fatigue and severe jaundice. Computed tomography revealed multiple hepatic masses with thick
Anti-EGFR antibody monotherapy is a safe and effective treatment for RAS wild-type CRC and hepatic metastases with severe hyperbilirubinemia.
Core Tip: We report the case of a patient with colorectal cancer and severe hyperbilirubinemia who was successfully treated with anti-EGFR antibody monotherapy. As this approach is safe and potentially lifesaving, it should be considered as a treatment option for hyperbilirubinemia due to hepatic metastases when biliary drainage is impossible.
- Citation: Tsurui T, Hirasawa Y, Kubota Y, Yoshimura K, Tsunoda T. Anti-EGFR antibody monotherapy for colorectal cancer with severe hyperbilirubinemia: A case report. World J Gastrointest Oncol 2024; 16(2): 557-562
- URL: https://www.wjgnet.com/1948-5204/full/v16/i2/557.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i2.557
Metastatic colorectal cancer (CRC) is the leading cause of cancer-related deaths worldwide[1]. The liver is the most common site of metastasis in CRC owing to the draining system of the portal vein. Hyperbilirubinemia is a serious consequence of hepatic metastasis and is a poor prognostic factor[2]. Biliary drainage is often difficult because of diffuse hepatic involvement. Clinical decision-making in these settings is challenging, because chemotherapeutic agents that are metabolized in the liver require dose adjustments and are contraindicated in severe hepatic failure. As more major clinical trials exclude the patients with severely impaired organ function, little evidence supports an optimal regimen and dose of chemotherapy for severe hyperbilirubinemia. Although the use of cytotoxic chemotherapy in patients with hepatic metastases and severe hyperbilirubinemia has been reported in a few case series, most cases did not achieve satisfactory outcomes and severe treatment-related adverse events were also reported. Therefore, no appropriate chemotherapeutic approach has yet been established for these patients. Herein, we report a case of CRC with hepatic metastases and severe hyperbilirubinemia that was successfully treated using panitumumab monotherapy. We suggest this approach as a novel treatment option for hyperbilirubinemia caused by hepatic metastases when biliary drainage is impossible.
A patient in his 50s experienced weight loss and progressive fatigue.
Laboratory tests revealed elevated transaminase and bilirubin levels, prompting a prompt referral to our hospital.
He had a history of type 2 diabetes mellitus, hypertension, and dyslipidemia.
The patient denied any family history of malignant tumors.
The patient was afebrile on admission. The Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 2. Physical examination revealed scleral icterus and yellow skin. The patient’s abdomen was flat and non-tender, with no palpable masses.
The results of laboratory blood tests revealed the following: Serum total bilirubin level: 8.4 mg/dL, conjugated bilirubin level: 6.2 mg/dL, aspartate aminotransferase: 141 U/L, alanine aminotransferase: 240 U/L, lactate dehydrogenase: 372 U/L, alkaline phosphatase: 447 U/L, gamma glutamyl transferase: 959 U/L, albumin: 3.3 g/dL, and prothrombin time: 99%. Carcinoembryonic antigen (CEA) was markedly elevated at 396 ng/mL.
A computed tomography (CT) scan demonstrated multiple hepatic masses with a thickened wall at the sigmoid colon (Figure 1).
Metastatic CRC was diagnosed, and obstructive jaundice with liver metastasis was suspected. Biomarker expression was as follows: RAS wild-type, BRAF wild-type, and microsatellite stable.
Endoscopic or percutaneous biliary drainage failed, owing to the diffuse involvement of the liver and the absence of a bile duct dilated enough to drain. These findings indicated the need for anticancer therapies. Consequently, panitumumab monotherapy (6 mg/kg, every two weeks) was initiated.
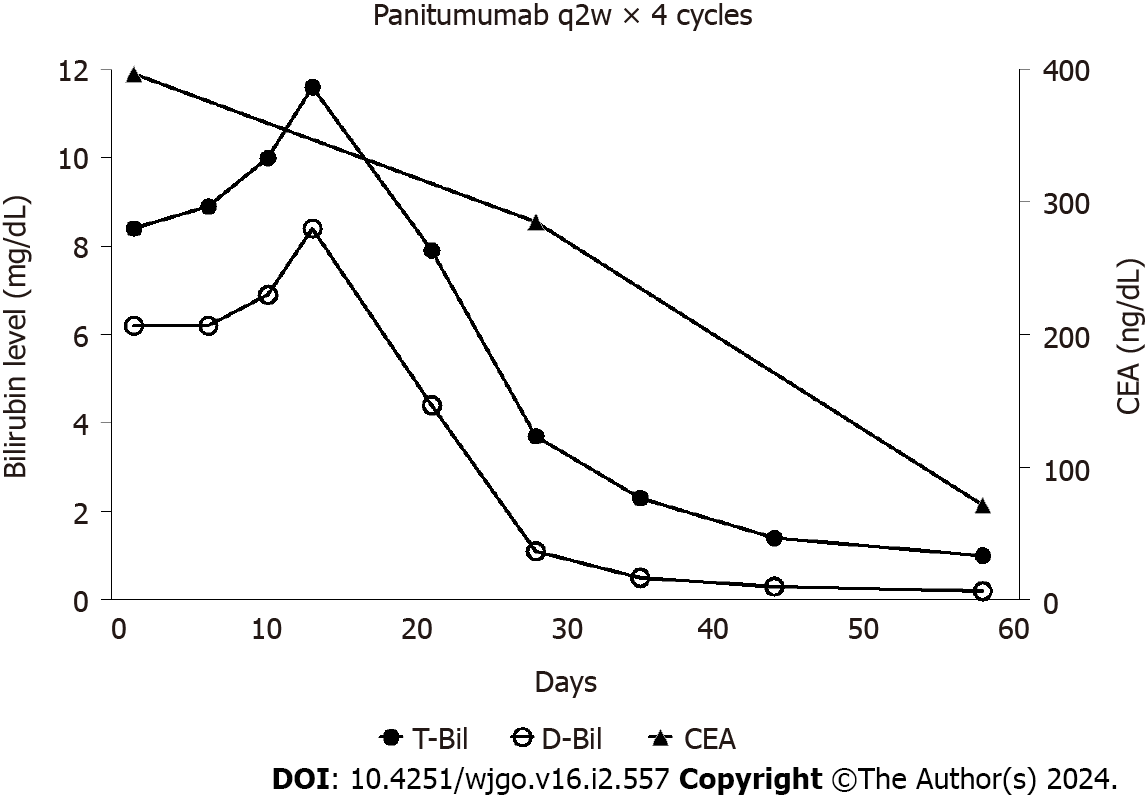
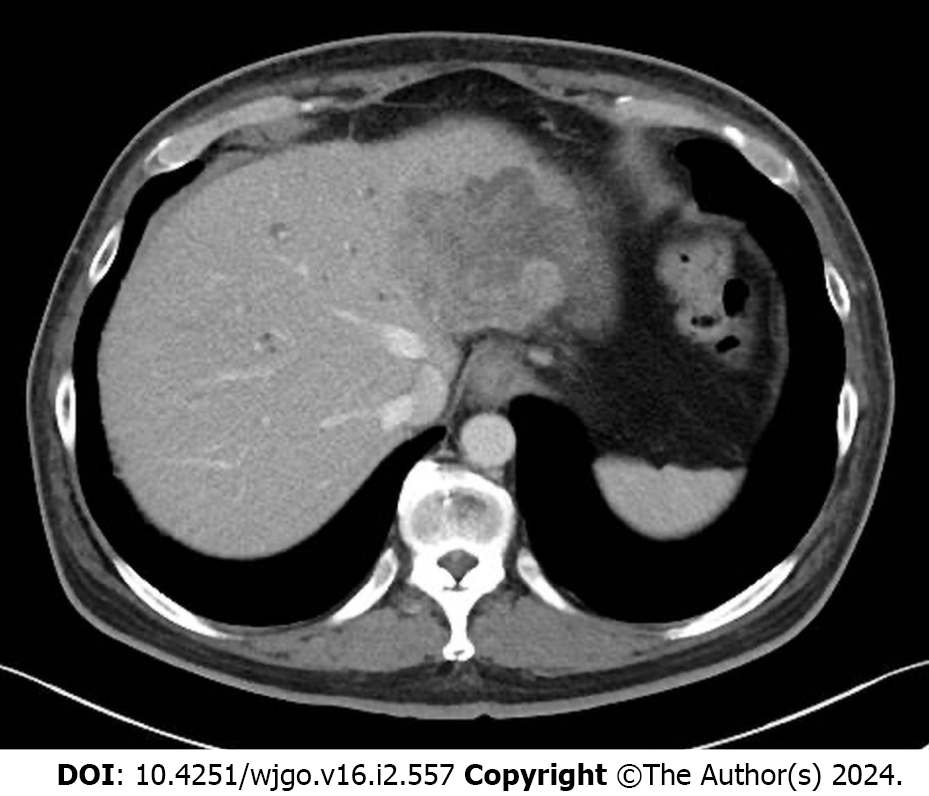
After two cycles of panitumumab monotherapy, the bilirubin level decreased markedly and completely normalized after four cycles (Figure 2). The patient’s ECOG PS improved to 0 and CEA levels decreased to 71 ng/mL. A CT tomography revealed shrinking hepatic masses (Figure 3). Therefore, fluorouracil plus leucovorin and oxaliplatin (a modified FOLFOX6 regimen) were added to panitumumab therapy. The patient received 22 cycles of this regimen over a year until the disease progressed. Thereafter, the patient declined further chemotherapy and received palliative care at a hospice.
Anti-EGFR antibody monotherapy was found to be a safe and effective treatment approach for patients with RAS wild-type CRC and hepatic metastases with severe hyperbilirubinemia.
In general, selecting a chemotherapy regimen for hyperbilirubinemia is particularly challenging. This is because hepatic dysfunction affects the pharmacokinetics of chemotherapeutic agents and may worsen their toxicity. Cytotoxic agents against CRC include fluoropyrimidines such as oxaliplatin and irinotecan. Fluoropyrimidines are mostly eliminated by hepatic metabolism, and some experts have suggested that they are contraindicated in patients with severe hepatic dysfunction[3]. Platinum derivatives are predominantly excreted through the renal tract. Although a dose-escalating pharmacological study showed that oxaliplatin can be administered without dose reduction in patients with severe liver dysfunction, several cases of oxaliplatin-induced hepatotoxicity have been reported[4,5]. Activation of irinotecan and detoxification of its metabolite, SN-38, occur predominantly in the liver; moreover, the toxicity of this drug may be increased in patients with reduced biliary excretion[5,6].
Recently, several studies have reported the use of cytotoxic chemotherapy in patients with hepatic metastases and severe hyperbilirubinemia[7-10]. Some cases showed reasonable and durable responses; however, most had an average OS of only several months, and some experienced severe treatment-related adverse events. In addition, cytotoxic chemotherapy often requires dose reduction. This is a considerable concern, because intensive chemotherapy is required to achieve a sufficient response to alleviate hyperbilirubinemia[5,8]. Furthermore, the PS of patients with severe hyperbilirubinemia is often poor at the time of diagnosis and progressively worsens, making them more vulnerable to chemotherapy toxicity.
By contrast, mAbs are mostly eliminated via intracellular catabolism, and are relatively unaffected by hepatic or renal functions[11]. Anti-EGFR antibodies, such as panitumumab and cetuximab, are recommended as first-line treatments for patients with RAS/BRAF wild-type and left-sided CRCs[12,13]. Although panitumumab is usually administered in combination with cytotoxic chemotherapy, monotherapy with this antibody has shown a reasonable response rate. Even in patients with refractory disease, the response rate reached 30% in those with RAS wild-type CRC[14]. Recently, the efficacy and safety of panitumumab monotherapy in frail or elderly patients who are considered unsuitable for intensive chemotherapy has been reported. One of these reports showed a considerably high response rate (up to 65%) to RAS wild-type left-sided CRC as initial therapy[15]. Several studies have supported the use of anti-EGFR monoclonal antibodies in patients with severe hyperbilirubinemia[5,16] (Table 1).
| Age (yr), gender | ECOG PS | Primary site | KRAS | Previous treatment | Total bilirubin (mg/dL) | Treatment | Overall survival (month) |
| 63, M[16] | 2 | Unknown | WT | FOLFIRI, FOLFOX | 2.5 | Cetuximab | 1.3 |
| 66, M[16] | 2 | Unknown | WT | IRIS, FOLFOX + Bevacizumab | 2.3 | Cetuximab | 3.1 |
| 65, M[16] | 2 | Unknown | WT | FOLFIRI, FOLFOX | 2.6 | Cetuximab | 5.9 |
| 45, F[16] | 3 | Unknown | WT | FOLFOX, FOLFIRI + Bevacizumab | 7.9 | Cetuximab | 2.8 |
| 56, F[16] | 1 | Unknown | WT | FOLFOX, FOLFIRI + Bevacizumab | 13 | Cetuximab | 2.6 |
| 69, M[16] | 3 | Unknown | WT | Irinotecan | 7.4 | Cetuximab | 1.2 |
| 78, M[16] | 2 | Unknown | MT | FOLFOX + Bevacizumab, FOLFIRI | 9.7 | Cetuximab | 0.5 |
| 43, M[9] | 3 | Right | WT | No | 9.4 | FOLFOX + Panitumuab | 1.5 |
| 48, M[17] | Unknown | Left | WT | No | 6.2 | FOLFOX + Cetuximab | ≥ 7 |
However, the role of anti-EGFR antibodies in patients with right-sided CRCs remains unclear. While patients with right-sided CRC do not benefit as much from anti-EGFR antibodies as those with left-sided CRC in the setting of first-line treatment, the use of anti-EGFR antibodies as a later treatment is a widely accepted approach[12]. Thus, our approach to initial anti-EGFR monotherapy in the setting of severe hyperbilirubinemia requires careful consideration of right-sided CRC owing to its possible lower response rate[15].
Further studies should be conducted to validate this approach on both sides of the CRC.
This case demonstrates the safety and efficacy of anti-EGFR antibody monotherapy in a patient with CRC and hepatic metastases with severe hyperbilirubinemia. Anti-EGFR antibody monotherapy should be considered a treatment option for patients with RAS wild-type CRC with hyperbilirubinemia due to hepatic metastases when biliary drainage is impossible.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang GX, China; Wu HT, China S-Editor: Lin C L-Editor: A P-Editor: Yuan YY
| 1. | GBD 2019 Colorectal Cancer Collaborators. Global, regional, and national burden of colorectal cancer and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:627-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 157] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 2. | Yang L, Ge LY, Yu T, Liang Y, Yin Y, Chen H. The prognostic impact of serum bilirubin in stage IV colorectal cancer patients. J Clin Lab Anal. 2018;32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Floyd J, Mirza I, Sachs B, Perry MC. Hepatotoxicity of chemotherapy. Semin Oncol. 2006;33:50-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 4. | Synold TW, Takimoto CH, Doroshow JH, Gandara D, Mani S, Remick SC, Mulkerin DL, Hamilton A, Sharma S, Ramanathan RK, Lenz HJ, Graham M, Longmate J, Kaufman BM, Ivy P; National Cancer Institute Organ Dysfunction Working Group. Dose-escalating and pharmacologic study of oxaliplatin in adult cancer patients with impaired hepatic function: a National Cancer Institute Organ Dysfunction Working Group study. Clin Cancer Res. 2007;13:3660-3666. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Elsoueidi R, Craig J, Mourad H, Richa EM. Safety and efficacy of FOLFOX followed by cetuximab for metastatic colorectal cancer with severe liver dysfunction. J Natl Compr Canc Netw. 2014;12:155-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Raymond E, Boige V, Faivre S, Sanderink GJ, Rixe O, Vernillet L, Jacques C, Gatineau M, Ducreux M, Armand JP. Dosage adjustment and pharmacokinetic profile of irinotecan in cancer patients with hepatic dysfunction. J Clin Oncol. 2002;20:4303-4312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Walia T, Quevedo JF, Hobday TJ, Croghan G, Jatoi A. Colorectal cancer patients with liver metastases and severe hyperbilirubinemia: A consecutive series that explores the benefits and risks of chemotherapy. Ther Clin Risk Manag. 2008;4:1363-1366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Quidde J, Azémar M, Bokemeyer C, Arnold D, Stein A. Treatment approach in patients with hyperbilirubinemia secondary to liver metastases in gastrointestinal malignancies: a case series and review of literature. Ther Adv Med Oncol. 2016;8:144-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Kasi PM, Thanarajasingam G, Finnes HD, Villasboas Bisneto JC, Hubbard JM, Grothey A. Chemotherapy in the Setting of Severe Liver Dysfunction in Patients with Metastatic Colorectal Cancer. Case Rep Oncol Med. 2015;2015:420159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Yeh YS, Huang ML, Chang SF, Chen CF, Hu HM, Wang JY. FOLFIRI combined with bevacizumab as first-line treatment for metastatic colorectal cancer patients with hyperbilirubinemia after UGT1A1 genotyping. Med Princ Pract. 2014;23:478-481. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 715] [Cited by in F6Publishing: 711] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 12. | Holch JW, Ricard I, Stintzing S, Modest DP, Heinemann V. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur J Cancer. 2017;70:87-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 317] [Cited by in F6Publishing: 367] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 13. | Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, Heinemann V, Van Cutsem E, Pignon JP, Tabernero J, Cervantes A, Ciardiello F. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28:1713-1729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 477] [Cited by in F6Publishing: 562] [Article Influence: 93.7] [Reference Citation Analysis (0)] |
| 14. | Kim TW, Elme A, Kusic Z, Park JO, Udrea AA, Kim SY, Ahn JB, Valencia RV, Krishnan S, Bilic A, Manojlovic N, Dong J, Guan X, Lofton-Day C, Jung AS, Vrdoljak E. A phase 3 trial evaluating panitumumab plus best supportive care vs best supportive care in chemorefractory wild-type KRAS or RAS metastatic colorectal cancer. Br J Cancer. 2016;115:1206-1214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Terazawa T, Kato T, Goto M, Ohta K, Noura S, Satake H, Kagawa Y, Kawakami H, Hasegawa H, Yanagihara K, Shingai T, Nakata K, Kotaka M, Hiraki M, Konishi K, Nakae S, Sakai D, Kurokawa Y, Shimokawa T, Satoh T. Phase II Study of Panitumumab Monotherapy in Chemotherapy-Naïve Frail or Elderly Patients with Unresectable RAS Wild-Type Colorectal Cancer: OGSG 1602. Oncologist. 2021;26:17-e47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Shitara K, Takahari D, Yokota T, Shibata T, Ura T, Muro K, Inaba Y, Yamaura H, Sato Y, Najima M, Utsunomiya S. Case series of cetuximab monotherapy for patients with pre-treated colorectal cancer complicated with hyperbilirubinemia due to severe liver metastasis. Jpn J Clin Oncol. 2010;40:275-277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Utsunomiya S, Okumura A, Watanabe K, Kunii S, Ishikawa D, Hirosaki T, Yamada K, Kaga A. A case of liver metastases with hyperbilirubinemia that was safely treated with chemotherapy (FOLFOX plus Cetuximab). Ann Oncol. 2017;28 Suppl 9:ix105. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |