Published online Sep 15, 2023. doi: 10.4251/wjgo.v15.i9.1626
Peer-review started: April 26, 2023
First decision: June 7, 2023
Revised: June 17, 2023
Accepted: July 29, 2023
Article in press: July 29, 2023
Published online: September 15, 2023
The hemoglobin, albumin, lymphocyte, and platelet (HALP) score, derived from a composite evaluation of markers reflecting the tumor-inflammation relationship and nutritional status, has been substantiated as a noteworthy prognostic determinant for diverse malignancies.
To investigate how the HALP score relates to prognosis in patients with metastatic gastric cancer.
The cutoff values for the HALP score, neutrophil/lymphocyte ratio, and pla
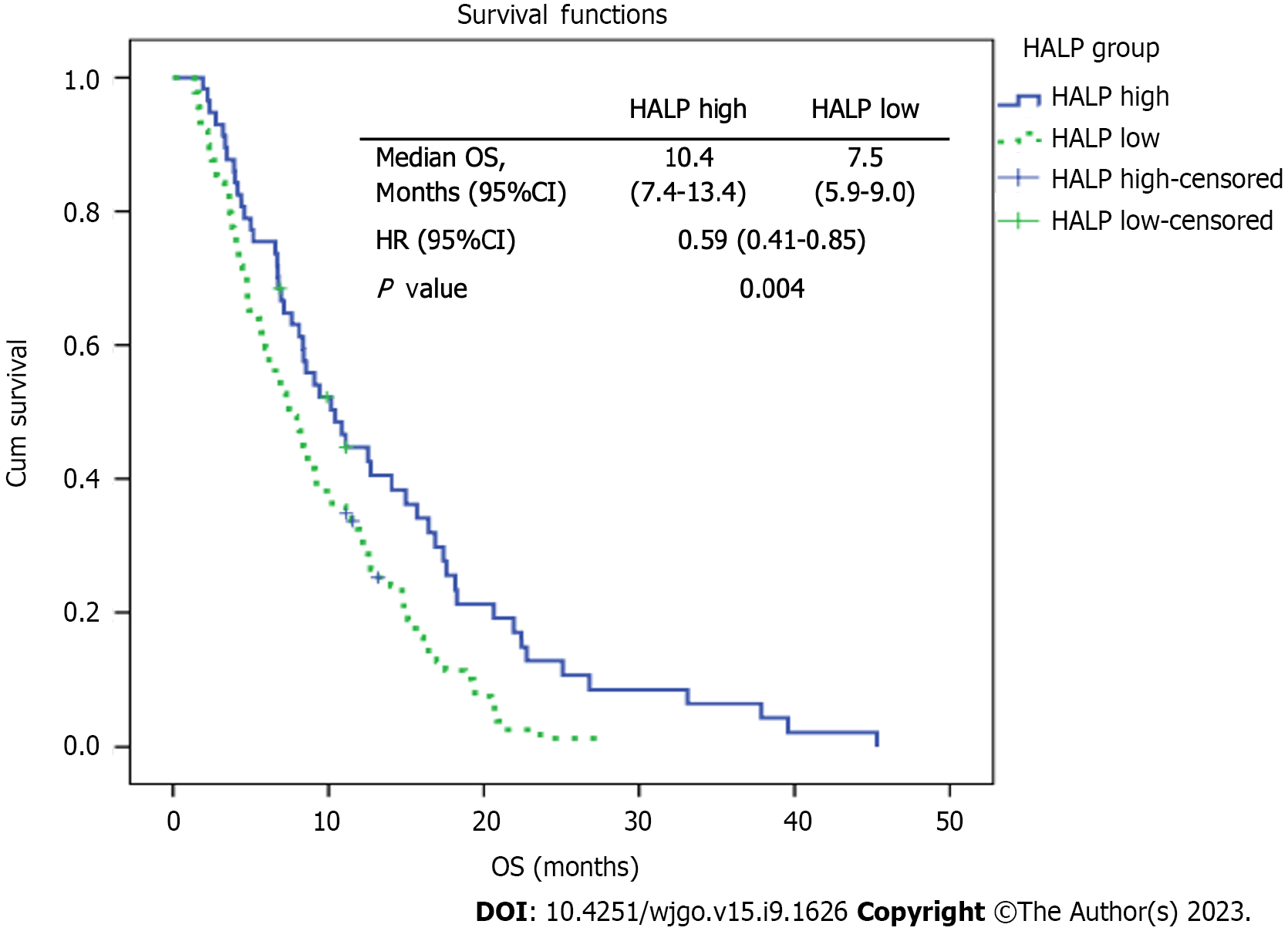
The study cohort comprised 147 patients and 110 of them (74.8%) were male. The patients' median age was 63 (22-89) years. The median overall survival was significantly superior in the patients with high HALP scores than in those with low HALP scores (10.4 mo vs 7.5 mo, respectively; P < 0.001)
The HALP score was found to be a prognostic factor in patients with metastatic gastric cancer.
Core Tip: Median overall survival (OS) was 10.4 mo in the high hemoglobin, albumin, lymphocyte, and platelet (HALP) group and 7.5 mo in the low HALP group. There was a statistically significant difference between the groups in terms of age (P < 0.001), second-line chemotherapy (P < 0.001), sex (P = 0.035), and HALP score (P = 0.004). The HALP score has been demonstrated to be useful as a prognostic factor in a variety of cancer types, including genitourinary and gastrointestinal malignancies. Our study is the first to investigate the HALP score in patients with metastatic gastric cancer. We found that patients with high HALP scores had longer OS. Given its simplicity and low cost, we think the HALP score can be utilized to manage patients with gastric cancer.
- Citation: Duzkopru Y, Kocanoglu A, Dogan O, Sahinli H, Cilbir E, Altinbas M. Hemoglobin, albumin, lymphocyte, and platelet score as a predictor of prognosis in metastatic gastric cancer. World J Gastrointest Oncol 2023; 15(9): 1626-1635
- URL: https://www.wjgnet.com/1948-5204/full/v15/i9/1626.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i9.1626
In Western countries, there has been a gradual decline in the prevalence of gastric cancer[1]. However, it remains a significant public health concern in certain regions of Eastern Asia[2]. Globally, gastric cancer ranks third in terms of cancer-related mortality and fifth in terms of overall prevalence[3]. Adenocarcinomas account for over 95% of all diagnosed cases of gastric cancer[4].
It is well known that gastric cancer has a poor prognosis. This is due to the disease usually being diagnosed at an advanced stage[5]. The most important factors in predicting the disease's prognosis are the stage of the tumor node metastasis (TNM), lymph node invasion, and the presence of distant metastases[6]. However, even among patients classified within the same stage, survival rates can significantly vary. Consequently, there is a pressing need for novel biomarkers to assist clinicians in accurately anticipating prognosis and making informed treatment decisions.
Numerous studies have demonstrated a significant association between systemic inflammation and the proliferation, invasion, and metastasis of the cancer[7]. At the same time, this inflammatory response around the tumor affects the formation and growth of the tumor[8]. Furthermore, blood cells trigger an adaptive immune response through the release of diverse cytokines, exerting an impact on tumor cells[9]. Based on the tumor inflammation relationship, biomarkers such as the neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), lymphocyte/monocyte ratio, and prognostic nutritional index are utilized to predict disease prognosis[10]. Combining these parameters to generate scores is thought to enhance the predictive value of prognosis compared to using individual biomarkers alone. The integration of multiple biomarkers enables a more comprehensive assessment and potentially provides a more accurate prediction of disease outcomes. One of these combinations is the hemoglobin, albumin, lymphocyte, and platelet (HALP) score, calculated using the HALP counts. Together, the immune system and nutritional status are assessed by the HALP score. By evaluating both the immune system and nutritional status, the HALP score has shown utility as a prognostic factor in various cancer types, including genitourinary and gastrointestinal malignancies[11,12]. In the context of gastric cancer, some retrospective studies have demonstrated the predictive value of the HALP score in the preoperative stage, providing foresights into the disease prognosis prior to surgical intervention[13,14].
A scoring system incorporating clinical and laboratory data may be useful in determining the prognosis of gastric cancer. In the present study, we aimed to investigate the prognostic effect of the HALP score in patients with metastatic gastric cancer.
A total of 158 patients were initially screened and, among them, 147 patients who met the inclusion criteria were included in the study. The data of patients diagnosed with metastatic gastric adenocarcinoma and followed up in the Medical Oncology Clinic of Diskapi Yildirim Beyazit Training and Research Hospital, Health Sciences University (Ankara, Turkey), between January 2010 and May 2021 were analyzed retrospectively. The inclusion criteria encompassed patients aged 18 years and older. However, those with heart failure, undergoing dialysis, having secondary malignancy, or suffering from any inflammatory disease were excluded.
Data for the study were obtained by collecting information from hospital records and patient files. Various variables were recorded and analyzed, including details regarding chemotherapy regimens, comorbidities, smoking and alcohol consumption histories, surgical procedures, pathological diagnoses, types of lymph node dissection, tumor sizes, metastasis sites, and patient survival durations. Overall survival (OS) was calculated as the time from the date of metastasis to the date of death or the last follow-up date. Also the HALP score was calculated using laboratory values at the time of metastasis. It was calculated by multiplying the hemoglobin albumin and lymphocyte/platelet ratio [hemoglobin (g/L) × albumin (g/L) × lymphocyte count/thrombocyte count][15].
Approval for the study was granted by the Diskapi Yıldırım Beyazıt Training and Research Hospital ethics committee (number: 116/21, date: 26.07.2021). The protocol of the study was prepared in accordance with the 1964 Declaration of Helsinki.
The statistical analyses were performed using IBM SPSS Statistics (version 22.0, IBM SPSS, United States). The clinical and demographic characteristics of the patients were subjected to descriptive analysis. Categorical and numerical variables were presented as numbers and percentages (n, %). Continuous data were expressed as means ± SD when the data were normally distributed; otherwise, they were expressed as median and range. Receiver operating characteristic (ROC) curve analysis was used to determine the optimal cutoff values of NLR, PLR, and HALP score. Survival outcomes were compared using the Kaplan–Meier method with the log-rank test (univariate analysis) or the Cox proportional hazards regression model (multivariate analysis). Only the parameters that demonstrated statistical significance in the univariate analysis were included in the multivariate analysis. A P value < 0.05 was considered statistically significant in all analyses. The statistical methods used were reviewed by Yakup Duzkopru from Ankara Etlik City Hospital.
A total of 147 patients diagnosed with metastatic gastric cancer were included. The majority of the patients (74.8%) were male. The median age of the patients was 63 (22-89). Among the participants, 74 patients (50.3%) had no additional diseases, while 78 patients (53.1%) had a history of smoking. A total of 90 patients (61.2%) had never undergone any surgical procedures.
Surgical interventions were performed in a subset of the patients. Specifically, total gastrectomy was conducted in 41 patients (27.9%), while subtotal gastrectomy was performed in 16 (10.9%). The histopathological examination revealed adenocarcinoma in 128 patients (87.1%). Among these patients, 74 (50.3%) were classified as having moderately differentiated adenocarcinoma. For the majority of the patients (38.1%), the primary tumor was in the corpus. The number of de novo metastatic patients was 103 (70.1%). Table 1 provides an overview of the clinicopathological characteristics of the patients in the study.
| Features | Frequency, n (%) |
| Gender | |
| Female | 37 (25.2) |
| Male | 110 (74.8) |
| ECOG PS | |
| 0 | 32 (21.8) |
| 1 | 83 (56.5) |
| 2 | 32 (21.8) |
| Comorbidity | |
| No | 74 (50.3) |
| Yes | 73 (49.7) |
| Smoking | |
| No | 69 (46.9) |
| Yes | 78 (53.1) |
| Surgery | |
| No | 90 (61.2) |
| Yes | 57 (38.8) |
| Type of surgery | |
| No | 90 (61.2) |
| Total gastrectomy | 41 (27.9) |
| Subtotal gstrectomy | 16 (10.9) |
| Patology | |
| Adenocarcinom | 128 (87.1) |
| Signet ring cell carcinom | 15 (10.2) |
| Musinoz adenocarcinom | 2 (1.4) |
| Mix carcinom | 2 (1.4) |
| Diferantiation | |
| Well | 5 (3.4) |
| Moderate | 74 (50.3) |
| Poorly | 53 (36.1) |
| Signet ring cell carcinoma | 15 (10.2) |
| Surgical margin | |
| No operation | 90 (61.2) |
| Positive | 6 (4.1) |
| Negative | 51 (34.7) |
| Tumor location | |
| Fundus, cardia | 48 (32.7) |
| Korpus | 56 (38.1) |
| Antrum, pylor | 43 (29.3) |
| De novo metastasis | |
| No | 44 (29.9) |
| Yes | 103 (70.1) |
| Age group | |
| ≤ 63 | 74 (50.3) |
| > 63 | 73 (49.7) |
Using ROC analysis, a cutoff value of 24.79 for the HALP score was determined, with 62.5% sensitivity and 62.3% specificity (AUC: 0.64, 95%CI: 0.48-0.80, P = 0.183). A HALP score of ≥ 24.79 was considered high and of < 24.79 low. The patients were divided into two groups: Those with low HALP scores (60.5%) and those with high HALP scores (39.5%).
The association between the HALP score and various characteristics of the patients was assessed, and the results indicate that there was no statistically significant relationship between the HALP score and sex (P = 0.816), smoking (P = 0.679), record of previous surgery (P = 0.804), type of operation performed (P = 0.783), pathological subtype (P = 0.18), presence of metastasis at the time of diagnosis (P = 0.894), and the tumor location (P = 0.142). However, statistically significant relationships were observed between the HALP score and other factors, specifically between the HALP score and the Eastern Cooperative Oncology Group Performance status (ECOG PS) (P = 0.02), the presence of additional diseases (P = 0.008), and the degree of differentiation (P = 0.045). The relevant patient characteristics related to the HALP score are summarized in Table 2.
| Features | HALP low, n (%) | HALP high, n (%) | P value |
| Gender | 0.816 | ||
| Female | 23 (25.8) | 14 (24.2) | |
| Male | 66 (74.2) | 44 (75.9) | |
| ECOG PS | 0.02 | ||
| 0 | 16 (18) | 16 (27.6) | |
| 1 | 47 (52.8) | 36 (62.1) | |
| 2 | 26 (29.2) | 6 (10.3) | |
| Comorbidity | 0.008 | ||
| No | 37 (41.6) | 37 (63.8) | |
| Yes | 52 (58.4) | 21 (36.2) | |
| Smoking | 0.679 | ||
| No | 43 (48.3) | 26 (44.8) | |
| Yes | 46 (51.7) | 32 (55.2) | |
| Surgery | 0.804 | ||
| No | 56 (62.9) | 34 (58.6) | |
| Yes | 33 (37.1) | 24 (41.4) | |
| Type of surgery | 0.783 | ||
| No | 55 (61.8) | 34 (58.6) | |
| Total gastrectomy | 23 (25.8) | 18 (31) | |
| Subtotal gastrectomy | 10 (11.2) | 6 (10.3) | |
| Patology | 0.18 | ||
| Adenocarcinom | 79 (88.8) | 49 (84.5) | |
| Signet ring cell carcinom | 8 (9) | 7 (12.1) | |
| Musinoz adenocarcinom | 2 (2.2) | 0 (0) | |
| Mix carcinom | 0 (0) | 2 (3.4) | |
| Diferantiation | 0.045 | ||
| Well | 1 (1.1) | 4 (6.9) | |
| Moderate | 42 (47.2) | 32 (55.2) | |
| Poorly | 38 (42.7) | 14 (24.1) | |
| Signet ring cell carcinoma | 8 (9) | 8 (13.8) | |
| Tumor location | 0.142 | ||
| Fundus, cardia | 25 (28.1) | 23 (39.7) | |
| Corpus | 33 (37.1) | 23 (39.7) | |
| Antrum, pylor | 31 (34.8) | 12 (20.7) | |
| De novo metastasis | 0.894 | ||
| No | 27 (30.3) | 17 (29.3) | |
| Yes | 62 (69.7) | 41 (70.7) |
The optimal cutoff values for NLR and PLR were determined by ROC analysis. NLR ≥ 2.88 was considered high (38.8% of patients) and < 2.88 low (61.2% of patients). PLR ≥ 166.1 was categorized as high (39.5% of patients) and < 166.1 low (60.5% of patients). The sensitivity and specificity for both NLR and PLR were 62.6% and 62.5%, respectively.
The median OS was 10.4 mo in the high HALP group and 7.5 mo in the low HALP group. The subgroups were further compared in terms of OS. In the univariate analysis, no statistically significant difference was observed in terms of NLR groups (P = 0.582), PLR groups (P = 0.350), differentiation groups (P = 0.06), and the presence of metastasis at the time of diagnosis (P = 0.754). However, statistically significant differences were found between the groups in terms of age (P < 0.001), second-line chemotherapy (P < 0.001), sex (P = 0.035), ECOG PS (P = 0.03), comorbidity (P = 0.004), and HALP score (P = 0.004) (Figure 1).
The multivariate analysis revealed that second-line chemotherapy (P < 0.001) and HALP score (P < 0.001) were statistically significant factors affecting OS. The HALP score was also statistically significant in the multivariate analysis (P = 0.001). The univariate and multivariate analyses of prognostic factors in terms of OS are presented in Table 3.
| Features | Median (months) | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | ||
| HALP groups | |||||
| HALP low | 7.5 | 0.59 (0.41-0.85) | 0.004 | Reference | 0.001 |
| HALP high | 10.4 | 0.53 (0.36-0.78) | |||
| NLR groups | |||||
| NLR low | 9.1 | 1.10 (0.78-1.56) | 0.582 | ||
| NLR high | 8.0 | ||||
| PLR groups | |||||
| PLR low | 8.6 | 1.18 (0.83-1.68) | 0.350 | ||
| PLR high | 8.4 | ||||
| Age groups | |||||
| ≤ 63 | 10.2 | 1.96 (1.37-2.8) | 0.000 | Reference | 0.060 |
| > 63 | 6.9 | 1.47 (0.98-2.19) | |||
| ECOG PS | |||||
| 0-1 | 9.1 | 1.57 (1.04-2.36) | 0.030 | Reference | 0.850 |
| 2 | 6.9 | 1.04 (0.66-1.64) | |||
| Comorbidity | |||||
| No | 10.2 | 1.67 (1.18-2.36) | 0.004 | Reference | 0.303 |
| Yes | 6.2 | 1.22 (0.84-1.78) | |||
| Diferantiation | |||||
| Well-moderate | 8.6 | 1.10 (0.78-1.53) | 0.600 | ||
| Poorly-Signet ring cell | 8.4 | ||||
| Second line CT | |||||
| No | 4.9 | 0.24 (0.16-0.35) | 0.000 | Reference | < 0.001 |
| Yes | 15.4 | 0.23 (0.16-0.34) | |||
| De novo metastasis | |||||
| No | 9.2 | 0.94 (0.65-1.37) | 0.754 | ||
| Yes | 8.2 | ||||
| Gender | |||||
| Female | 9.1 | 1.53 (1.03-2.28) | 0.035 | Reference | 0.051 |
| Male | 8.2 | 1.52 (0.99-2.31) | |||
| Tumor location | |||||
| Fundus, cardia | 7.5 | 0.056 | |||
| Corpus | 8.8 | - | - | ||
| Antrum, pylor | 9.2 | ||||
The HALP score, derived from the levels of hemoglobin, albumin, lymphocytes, and platelets, serves as an indicator of the patient's immunological and nutritional status. Anemia, commonly observed in cancer patients, particularly in gastric cancer, is recognized as a prevalent paraneoplastic syndrome. Chronic bleeding associated with gastric cancer often contributes to the development of anemia[16]. Anemia is thought to affect the performance status, chemotherapy tolerance, and course of the disease in patients with gastric cancer[17]. Additionally, hypoalbuminemia has been identified as an independent prognostic factor linked to poor outcomes in several studies[18]. It is known that immune system suppression raises the probability of cancer development[19]. Therefore, the HALP score, which encompasses both immunological and nutritional components, is promising as a valuable marker for predicting prognosis in patients with gastric cancer. Previous studies have demonstrated the predictive value of the HALP score in terms of lymph node involvement and the likelihood of recurrence in gastric cancer patients during the preoperative period[13,14]. In the present study, our objective was to explore the relationship between the HALP score and OS in patients diagnosed with metastatic gastric cancer.
In a study conducted by Chen et al[14], involving a cohort of 888 patients diagnosed with gastric cancer, a HALP score cutoff value of 56.8 was adopted. That study demonstrated that patients with high HALP scores had significantly longer OS times compared to those with low HALP scores. The authors also identified tumor size and T stage as independent factors associated with the HALP score. Subgroup analysis based on TNM stages revealed that there was no significant difference in survival between stage 4 patients with high HALP scores and those with low HALP scores. It is important to note that their study included a relatively small number of metastatic patients, with only 5 (1.9%) in the high HALP score group and 36 (6.1%) in the low HALP score group[14]. The lack of a difference in survival observed in the metastatic gastric cancer patients with high and low HALP scores in Chen's study could potentially be attributed to the small number of stage 4 patients and the imbalanced distribution of patients in the study cohort.
In a study conducted by Wang et al[13], the prognostic significance of the HALP score in the preoperative period was investigated in patients diagnosed with gastric cancer. A cutoff value of 35.3 was determined for the HALP score. Their study revealed that the HALP score, calculated prior to surgery, served as an effective marker for predicting lymph node status in gastric cancer patients. The authors emphasized that the HALP score could be utilized to personalize the surgical approach, providing valuable information for treatment planning and decision-making[13].
Sargın and Dusunceli[19] conducted a retrospective evaluation of 204 patients diagnosed with gastric cancer. Through the use of ROC analysis, they determined a cutoff of 23.8 for the HALP score. Their study revealed a significant difference in OS between patients with high HALP scores and those with low HALP scores (P = 0.05). Among the patient cohort, 136 individuals (66.7%) received adjuvant chemotherapy, while palliative chemotherapy was administered to 68 (33.3%). However, there was no statistically significant difference in OS between patients with high and low HALP scores in metastatic patients receiving palliative therapy. It is worth noting that the limited number of patients with metastatic disease in their study might have contributed to this lack of statistical significance[19].
In our study, which included 147 patients with metastatic gastric cancer, the patients with high HALP scores exhibited a significantly longer OS compared to those with low HALP scores. The median OS was 10.4 mo in the high HALP score group and 7.5 mo in the low HALP score group. These findings suggest that higher HALP scores are associated with improved OS in patients with metastatic gastric cancer.
When reviewing previous studies on NLR and PLR, it is generally accepted that higher levels of NLR and PLR are associated with worse survival outcomes. However, the literature reveals conflicting results[7,20,21]. In the study conducted by Magdy et al[10], a borderline significant association was observed between NLR levels and OS, while no significant association was found with progression-free survival[10]. In the current study, there was no significant difference in OS between patients with high and low NLR, or between patients with high and low PLR. These findings support the hypothesis that the HALP score, which is obtained by combining nutritional and inflammatory markers, provides a better prognosis prediction for metastatic gastric cancer compared to other known inflammation-related markers.
Previous studies have consistently demonstrated that the HALP score, calculated based on preoperative values, serves as a valuable marker for predicting lymph node involvement, prognosis, and OS[13,14,19]. However, it did not reach statistical significance in the metastatic subgroups, which generally constitute a small portion of the patients in the studies. In the present study, our results indicate that the HALP score, determined using values obtained during the metastatic process, holds significant utility as a biomarker for predicting OS. By focusing specifically on patients with metastatic gastric cancer, we were able to evaluate the direct impact of the HALP score in this specific subgroup. Our findings highlight the prognostic value of the HALP score in the context of metastatic gastric cancer, further supporting its potential as a clinically useful biomarker in this setting.
The present study has some limitations. Firstly, the study was retrospective and conducted in a single-center setting, which inherently carries the risk of bias and compromises the generalizability of the findings. Secondly, the cutoff values from the ROC analysis did not exhibit the desired level of sensitivity and specificity, thus potentially affecting the accuracy of the results. Additionally, the exclusion of patients with missing records from the analyses reveals the possibility of bias. Hence, it is crucial to consider these limitations when interpreting the outcomes of our study, recognizing the need for further research with robust designs and larger, more diverse patient cohorts to enhance the validity and generalizability of the results.
HALP score is a biomarker that can be easily calculated by routine tests and is known to predict prognosis in many tumors. This is the first study to demonstrate the prognostic value of the HALP score in patients with metastatic gastric cancer. This score is a potential biomarker to utilize in the management of patients with metastatic gastric cancer. However, multicenter and prospective studies with more patients are required.
The hemoglobin, albumin, lymphocyte, and platelet (HALP) score, derived from a composite evaluation of markers reflecting the tumor-inflammation relationship and nutritional status, has been substantiated as a noteworthy prognostic determinant for diverse malignancies. A scoring system incorporating clinical and laboratory data may hold utility in determining the prognosis of gastric cancer.
The need for healthcare professionals to utilize supportive tools in predicting prognosis and making treatment decisions in metastatic gastric cancer.
To investigate how the HALP score relates to prognosis in patients with metastatic gastric cancer.
This retrospective study cohort comprised 147 patients with metastatic gastric cancer. The cutoff values for the HALP score, neutrophil/lymphocyte ratio, and platelet/lymphocyte ratio were determined using receiver operating characteristic analysis. Low HALP scores were defined as those less than 24.79 and high HALP scores as those greater than 24.79.
The median overall survival was significantly superior in patients with high HALP score than those with low HALP score (10.4 mo vs 7.5 mo, respectively; P < 0.001).
The HALP score was found to be a prognostic factor in patients with metastatic gastric cancer.
Given its simplicity and low cost, we think the HALP score can be utilized to manage patients with gastric cancer.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lin Q, China; MD UF, Italy; Shahid M, Pakistan S-Editor: Fan JR L-Editor: Filipodia P-Editor: Zhang XD
| 1. | National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Stat Facts: Stomach Cancer. Surveillance, Epidemiology, and End Results (SEER) Program Cancer Stat Facts: Stomach Cancer, 2021. [Cited in This Article: ] |
| 2. | GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol. 2020;5:42-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 336] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. International Agency for Research on Cancer. GLOBOCAN 2020: Stomach cancer fact sheet, 2020. [Cited in This Article: ] |
| 4. | Ma J, Shen H, Kapesa L, Zeng S. Lauren classification and individualized chemotherapy in gastric cancer. Oncol Lett. 2016;11:2959-2964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 5. | Dicken BJ, Bigam DL, Cass C, Mackey JR, Joy AA, Hamilton SM. Gastric adenocarcinoma: review and considerations for future directions. Ann Surg. 2005;241:27-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 453] [Cited by in F6Publishing: 482] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 6. | Sano T, Coit DG, Kim HH, Roviello F, Kassab P, Wittekind C, Yamamoto Y, Ohashi Y. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20:217-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 302] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 7. | Zhang X, Zhao W, Yu Y, Qi X, Song L, Zhang C, Li G, Yang L. Clinicopathological and prognostic significance of platelet-lymphocyte ratio (PLR) in gastric cancer: an updated meta-analysis. World J Surg Oncol. 2020;18:191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1802] [Cited by in F6Publishing: 1962] [Article Influence: 130.8] [Reference Citation Analysis (0)] |
| 9. | Varga G, Foell D. Anti-inflammatory monocytes-interplay of innate and adaptive immunity. Mol Cell Pediatr. 2018;5:5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Magdy M, Hussein T, Ezzat A, Gaballah A. Pre-treatment Peripheral Neutrophil-Lymphocyte Ratio as a Prognostic Marker in Gastric Cancer. J Gastrointest Cancer. 2019;50:763-768. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Jiang H, Li H, Li A, Tang E, Xu D, Chen Y, Zhang Y, Tang M, Zhang Z, Deng X, Lin M. Preoperative combined hemoglobin, albumin, lymphocyte and platelet levels predict survival in patients with locally advanced colorectal cancer. Oncotarget. 2016;7:72076-72083. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Peng D, Zhang CJ, Gong YQ, Hao H, Guan B, Li XS, Zhou LQ. Prognostic significance of HALP (hemoglobin, albumin, lymphocyte and platelet) in patients with bladder cancer after radical cystectomy. Sci Rep. 2018;8:794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Wang X, He Q, Liang H, Liu J, Xu X, Jiang K, Zhang J. A novel robust nomogram based on preoperative hemoglobin and albumin levels and lymphocyte and platelet counts (HALP) for predicting lymph node metastasis of gastric cancer. J Gastrointest Oncol. 2021;12:2706-2718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Chen XL, Xue L, Wang W, Chen HN, Zhang WH, Liu K, Chen XZ, Yang K, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Prognostic significance of the combination of preoperative hemoglobin, albumin, lymphocyte and platelet in patients with gastric carcinoma: a retrospective cohort study. Oncotarget. 2015;6:41370-41382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Hasselmann M, Alix E. Tools and procedures for screening for malnutrition and its associated in risks in hospital. Nutrition Clinique et Metabolisme. 2003;17:218-226. [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. Am J Med. 2004;116 Suppl 7A:11S-26S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 325] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 17. | Tomita M, Shimizu T, Hara M, Ayabe T, Onitsuka T. Impact of preoperative hemoglobin level on survival of non-small cell lung cancer patients. Anticancer Res. 2008;28:1947-1950. [PubMed] [Cited in This Article: ] |
| 18. | Crumley AB, Stuart RC, McKernan M, McMillan DC. Is hypoalbuminemia an independent prognostic factor in patients with gastric cancer? World J Surg. 2010;34:2393-2398. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Sargin ZG, Dusunceli I. The Effect of HALP Score on the Prognosis of Gastric Adenocarcinoma. J Coll Physicians Surg Pak. 2022;32:1154-1159. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 20. | Wang W, Tong Y, Sun S, Tan Y, Shan Z, Sun F, Jiang C, Zhu Y, Zhang J. Predictive value of NLR and PLR in response to preoperative chemotherapy and prognosis in locally advanced gastric cancer. Front Oncol. 2022;12:936206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 9] [Reference Citation Analysis (0)] |
| 21. | Gunaldi M, Goksu S, Erdem D, Gunduz S, Okuturlar Y, Tiken E, Kahraman S, Inan YO, Genc TB, Yildirim M. Prognostic impact of platelet/lymphocyte and neutrophil/lymphocyte ratios in patients with gastric cancer: a multicenter study. Int J Clin Exp Med. 2015;8:5937-5942. [PubMed] [Cited in This Article: ] |