Published online Nov 15, 2022. doi: 10.4251/wjgo.v14.i11.2170
Peer-review started: July 15, 2022
First decision: August 8, 2022
Revised: September 5, 2022
Accepted: October 12, 2022
Article in press: October 12, 2022
Published online: November 15, 2022
Gastric cancer (GC) is a common type of digestive cancer with high morbidity and mortality rates worldwide. Considerable effort has been expended in under
To explore the expression and function of miR-30e-3p in GC development.
MiR-30e-3p was found to be downregulated in GC, with low levels thereof predicting poor outcomes among patients with GC. Functionally, we revealed that miR-30e-3p suppressed cell growth and metastatic behaviors of GC cells. Bioinformatics analysis predicted that THO complex 2 (THOC2) was a direct target of miR-30e-3p, and the interaction between miR-30e-3p and THOC2 was further validated by a luciferase reporter assay.
Our findings revealed that knockdown of THOC2 inhibited the growth and metastatic behaviors of GC cells. After investigating signaling pathways involved in miR-30e-3p regulation, we found that the miR-30e-3p/THOC2 axis regulated the PI3K/AKT/mTOR pathway in GC.
Our findings suggest the novel functional axis miR-30e-3p/THOC2 is involved in GC development and progression. The miR-30e-3p/THOC2 axis could be utilized to develop new therapies against GC.
Core Tip: Gastric cancer (GC) is a common digestive cancer with high morbidity and mortality rates worldwide. Considerable effort has been expended in understanding the mechanism of GC development and metastasis. Given that microRNAs have been found to participate in the regulation of GC progression, we explored the expression and function of miR-30e-3p in GC development and revealed that knockdown of THO complex 2 (THOC2) inhibited the growth and metastatic behaviors of GC cells. After investigating signaling pathways involved in miR-30e-3p regulation were investigated, we found that the miR-30e-3p/THOC2 axis regulated the PI3K/AKT/mTOR pathway in GC.
- Citation: Gu XJ, Li YJ, Wang F, Ye T. MiR-30e-3p inhibits gastric cancer development by negatively regulating THO complex 2 and PI3K/AKT/mTOR signaling. World J Gastrointest Oncol 2022; 14(11): 2170-2182
- URL: https://www.wjgnet.com/1948-5204/full/v14/i11/2170.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i11.2170
Gastric cancer (GC) is a common type of digestive cancer with high morbidity and mortality rates worldwide[1]. Helicobacter pylori (H. pylori) infection has been considered the most significant risk factor for GC, especially in China[2,3]. Nonetheless, the incidence of GC has substantially declined with improvements in the treatment of H. pylori infection over the past decades[4]. Technical advances of diagnosis and therapy improve the survival of patients with GC[5,6]. However, the prognosis remains unsatisfactory as patients with GC are mostly diagnosed at later stages and develop drug-resistance after treatment. Thus, it is essential to understand the tumorigenesis of GC and identify novel early diagnostic biomarkers and therapeutic targets for its treatment.
MicroRNAs (miRNAs) are small non-coding RNAs that participate in various biological processes[7]. Mounting evidence has shown that miRNA regulates tumorigenesis of multiple cancers, including GC[8,9]. MiRNAs exert their functions in GC development and metastasis by modulating tumor cell growth and malignancy[10]. For instance, miR-181a acts as an oncogenic miRNA in GC by negatively regulating caprin-1 expression, and overexpression of miR-181a predicts poor patient survival[11]. In contrast, microRNA profiling has identified that miR-6165 is a tumor suppressor and inhibits GC progression by regulating STRN4[12]. Moreover, miRNAs can be utilized as biomarkers for GC diagnosis and patient prognosis[13]. Our preliminary screening identified low miR-30e-3p expression in GC tissues. It has been reported that miR-30e-3p suppressed clear cell renal cell carcinoma (ccRCC) development and metastasis via targeting snail 1[14]. Nevertheless, the function of miR-30e-3p in GC has yet to be studied.
THO complex 2 (THOC2) is an RNA-binding protein involved in mRNA export, genomic stability, and mitotic progression[15]. THOC2 is essential for the early embryonic development of zebrafish[16]. Zhou et al[17] reported that THOC2 serves as an oncogene in melanoma and that knockdown of THOC2 inhibits the growth and metastasis of melanoma. Nevertheless, the function and regulation of THOC2 in GC remain unclear.
In the current study, we showed that miR-30e-3p was downregulated in GC tissues and cell lines, and low miR-30e-3p levels predicted unfavorable prognosis in patients with GC. Overexpression of miR-30e-3p suppressed the malignant behaviors of GC cells by negatively regulating THOC2. We also confirmed that the miR-30e-3p/THOC2 axis regulated the PI3K/AKT/mTOR pathway in GC. These findings indicate that miR-30e-3p/THOC2 could serve as a novel diagnosis biomarker and therapeutic target for GC treatment.
Human GC tissues and matched adjacent tissues (20 pair) were obtained during surgery on patients with GC at General Hospital of Ningxia Medical University. The primary clinicopathological features of the patients are summarized in Supplementary Table 1. The tissues were stored in liquid nitrogen until further use. All tissue samples were validated by two independent pathologists. All patients participating in the study provided written informed consent. The Institutional Review Board and ethics committee of Ningxia Medical University reviewed and approved the protocol.
Human GC cells (MGC803, AGS, MKN45, and BGC-823) and control human GES-1 cells were obtained from American Type Culture Collection (ATCC, VA, United States) and cultured following the guidelines provided by ATCC in an incubator at 37 °C and 5% CO2.
RNA from tissue specimens or cells were extracted using Trizol (Thermo Scientific, United States) and reverse-transcribed into cDNA using the TaqMan microRNA RT Kit (Thermo Scientific, United States). Quantitative real-time polymerase chain reaction (qRT-PCR) was conducted using the PowerUP SYBR Master Mix (Applied Biosystems, United States). The 2−ΔΔCT method was used to calculate the relative expression of miR-30e-3p or THOC2. The sequences of primers were as follows: miR-30e-3p: 5′-GCGTCTTTCAGTCGGATGTTTACAGC-3′; COLEC12: 5′-TCTCCTCCGTCAGTAACCGT-3′, and 5′-CAGGCTTGATTGACACTGGC-3′; CNPY2: 5′-GGCCACTCCTATTCTACGGC-3′, and 5′-CATCC
MiR-30e-3p mimics (5′-CUUUCAGUCGGAUGUUUACAGC-3′), miR-30e-3p inhibitors (5′-CTGTAAACATCCGACTGAAA-3′), and the relative negative controls (5′-UCACAACCUCCUAGAAAGAGUAGA-3′) were purchased from GenePharma (Shanghai, China). SiRNA targeting THOC2 (siRNA1: CGAAUUUUUGCAUUUAUGUCG-3′, siRNA1: 5′-CAUGAUAGUUCAACAUACAGA-3′) and scramble negative control (5′-UUCUCCGAACGUGUCACGUTT-3′) were obtained from Gene
Cell growth was assessed using a colony formation assay and CCK-8 assay as previously described[18].
Transwell assay was conducted using a transwell 24-well plate (Corning, United States) with or without matrigel precoating (BD Bioscience, United States). Cells were suspended in medium without serum and added into the upper chamber. The bottom chamber was filled with 500 µL complete medium. After 48 h, the migration or invasive cells were fixed, stained with crystal violet, and then calculated.
The putative WT or mutated 3′-UTR of THOC2 was cloned into the pGL3 plasmid (Promega, United States). The luciferase reporter vector and control Renilla vector were co-transfected into AGS or BGC-823 cells with miR-30e-3p mimics or control. The relative luciferase activity was assessed 48 h later using the Dual-Glo kit (Promega, United States).
Protein lysate preparation, SDS-PAGE, and Western blot were performed following the standard protocol. The following primary antibodies were used: THOC2 (ab129485) from Abcam, United States and mTOR (#2983), Akt (#4685), and PI3K (#17366) from Cell Signaling Technology, United States. E-cadherin (20874-1-AP), N-cadherin (22018-1-AP), vimentin (10366-1-AP), CNPY2 (14635-1-AP), YTHDF3 (25537-1-AP), SLC25A33 (17794-1-AP), CDKN1B (25614-1-AP), and β-actin (81115-1-RR) were purchased from Proteintech; COLEC12 (SAB1403383) was purchased from Sigma; and was purchased from NPY2R (PA5-102110) from Invitrogen.
Four-week-old male BALB/c nude mice were obtained from Jiangsu Aniphe Biolaboratory Inc. The guidelines for the animal studies were approved by the Animal Care Committee of Ningxia Medical University Hospital. BGC-823 cells were selected for tumor implantation. Around 48 h after transfection of miR-30e-3p mimic, approximately 1 ×107 BGC-823 cells were harvested and implanted sub
All results were presented as mean ± SD. Statistical analysis was conducted using GraphPad Prism V6.0 (GraphPad, United States). Student t-test or one-way ANOVA was used as necessary. A P < 0.05 indicated statistical significance.
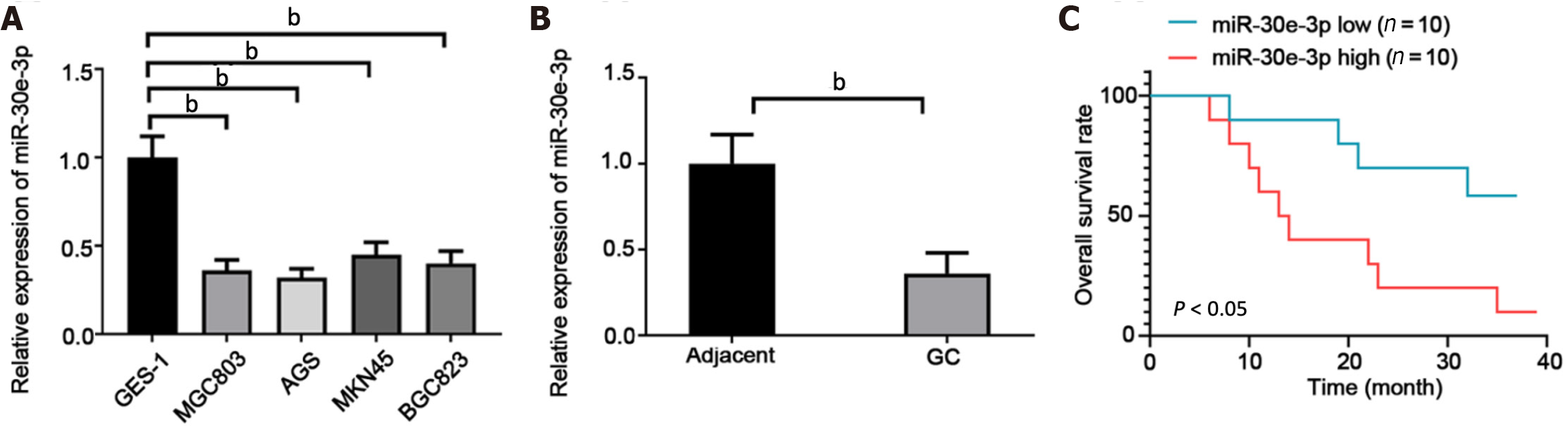
We first examined the miR-30e-3p expression in several GC cell lines. As detailed in Figure 1A, miR-30e-3p levels were lower in GC cells (MGC803, AGS, MKN45, and BGC-823) than in control GES-1 cells. Additionally, we demonstrated that miR-30e-3p expression was much lower in GC tissues than in matched adjacent non-tumor tissues (Figure 1B). Kaplan–Meier survival analysis suggested that patients with low miR-30e-3p expression had worse overall survival rate compared to those with high miR-30e-3p expression (Figure 1C). Thus, miR-30e-3p might function as a tumor suppressor in GC.
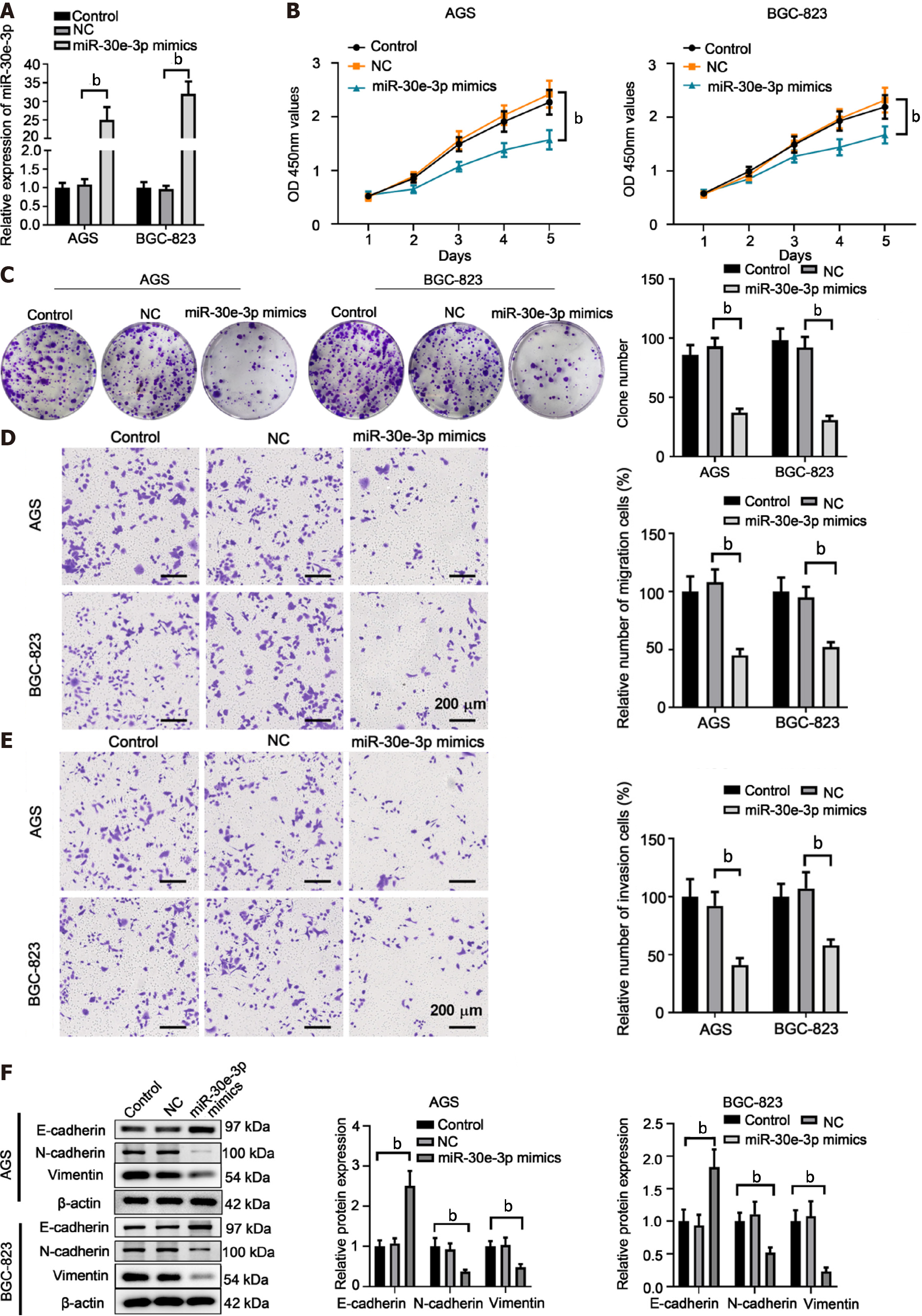
To explore miR-30e-3p function in GC, we transfected miR-30e-3p mimics into AGS or BGC-823 cells, after which the overexpression of miR-30e-3p was validated using qRT-PCR (Figure 2A). Functionally, we found that miR-30e-3p overexpression suppressed cell proliferation and colony formation of AGS or BGC-823 (Figure 2B and C). Furthermore, transfection of miR-30e-3p mimics resulted in decreased metastatic abilities of GC cells, with lesser cell migration or invasion (Figure 2D and E). In addition, transfection of miR-30e-3p mimics increased E-cadherin expression but decreased the expression of N-cadherin and vimentin (Figure 2F). These findings suggest that overexpression of miR-30e-3p suppresses the growth and metastatic behaviors of GC cells.
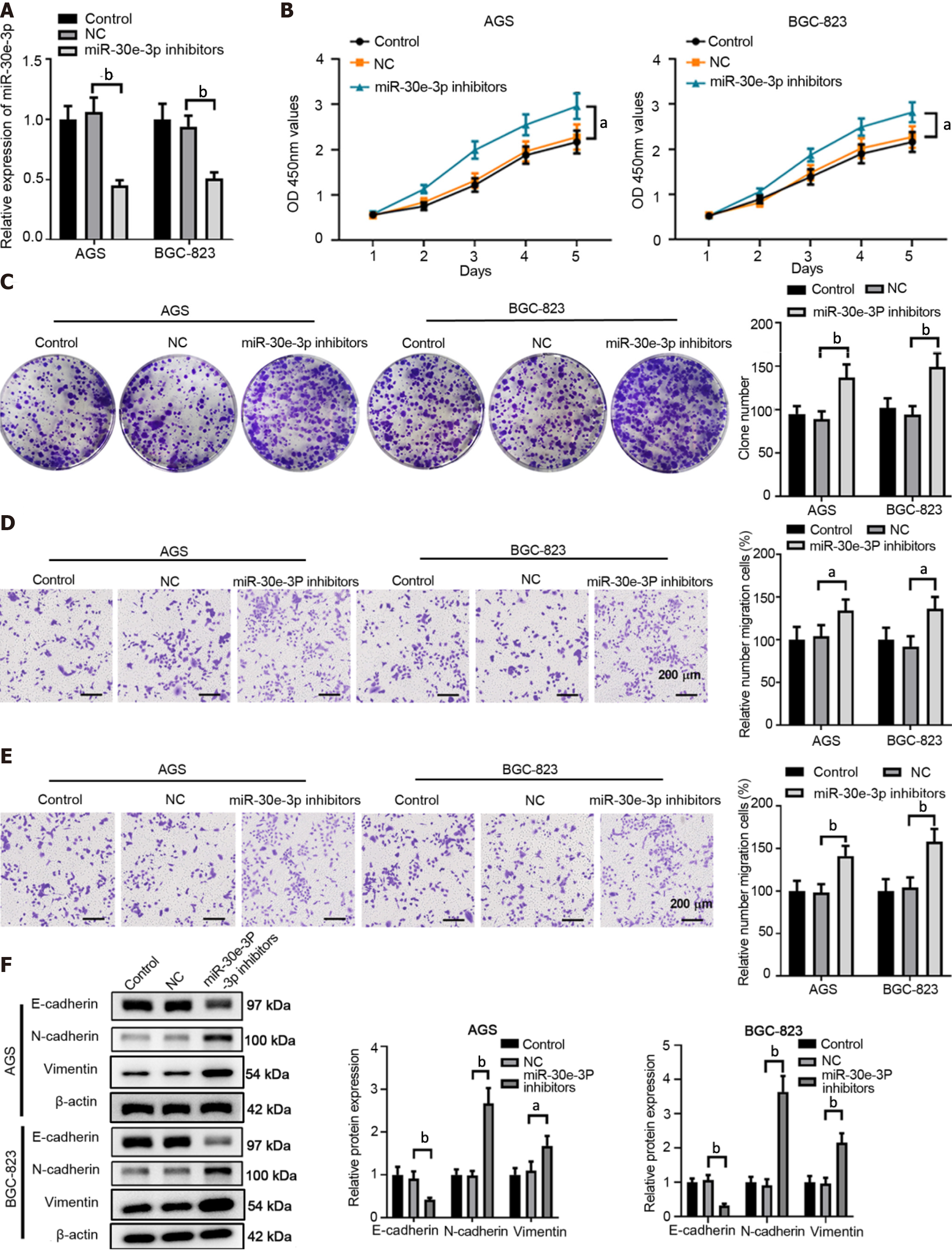
Conversely, we utilized miR-30e-3p inhibitors to suppress miR-30e-ep expression in GC cells (Figure 3A). MiR-30e-3p inhibitors also dampened the cell proliferation of GC cells (Figure 3B). Inhibition of miR-30e-3p promoted a higher colony number in AGC or SGC-823 cells compared with the control or negative control group (Figure 3C). Consistently, inhibition of miR-30e-3p enhanced GC cell migration and invasion (Figure 3D and E). In addition, transfection of miR-30e-3p inhibitors suppressed E-cadherin expression but promoted N-cadherin and Vimentin expression (Figure 3F). Taken together, both the overexpression and knockdown experiments demonstrated that miR-30e-3p negatively regulated GC cell growth and metastasis.
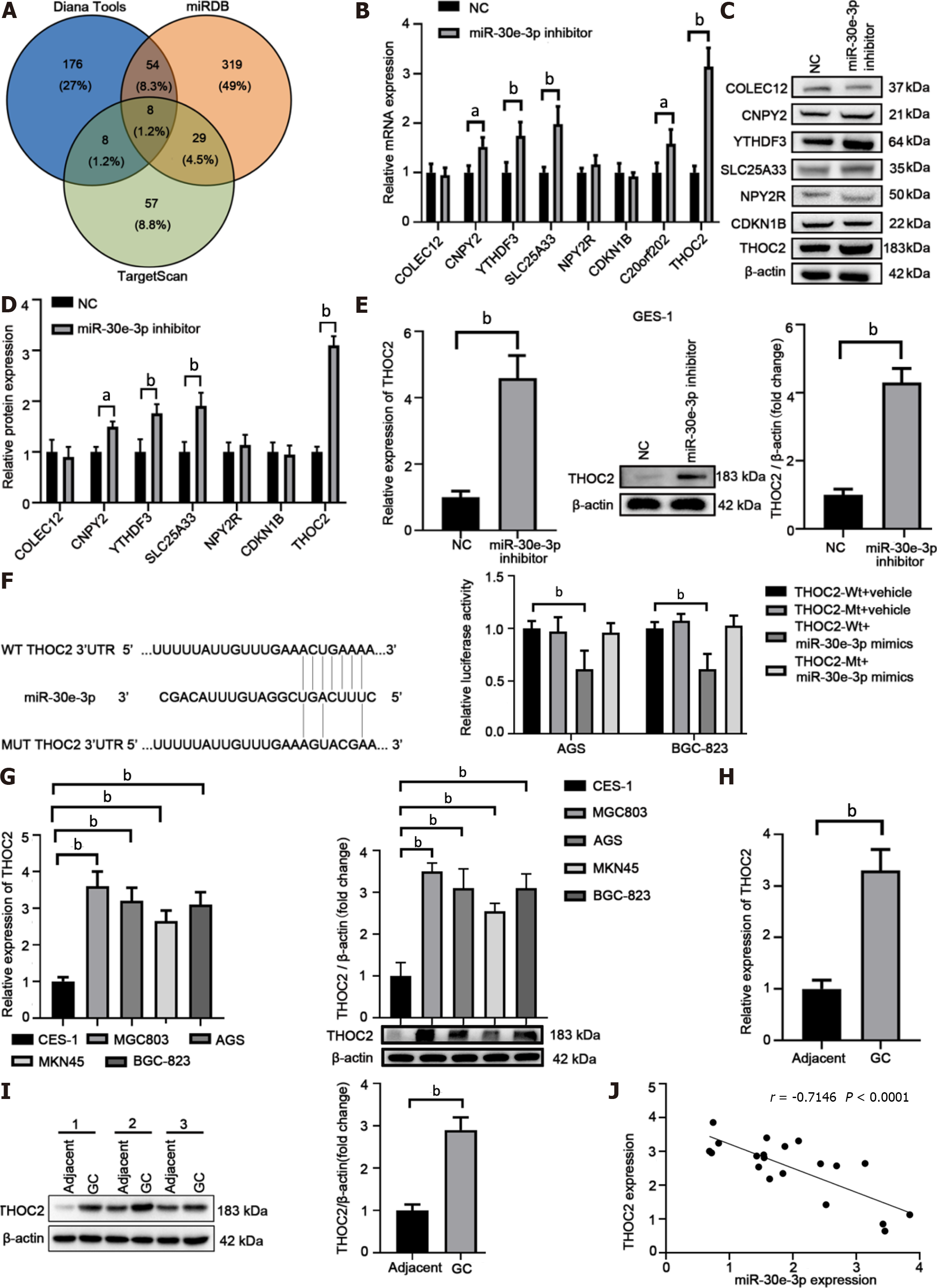
Bioinformatics analysis identified THOC2 as a top candidate of miR-30e-3p (Figure 4A and Supplementary Table 2). Overexpression of miR-30e-3p inhibitor significantly promoted CNPY2, YTHDF3, SLC25A33, C20orf202, and THOC2 expression, among which THOC2 demonstrated the greatest upregulation (Figure 4B). After also detecting the protein levels of these potential target genes, we found that the results were consistent with the mRNA expression level of these genes (Figure 4C and D). Moreover, suppression of miR-30e-3p also upregulated THOC2 expression in GES-1 cells (Figure 4E), suggesting that miR-30e-3p could bind to the 3′-URT of THOC2 (Figure 4F). Luciferase reporter assay further confirmed the binding between miR-30e-3p and the wild-type 3′-UTR of THOC2 (Figure 4F). We found that THOC2 expression was significantly higher in GC cells than in GES-1 cells (Figure 4G). Furthermore, both THOC2 mRNA and protein levels were markedly enhanced in GC tissues (Figure 4H–J). THOC2 expression was negatively associated with miR-30e-3p expression in GC tissues (Figure 4J). Hence, THOC2 is a direct target of miR-30e-3p in GC cells.
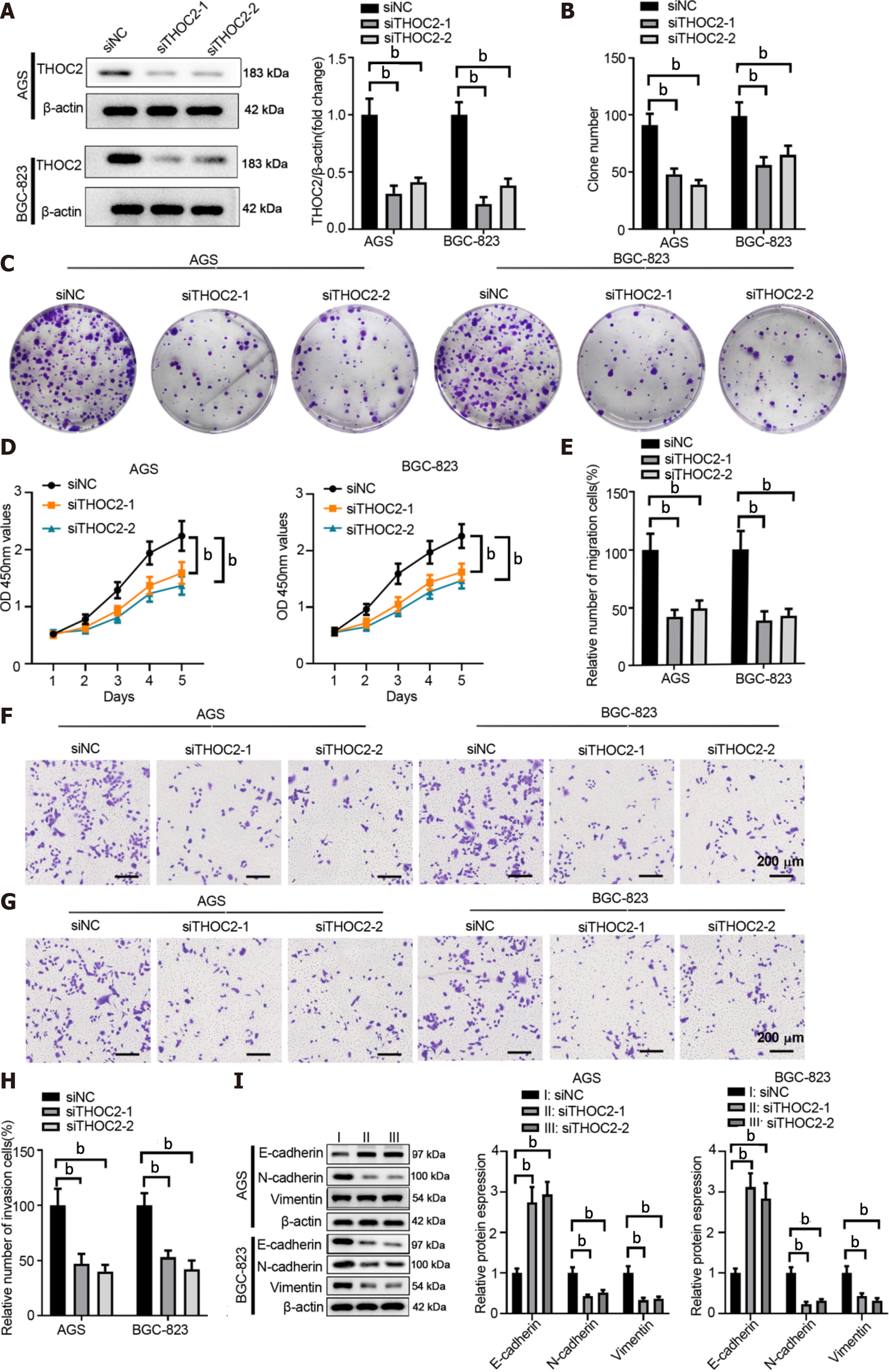
To evaluate the function of THOC2 in GC, we silenced THOC2 expression in AGS or BGC-823 cells using siRNA targeting THOC2 (Figure 5A). THOC2 knockdown significantly dampened colony formation and cell viability of GC cells (Figure 5B and 5C). The CCK-8 assay showed that knockdown of THOC2 significantly inhibited GC cells proliferation (Figure 5D). Furthermore, the metastasis behaviors of GC cells transfected with siTHOC2 were drastically inhibited (Figure 5E-H). In addition, silencing THOC2 significantly increase E-cadherin expression but decreased the expression of N-cadherin and Vimentin in AGS and BGC-823 cells (Figure 5I). In summary, the provided data indicated that knockdown of THOC2 suppresses the malignant behaviors of GC cells.
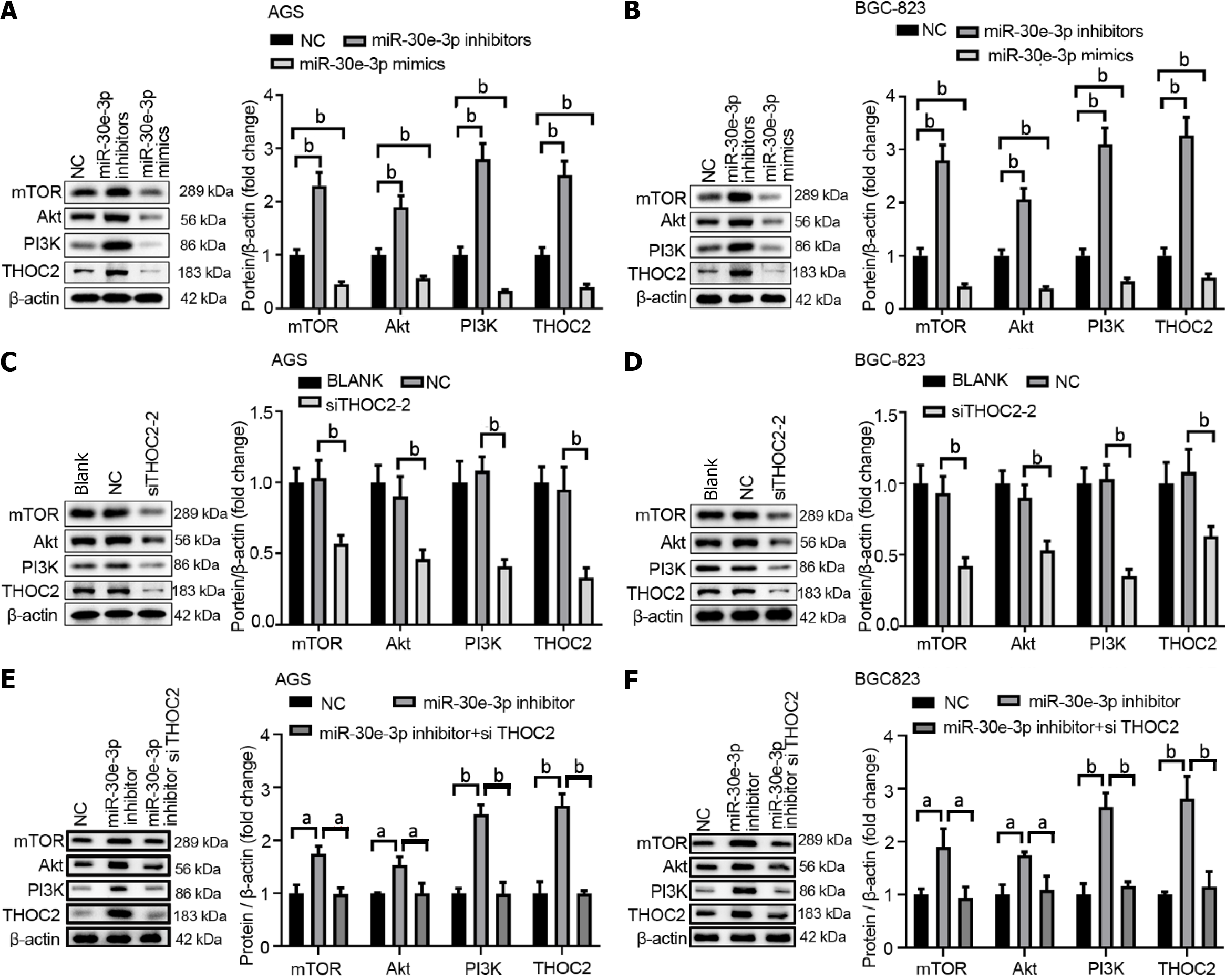
A previous study has demonstrated that the THO complex participates in the modulation of the p53 and PI3K/AKT pathways[19]. As such, we examined the PI3K/AKT/mTOR pathway in GC and analyzed the functional role of miR-30e-3p/THOC2. As shown in Figure 6A and B, inhibition of miR-30e-3p enhanced PI3K/AKT/mTOR expression, whereas overexpression of miR-30e-3p using miR-30e-3p mimics significantly decreased the expression of PI3K/AKT/mTOR in AGS or BGC-823 cells. Additionally, we demonstrated that THOC2 knockdown inhibited the expression of PI3K/AKT/mTOR (Figure 6C and D). Moreover, a restoration assay designed by our group showed that inhibition of miR-30e-3p interfered with THOC2 siRNA and that miR-30e-3p played a regulatory role by directly targeting THOC2 to regulate PI3K/AKT/mTOR signaling pathway in gastric cancer (Figure 6E and F). Thus, miR-30e-3p/THOC2 might regulate GC cell growth and metastasis by modulating the PI3K/ AKT/mTOR signaling pathway.
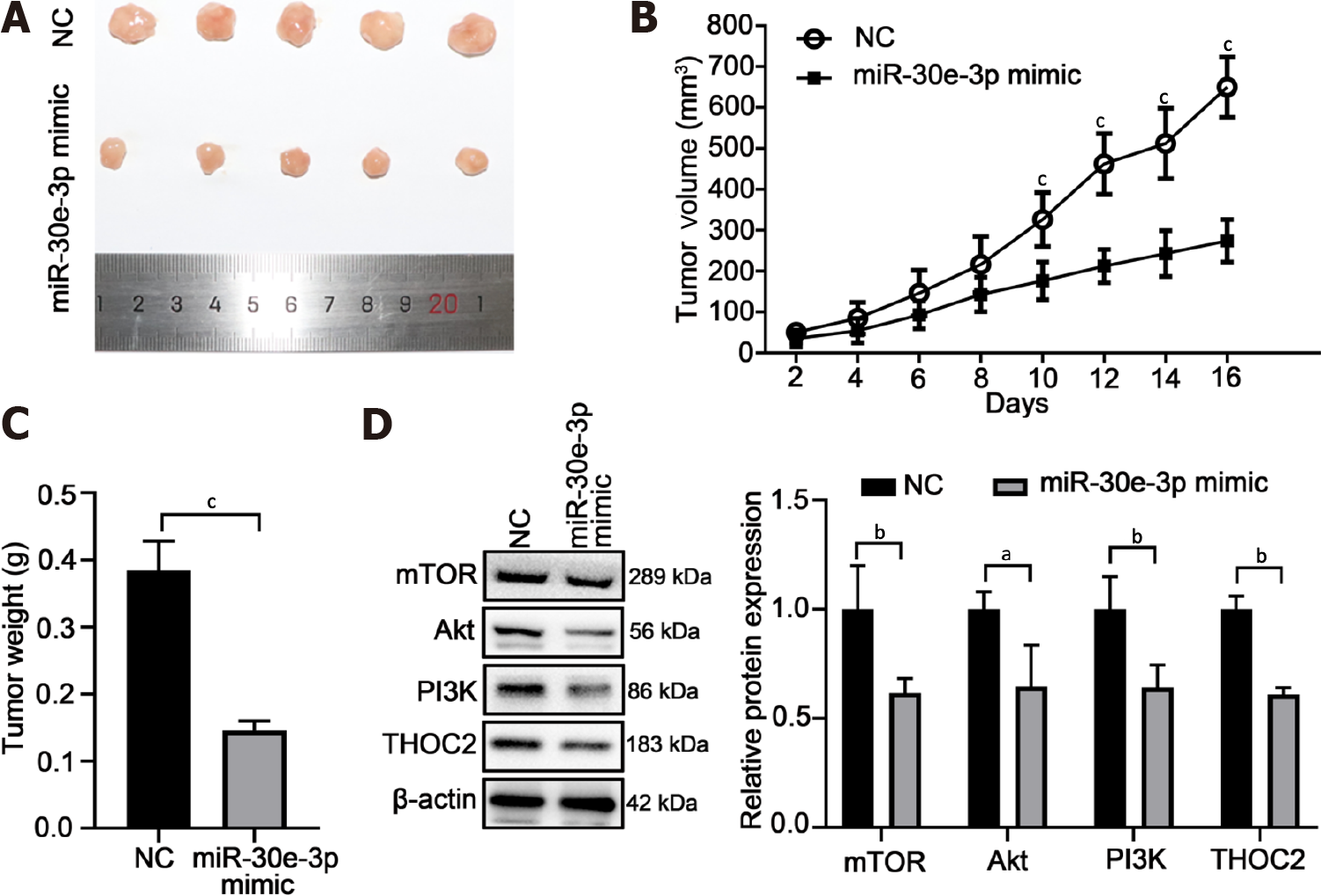
To investigate the function of miR-30e-3p on GC cell growth in vivo, BGC-823 cells transfected with miR-30e-3p mimics after 48 h were injected subcutaneously into BALB/c nude mice. After 16 d, the growth status of the tumor was analyzed. The tumor diameter of the miR-30e-3p mimic group was significantly smaller than that of the control group (Figure 7A). The tumor volume and weight of the miRNA mimic group were smaller than that of the control group (Figure 7B and C). In addition, we also detected the protein expression of THOC2, the target gene of miR-30e-3p, and genes associated with the PI3K/AKT/mTOR signaling pathway (Figure 7D). Accordingly, we found that the protein expression of these genes was consistent with that in vitro. Taken together, the presented data showed that miR-30e-3p inhibits GC cells growth in vivo.
Accumulating evidence has revealed that miRNAs play important regulatory roles in GC development and metastasis[20]. MiRNAs can also serve as non-invasive diagnostic biomarkers for GC[21]. In line with this, the current study sought to further investigate the expression and function of miR-30e-3p. Notably, we found that miRNA-30e-3p functioned as a tumor suppressor in GC and that miR-30e-3p overexpression inhibited GC cell growth and invasion. Additionally, we identified that THOC2 was a target of miR-30e-3p and that knockdown of THOC2 suppressed the malignant behaviors of GC cells. Taken together, our results demonstrated that miR-30e-3p/THOC2 could be utilized to develop new diagnostic biomarker and therapeutic target for GC treatment.
The current study found that GC tissues and cells had lower miR-30e-3p levels compared to matched normal tissues and control cells. Previous studies have shown that miR-30e-3p expression decreased in hepatocellular carcinoma (HCC) and that miR-30e-3p regulated HCC development by modulating MDM2/TP53 signaling[22]. Moreover, low expression of miR-30e-3p in HCC predicted drug-resistance to sorafenib treatment[22]. Similarly, evidence has shown that miR-30e inhibited breast cancer development and progression by targeting IRS1, and low miR-30e expression mediated the chemosensitivity of paclitaxel treatment in breast cancer[23]. We also showed that miR-30e-3p decreased GC cell proliferation, migration, and invasion. Low levels of miR-30e-3p in patients with GC indicated poor prognosis. Thus, miR-30e-3p functions as a tumor suppressor in GC. However, further investigations are needed to determine whether miR-30e-3p exerts regulatory effects on drug sensitivity during GC treatment. One intriguing finding of the current stud was the regulation of miR-30e-3p expression in GC. In ovarian cancer, lncRNA MEG3 had been found to sponge miR-30e-3p and regulate LAMA4 expression[24].
Multiple targets of miR-30e-3p have been identified, including Ubc9, P4HA1, IRS1, and ATG5[23,25-27]. We found that THOC2 was directly targeted by miR-30e-3p. Studies on the function of THOC2 in patients with psychomotor retardation showed that THOC2 was involved in mRNA-Export pathway in X-linked intellectual disability[28,29]. The dysregulated expression of THOC2 has been reported in severe neurocognitive and growth disorders[30]. Recently, the oncogenic function of THOC2 has been revealed in malignancies such as melanoma[17]. To the best of our knowledge, this has been the first study to demonstrated that THOC2 exhibited oncogenic function in GC development. Knockdown of THOC2 suppressed GC cell growth and metastasis. The regulatory axis of miR-30e-3p/THOC2 has been validated using the luciferase reporter assay, and Pearson analysis indicated that miR-30e-3p expression was negatively associated with THOC2 expression. Although our data confirmed that THOC2 was regulated by miR-30e-3p, other miRNAs might regulate THOC2, which could suppress GC development and progression.
Bioinformatics analysis was conducted to study the signaling pathways involved in miR-30e-3p/THOC2 regulation in GC. One study showed that the THO complex participates in the regulation of p53 and PI3K/AKT signaling[19]. The PI3K/AKT pathway is critical for cancer cell survival, proliferation, and apoptosis[31,32]. Consistently, we also found that the PI3K/AKT/mTOR signaling pathway was regulated by miR-30e-3p/THOC2 axis in vitro and in vivo. Thus, our findings provide a new approach in regulating the PI3K/AKT/mTOR pathway.
In conclusion, our findings suggested that miR-30e-3p directly targets THOC2 and that THOC2 mediates the tumor suppression function of miR-30e-3p in GC. Low expression of miR-30e-3p or upregulation of THOC2 predicts poor prognosis of patients with GC. The diagnostic and therapeutic value of miR-30e-3p/THOC2 in GC should be further investigated in future studies.
Gastric cancer (GC) is a common type of digestive cancer with high morbidity and mortality rates worldwide.
Considerable effort has been expended in understanding the mechanism of GC development and metastasis.
We explored the expression and function of miR-30e-3p in GC development.
We conducted quantitative real-time polymerase chain reaction, transfection, cell growth assays, transwell assay, luciferase reporter assay, western blot assays to explore the expression and function of miR-30e-3p.
Our findings revealed that knockdown of THO complex 2 (THOC2) inhibited the growth and metastatic behaviors of GC cells. After investigating signaling pathways involved in miR-30e-3p regulation, we found that the miR-30e-3p/THOC2 axis regulated the PI3K/AKT/mTOR pathway in GC.
Our findings suggested that miR-30e-3p directly targets THOC2 and that THOC2 mediates the tumor suppression function of miR-30e-3p in GC. Low expression of miR-30e-3p or upregulation of THOC2 predicts poor prognosis of patients with GC. The diagnostic and therapeutic value of miR-30e-3p/THOC2 in GC should be further investigated in future studies.
Considerable effort has been expended in understanding the mechanism of GC development and metastasis. Given that microRNAs have been found to participate in the regulation of GC progression.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu YQ, United States; Tanabe S, Japan S-Editor: Zhang H L-Editor: A P-Editor: Zhang XD
| 1. | Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 584] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 2. | Khasag O, Boldbaatar G, Tegshee T, Duger D, Dashdorj A, Uchida T, Matsuhisa T, Yamaoka Y. The prevalence of Helicobacter pylori infection and other risk factors among Mongolian dyspeptic patients who have a high incidence and mortality rate of gastric cancer. Gut Pathog. 2018;10:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Fuccio L, Eusebi LH, Bazzoli F. Gastric cancer, Helicobacter pylori infection and other risk factors. World J Gastrointest Oncol. 2010;2:342-347. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 53] [Cited by in F6Publishing: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Balakrishnan M, George R, Sharma A, Graham DY. Changing Trends in Stomach Cancer Throughout the World. Curr Gastroenterol Rep. 2017;19:36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 246] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 5. | Necula L, Matei L, Dragu D, Neagu AI, Mambet C, Nedeianu S, Bleotu C, Diaconu CC, Chivu-Economescu M. Recent advances in gastric cancer early diagnosis. World J Gastroenterol. 2019;25:2029-2044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 191] [Cited by in F6Publishing: 219] [Article Influence: 43.8] [Reference Citation Analysis (3)] |
| 6. | Xu HM, Wang X. [Current status and prospects of clinical research on diagnosis and treatment of gastric cancer in China]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:109-114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 2] [Reference Citation Analysis (0)] |
| 7. | O'Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol (Lausanne). 2018;9:402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2111] [Cited by in F6Publishing: 2608] [Article Influence: 434.7] [Reference Citation Analysis (0)] |
| 8. | Zheng Q, Chen C, Guan H, Kang W, Yu C. Prognostic role of microRNAs in human gastrointestinal cancer: A systematic review and meta-analysis. Oncotarget. 2017;8:46611-46623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016;1:15004. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1289] [Cited by in F6Publishing: 1451] [Article Influence: 181.4] [Reference Citation Analysis (0)] |
| 10. | Yoda Y, Takeshima H, Niwa T, Kim JG, Ando T, Kushima R, Sugiyama T, Katai H, Noshiro H, Ushijima T. Integrated analysis of cancer-related pathways affected by genetic and epigenetic alterations in gastric cancer. Gastric Cancer. 2015;18:65-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 11. | Lu Q, Chen Y, Sun D, Wang S, Ding K, Liu M, Zhang Y, Miao Y, Liu H, Zhou F. MicroRNA-181a Functions as an Oncogene in Gastric Cancer by Targeting Caprin-1. Front Pharmacol. 2018;9:1565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 12. | Wang Z, Li Y, Cao J, Zhang W, Wang Q, Zhang Z, Gao Z, Ye Y, Jiang K, Wang S. MicroRNA Profile Identifies miR-6165 Could Suppress Gastric Cancer Migration and Invasion by Targeting STRN4. Onco Targets Ther. 2020;13:1859-1869. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Yuan HL, Wang T, Zhang KH. MicroRNAs as potential biomarkers for diagnosis, therapy and prognosis of gastric cancer. Onco Targets Ther. 2018;11:3891-3900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Wang D, Zhu C, Zhang Y, Zheng Y, Ma F, Su L, Shao G. MicroRNA-30e-3p inhibits cell invasion and migration in clear cell renal cell carcinoma by targeting Snail1. Oncol Lett. 2017;13:2053-2058. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Francisco-Mangilet AG, Karlsson P, Kim MH, Eo HJ, Oh SA, Kim JH, Kulcheski FR, Park SK, Manavella PA. THO2, a core member of the THO/TREX complex, is required for microRNA production in Arabidopsis. Plant J. 2015;82:1018-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci U S A. 2004;101:12792-12797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 640] [Cited by in F6Publishing: 625] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 17. | Zhou X, Liu X, Zhang G, Zhang Q, Chen H, Wang Y, Fang F, Sun J. Knockdown THOC2 suppresses the proliferation and invasion of melanoma. Bioengineered. 2019;10:635-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Recent advances in the management of children with Wilms' tumor. Prog Clin Biol Res. 1983;132E:153-158. [PubMed] [Cited in This Article: ] |
| 19. | Moon S, Chung YD. p53 and PI3K/AKT signalings are up-regulated in flies with defects in the THO complex. Mol Cells. 2013;35:261-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Hwang J, Min BH, Jang J, Kang SY, Bae H, Jang SS, Kim JI, Kim KM. MicroRNA Expression Profiles in Gastric Carcinogenesis. Sci Rep. 2018;8:14393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 21. | Link A, Kupcinskas J. MicroRNAs as non-invasive diagnostic biomarkers for gastric cancer: Current insights and future perspectives. World J Gastroenterol. 2018;24:3313-3329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 73] [Cited by in F6Publishing: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 22. | Gramantieri L, Pollutri D, Gagliardi M, Giovannini C, Quarta S, Ferracin M, Casadei-Gardini A, Callegari E, De Carolis S, Marinelli S, Benevento F, Vasuri F, Ravaioli M, Cescon M, Piscaglia F, Negrini M, Bolondi L, Fornari F. MiR-30e-3p Influences Tumor Phenotype through MDM2/TP53 Axis and Predicts Sorafenib Resistance in Hepatocellular Carcinoma. Cancer Res. 2020;80:1720-1734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 23. | Liu MM, Li Z, Han XD, Shi JH, Tu DY, Song W, Zhang J, Qiu XL, Ren Y, Zhen LL. MiR-30e inhibits tumor growth and chemoresistance via targeting IRS1 in Breast Cancer. Sci Rep. 2017;7:15929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Liu Y, Xu Y, Ding L, Yu L, Zhang B, Wei D. LncRNA MEG3 suppressed the progression of ovarian cancer via sponging miR-30e-3p and regulating LAMA4 expression. Cancer Cell Int. 2020;20:181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Wu F, Zhu S, Ding Y, Beck WT, Mo YY. MicroRNA-mediated regulation of Ubc9 expression in cancer cells. Clin Cancer Res. 2009;15:1550-1557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Feng G, Shi H, Li J, Yang Z, Fang R, Ye L, Zhang W, Zhang X. MiR-30e suppresses proliferation of hepatoma cells via targeting prolyl 4-hydroxylase subunit alpha-1 (P4HA1) mRNA. Biochem Biophys Res Commun. 2016;472:516-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Ye Y, Fang Y, Xu W, Wang Q, Zhou J, Lu R. 3,3'-Diindolylmethane induces anti-human gastric cancer cells by the miR-30e-ATG5 modulating autophagy. Biochem Pharmacol. 2016;115:77-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 28. | Kumar R, Corbett MA, van Bon BW, Woenig JA, Weir L, Douglas E, Friend KL, Gardner A, Shaw M, Jolly LA, Tan C, Hunter MF, Hackett A, Field M, Palmer EE, Leffler M, Rogers C, Boyle J, Bienek M, Jensen C, Van Buggenhout G, Van Esch H, Hoffmann K, Raynaud M, Zhao H, Reed R, Hu H, Haas SA, Haan E, Kalscheuer VM, Gecz J. THOC2 Mutations Implicate mRNA-Export Pathway in X-Linked Intellectual Disability. Am J Hum Genet. 2015;97:302-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Di Gregorio E, Bianchi FT, Schiavi A, Chiotto AM, Rolando M, Verdun di Cantogno L, Grosso E, Cavalieri S, Calcia A, Lacerenza D, Zuffardi O, Retta SF, Stevanin G, Marelli C, Durr A, Forlani S, Chelly J, Montarolo F, Tempia F, Beggs HE, Reed R, Squadrone S, Abete MC, Brussino A, Ventura N, Di Cunto F, Brusco A. A de novo X;8 translocation creates a PTK2-THOC2 gene fusion with THOC2 expression knockdown in a patient with psychomotor retardation and congenital cerebellar hypoplasia. J Med Genet. 2013;50:543-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Kumar R, Gardner A, Homan CC, Douglas E, Mefford H, Wieczorek D, Lüdecke HJ, Stark Z, Sadedin S; Broad CMG, Nowak CB, Douglas J, Parsons G, Mark P, Loidi L, Herman GE, Mihalic Mosher T, Gillespie MK, Brady L, Tarnopolsky M, Madrigal I, Eiris J, Domènech Salgado L, Rabionet R, Strom TM, Ishihara N, Inagaki H, Kurahashi H, Dudding-Byth T, Palmer EE, Field M, Gecz J. Severe neurocognitive and growth disorders due to variation in THOC2, an essential component of nuclear mRNA export machinery. Hum Mutat. 2018;39:1126-1138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Zhang H, Xu W, Li B, Zhang K, Wu Y, Xu H, Wang J, Zhang J, Fan R, Wei J. Curcumin Promotes Cell Cycle Arrest and Inhibits Survival of Human Renal Cancer Cells by Negative Modulation of the PI3K/AKT Signaling Pathway. Cell Biochem Biophys. 2015;73:681-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | Zhou C, Qiu L, Sun Y, Healey S, Wanebo H, Kouttab N, Di W, Yan B, Wan Y. Inhibition of EGFR/PI3K/AKT cell survival pathway promotes TSA's effect on cell death and migration in human ovarian cancer cells. Int J Oncol. 2006;29:269-278. [PubMed] [Cited in This Article: ] |