Published online Jun 15, 2021. doi: 10.4251/wjgo.v13.i6.612
Peer-review started: December 6, 2020
First decision: January 29, 2021
Revised: February 11, 2021
Accepted: May 19, 2021
Article in press: May 19, 2021
Published online: June 15, 2021
There is no established correlation between 24-h esophageal pH-metry (Eso-pH) and the new laryngopharyngeal pH-monitoring system (Restech) as only small case series exist. Eso-pH was not designed to detect laryngopharyngeal reflux (LPR) and Restech may detect LPR better. We have previously published a dataset using the two techniques in a large patient collective with gastroesophageal reflux disease. Anatomically, patients after esophagectomy were reported to represent an ideal human reflux model as no reflux barrier exists.
To use a human reflux model to examine our previously published correlation in these patients.
Patients after Ivor Lewis esophagectomy underwent our routine follow-up program with surveillance endoscopies, computed tomography scans and further exams following surgery. Only patients with a complete check-up program and reflux symptoms were offered inclusion into this prospective study and evaluated using Restech and simultaneous Eso-pH. Subsequently, the relationship between the two techniques was evaluated
A total of 43 patients from May 2016 - November 2018 were included. All patients presented with mainly typical reflux symptoms such as heartburn (74%), regurgitation (84%), chest pain (58%), and dysphagia (47%). Extraesophageal symptoms such as cough, hoarseness, asthma symptoms, and globus sensation were also present. Esophageal 24-hour pH-metry was abnormal in 88% of patients with a mean DeMeester Score of 229.45 [range 26.4-319.5]. Restech evaluation was abnormal in 61% of cases in this highly selective patient cohort. All patients with abnormal supine LPR were also abnormal for supine esophageal reflux measured by conventional Eso-pH.
Patients following esophagectomy and reconstruction with gastric interposition can ideally serve as a human reflux model. Interestingly, laryngopharyngeal reflux phases occur mainly in the upright position. In this human volume-reflux model, results of simultaneous esophageal and laryngopharyngeal (Restech) pH-metry showed 100% correlation as being explicable by one of our reflux scenarios.
Core Tip: There is no established correlation between 24-h esophageal pH-metry (Eso-pH) and the new laryngopharyngeal pH-monitoring system (Restech) as only small case series exist. Anatomically, patients after esophagectomy were reported to represent an ideal human reflux model as no reflux barrier exists. Patients after esophagectomy were evaluated using Restech and simultaneous Eso-pH. In this human volume-reflux model, Eso-pH correlated completely with laryngopharyngeal pH-metry (Restech).
- Citation: Babic B, Müller DT, Gebauer F, Schiffmann LM, Datta RR, Schröder W, Bruns CJ, Leers JM, Fuchs HF. Gastrointestinal function testing model using a new laryngopharyngeal pH probe (Restech) in patients after Ivor-Lewis esophagectomy. World J Gastrointest Oncol 2021; 13(6): 612-624
- URL: https://www.wjgnet.com/1948-5204/full/v13/i6/612.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i6.612
Gastroesophageal reflux disease (GERD) is a common disorder of the upper gastrointestinal tract with a high prevalence, especially in the western world[1]. Symptoms are usually defined as typical/esophageal and atypical/extraesophageal symptoms with the most common and typical symptoms being heartburn and regurgitation. Still, a significant number of patients suffers from atypical/extraesophageal symptoms such as chronic cough, hoarseness, sore throat, and pharyngeal burning. Other, more unspecific symptoms like a burning sensation of the tongue and mouth, a globus sensation, and dental erosions may also be present. A causal association of extraesophageal symptoms with GERD or nasopharyngeal etiologies remains a major diagnostic challenge in these patients. In consequence, a satisfying therapy of patients with extraesophageal symptoms is not easy to offer[2,3]. A positive response to a medical therapy with proton pump inhibitors seems to be a positive prognostic predictor for connecting GERD to extraesophageal symptoms. Still the level of evidence for respiratory diseases caused by GERD remains rather low[1,4].
Esophageal 24-h pH monitoring (Eso-pH) is the gold standard for the detection of GERD. Herewith, the acid exposure of the lower esophagus can be identified and quantified[1]. To improve measurement of episodes caused by proximal esophageal reflux, a dual-probe pH monitoring was introduced in the late 1990’s[5,6]. With the necessity of a high esophageal positioning of the pH-probe for the detection of laryngopharyngeal reflux (LPR), existing pH-metry devices designed for lower esophageal pH-metry were not always reliable and valid. The development and implementation of pH-impedance monitoring made it possible to distinguish between acid and non-acid reflux and furthermore allowed a quantification of proximal esophageal reflux. In addition, correlation between symptoms and episodes of reflux can be seen.
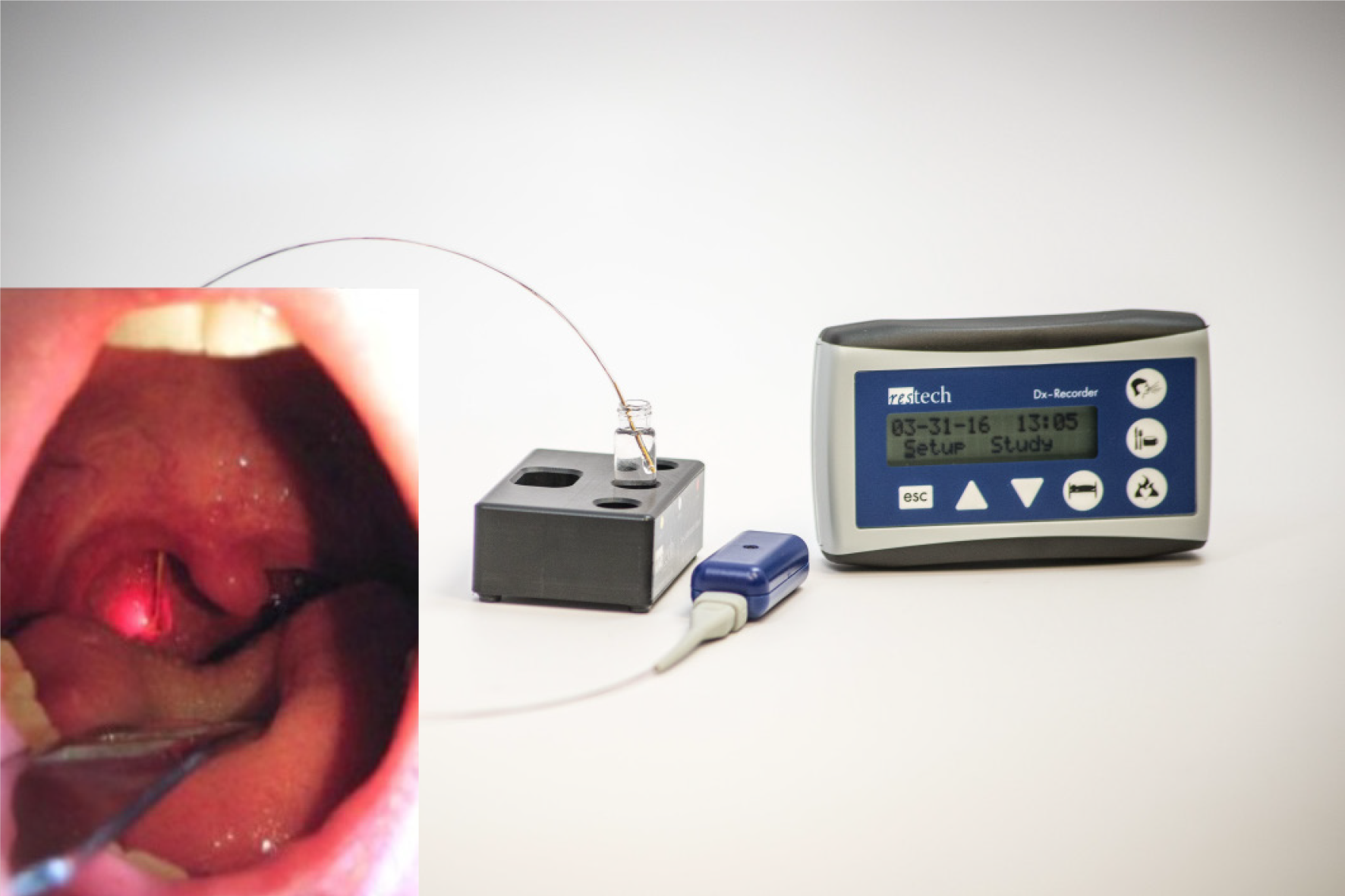
Recently a novel pH device (Restech pH measurement system, Respiratory Technology Corp., Houston, TX, United States = Restech) has been developed and normal values were published in 2009[7]. This device is designed to be positioned above the upper esophageal sphincter in the oropharynx. The teardrop design prevents drying of the catheter, a common problem of the Eso-pH catheter when placed high in the oropharynx. Restech was created to detect both liquid and acidic gas vapor, and the oropharyngeal placement may lead to more accurate results[8]. Worrell et al[9] published that Restech may help to achieve a better patient selection for a successful outcome for extraesophageal reflux symptoms after laparoscopic anti-reflux surgery. Our group recently published the largest case series and validation study using Restech and classic esophageal pH-metry simultaneously in more than 100 patients with GERD[10] showing that. various reflux scenarios exist in patients with reflux disease.
Other researchers developed a human reflux model in the early 2000’s[11]. We further evolved this idea and developed the University of Cologne human reflux model. Patients that underwent esophagectomy were followed up thoroughly after surgery over a long period of time and data on those bothersome symptoms was collected[12]. Interestingly, patients who had undergone esophagectomy for adenocarcinoma of the esophagus showed a faster progression to so-called Neo-Barretts in the esophageal remnant.
Therefore, it is the aim of this current study to further validate the new technology (Restech) using a prospective cohort of patients after Ivor-Lewis esophagectomy clinically presenting with severe GERD.
Our academic center is a certified center of excellence for surgery of the upper gastrointestinal tract. A prospective analysis of patient data during follow-up after hybrid minimally invasive Ivor Lewis esophagectomy for cancer was performed. To obtain a homogenous population, only patients at least 3 mo out of surgery and disease-free survival were included in this study to eliminate immediate postoperative effects. The study was conducted with approval from the institutional review board at the University of Cologne (IRB reference 16-727) and subjects gave written informed consent prior to participation in the study.
Demographics, endoscopic findings, biopsies at different follow-up time points, as well as tumor histology and stage were recorded in the prospective database. Additionally, symptoms were recorded at all times of follow-up. Only patients that presented with reflux-related symptoms or those showing endoscopic proof of mucosal damage in the esophageal remnant were offered inclusion in this study. To minimize the risks and inconvenience to our patients and to follow current ethical principles for research, no control group without symptoms or proof of reflux associated changes was investigated for this study.
Esophageal cancer was treated according to previously published guidelines[13-15]. In a multimodal setting, surgery was scheduled 4 to 8 wk after neoadjuvant treatment and was typically performed as a hybrid or totally minimally invasive Ivor Lewis procedure with high intrathoracic esophagogastric anastomosis and two-field lymphadenectomy. We performed hybrid and totally minimally invasive procedures according to international guidelines[16-18]. All patients underwent our previously published risk assessment before surgery[19]. Esophagitis was recorded according to the Los Angeles classification during follow-up endoscopy[20]. The definition of Barrett’s mucosa included both specialized and non-specialized columnar epithelium from the esophageal remnant and was only diagnosed when goblet cells were present[21]. We published our management if mucosal inflammation was encountered during follow-up of esophageal cancer before[12].
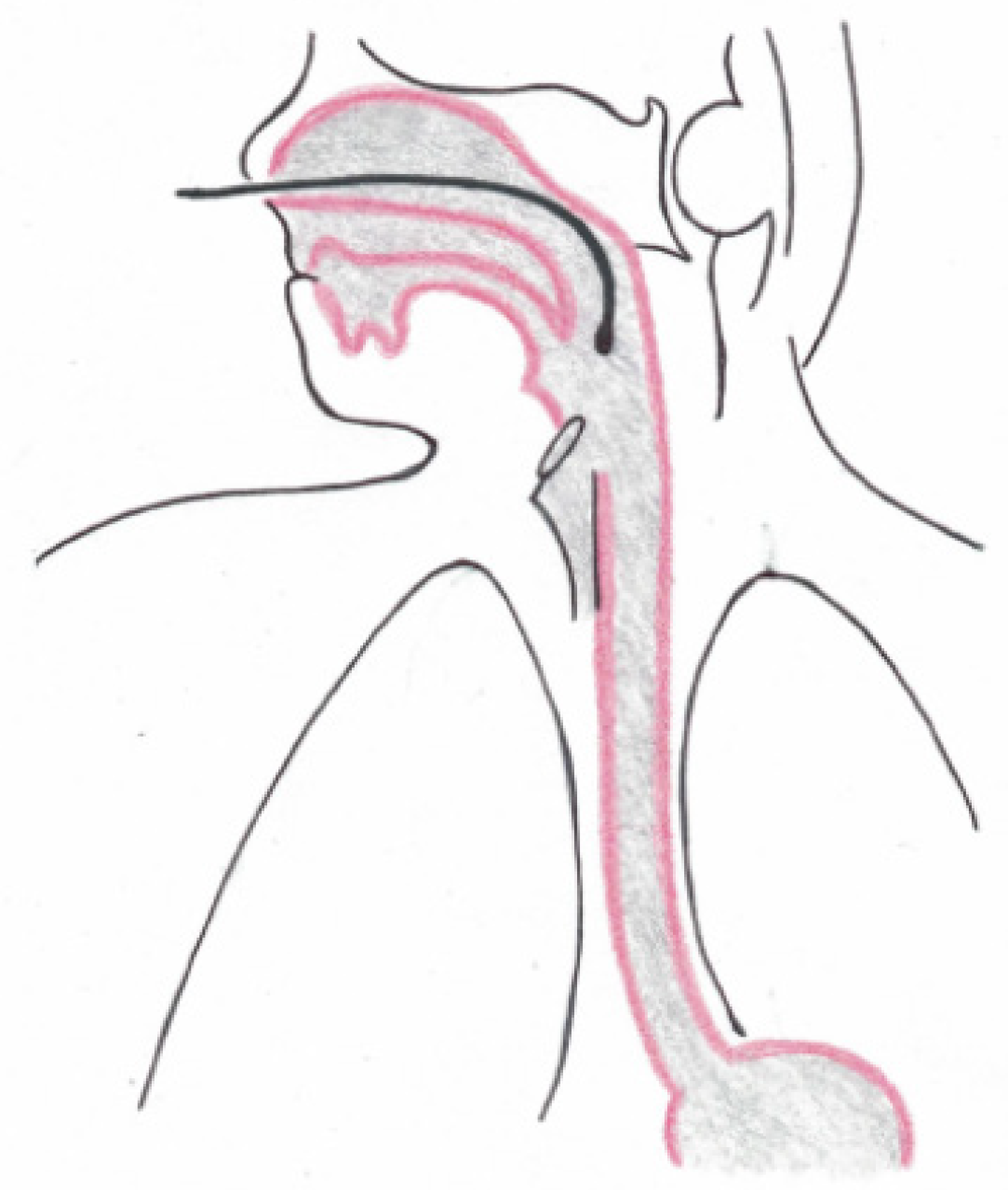
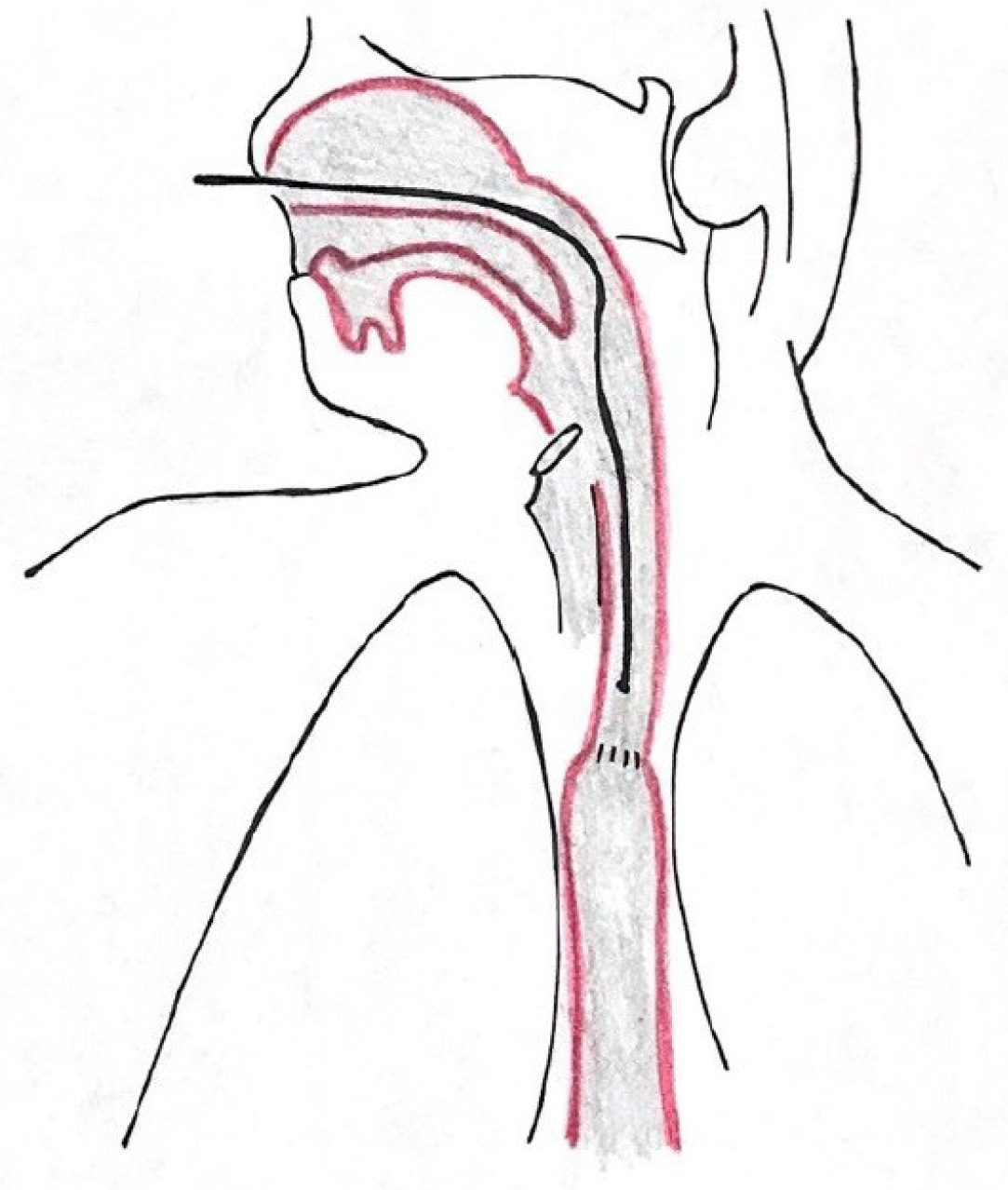
All patients with GERD symptoms were offered inclusion to our study. They were subsequently seen in a specialized surgical outpatient clinic with all laboratory instruments for up-to-date gastrointestinal function testing (High Resolution Manometry (HRM), upper-gastrointestinal endoscopy, contrast radiography, and 24-h impedance-pH-monitoring with simultaneous 24-h Restech pH-monitoring). All patients underwent a standardized interview about quality of life (GIQLI, GERD-HRQL), the presence of heartburn, regurgitation, dysphagia, and atypical symptoms, as reported by others before[22,23]. Gastrointestinal function testing was performed according to the current EAES (European Association of Endoscopic Surgery) recommendations for management of GERD[1]. No HRM or barium swallow was performed in this study. We have published a detailed description of upper-gastrointestinal endoscopy, esophageal pH-monitoring (Eso-pH), and simultaneous laryngopharyngeal pH-monitoring as performed in our center before[10]. The esophagogastric anastomosis was defined as esophagogastric junction for placement of the pH-metry probe. Placement of both laryngopharyngeal and esophageal pH probes are shown in Figures 1-3. In addition, a standardized protocol was followed during the measurements to ensure valid study data. Patients were asked to maintain their regular diet and instructed to eat three meals per day with drinking allowed only at mealtimes. Mealtimes were then excluded from the analysis.
Based on gastrointestinal function testing, and previous studies from our group[10], patients were subdivided in groups A-D (Table 1). Group A consists of patients with an abnormal esophageal but normal oropharyngeal acid exposure, Group B consists of patients with a normal esophageal but abnormal oropharyngeal acid exposure, Group C of patients with both abnormal esophageal and oropharyngeal acid exposure, and Group D of patients with no abnormal esophageal and oropharyngeal acid exposure.
| Restech pH-metry | Esophageal pH-metry | |
| Normal | Abnormal | |
| Normal | D (n = 4) | A (n = 9) |
| Abnormal | B (n = 0) | C (n = 20) |
Data were collected prospectively, including but not limited to, age, gender, Body Mass Index, esophageal pH-metry results, Restech pH-metry results, endoscopic findings, oncological parameters, and surgical therapy.
Main outcome of measure was the correlation of esophageal and laryngopharyngeal pH-metry results in this human reflux model. Continuous variables are presented as means and range. Categorical data are presented as numbers and percentages. The Student t-test, (for continuous variables), and Chi-square test, (for nominal or categorical variables), were used for all bivariate analyses. All tests were 2-sided, with statistical significance set at P ≤ 0.05. Data were analyzed by GraphPad (GraphPad Software, San Diego, CA, United States). Statistical review of this study was performed by a biomedical statistician.
A total of 413 patients underwent Ivor-Lewis esophagectomy at our institution between May 2016 and November 2018. In the same period, 43 patients after Ivor-Lewis esophagectomy (9 females) with a mean age of 61 years (range 39-79) consented for the present study and were completely followed up including gastrointestinal function testing and subsequently included in this study. Typical GERD symptoms such as regurgitation, dysphagia, and heartburn were present in a large proportion of our collective. Some patients also suffered from extraesophageal reflux symptoms such as chronic cough, hoarseness, sore throat and pharyngeal burning. Unwanted weight loss and retrosternal pain were other chief complaints of this study cohort. The detailed follow-up information is given in Table 2. Of the total 43 patients, 34 patients (79%) had adenocarcinoma and 9 patients (21%) had squamous cell carcinoma of the esophagus. All patients were routinely on proton pump inhibitors at a daily dose of 40mg and off PPIs for at least 7 d for the measurement. Mean level of intrathoracic anastomosis was at 24 cm (range 20-33 cm) from incisors.
| n | % | |
| Total | 43 | |
| Follow up time (d) | Mean 790 (median 574) | Range 106-3640 |
| Mucosal disease | ||
| Reflux esophagitis | ||
| None | 18 | 42 |
| LA grade A | 9 | 21 |
| LA Grade B | 7 | 16 |
| LA Grade C | 5 | 12 |
| LA Grade D | 4 | 9 |
| Barrett’s | 1 | 2 |
| Symptoms | ||
| Heartburn | 32 | 74 |
| Regurgitation | 36 | 84 |
| Dysphagia | 20 | 47 |
| Chest pain | 25 | 58 |
| Atypical symptoms | 10 | 23 |
| Weight loss | 20 | 47 |
Complete workup consisting of esophagogastroduodenoscopy, 24-h esophageal pH-metry and Restech pH-metry, was available for 33 patients. Two patients did not tolerate the pH probe and 8 patients did not have a complete data set available for analysis. A total of 29 (88%) patients had an abnormal pH-metry as defined by a DeMeester Score of > 14.7. Restech pH-metry was abnormal in 20 (61%) patients as defined by a RYAN Score of 9.4 in upright and/or 6.8 in supine position using DataView 3 for analysis of measured pH data. Restech pH-metry was more commonly abnormal in upright position (n = 20) than in supine (n = 4) position (61 % vs 12%; P < 0.0001). However, patients with an abnormal supine RYAN score also had an abnormal upright score. All patients with an abnormal supine RYAN score also had abnormal acid exposure in supine position measured with esophageal pH-metry. Endoscopic findings were esophagitis in the esophageal remnant in half of the included patients, and Barrett’s esophagus in 1 patient.
Group A (abnormal Eso-pH, normal Restech): A total of 9 patients, (1 female), fulfilled inclusion criteria of subgroup A. All patients complained of heartburn. Other symptoms reported were primarily regurgitation, (89%), and chest pain. Two patients also reported extraesophageal reflux symptoms (cough). Patients had a severely abnormal esophageal acid exposure with a mean DeMeester score of 202.9 (range 27-308.5) and unobtrusive Restech results. Further details are depicted in Tables 3 and 4.
| Group A | Group C | Group D | ||||
| n | % | n | % | n | % | |
| Total | 9 | 100 | 20 | 100 | 4 | 100 |
| Females | 1 | 11 | 3 | 15 | 2 | 50 |
| Heartburn | 9 | 100 | 15 | 75 | 0 | 0 |
| Regurgitation | 8 | 89 | 17 | 85 | 1 | 25 |
| Dysphagia | 4 | 44 | 11 | 55 | 2 | 50 |
| Chest Pain | 8 | 89 | 11 | 55 | 0 | 0 |
| Weight loss | 5 | 56 | 10 | 50 | 2 | 50 |
| Extraesophageal reflux symptoms | 2 | 22 | 4 | 20 | 1 | 25 |
| Mean | Range | Mean | Range | Mean | Range | |
| Age (yr) | 59 | 48-66 | 61 | 46-77 | 69 | 58-77 |
| BMI (kg/m2) | 24.9 | 19.2-29.1 | 25 | 20.5-29.6 | 23.1 | 17.6-26.3 |
| Restech | ||||||
| RYAN upright | 2.36 | 2.12-4.26 | 84.8 | 10.6-381 | 2.12 | - |
| RYAN supine | 2.17 | - | 14.4 | 2.17 – 149.1 | 2.17 | - |
| Eso-pH | ||||||
| DeMeester score | 202.9 | 27-308.5 | 242 | 26.4-319.5 | 0 | - |
| % time pH < 4 | 36.4 | 6.2-71 | 29.1 | 3.7-90.7 | 0.55 | 0-1.9 |
| Gastric pH | 3.2 | 1.6-6.7 | 2.62 | 1.2-5.6 | 3.9 | 2-6.7 |
| Group A | Group C | Group D | ||||
| n | % | n | % | n | % | |
| Total | 9 | 100 | 20 | 100 | 4 | 100 |
| Anastomosis (cm) mean/range | 25 | 20-29 | 25 | 20-33 | 22 | 21-23 |
| Esophagitis | ||||||
| LA Grade A | 4 | 44 | 1 | 5 | 1 | 25 |
| LA Grade B | 1 | 11 | 4 | 20 | 0 | 0 |
| LA Grade C | 1 | 11 | 3 | 15 | 0 | 0 |
| LA Grade D | 1 | 11 | 2 | 10 | 0 | 0 |
| Barrett’s | 0 | 0 | 1 | 5 | 0 | 0 |
Group B (normal Eso-pH, abnormal Restech): No patients fulfilling criteria for this group were found in this collective of heavy volume reflux patients (University of Cologne Human Reflux model).
Group C (abnormal Eso-pH, abnormal Restech): A total of 20 patients, (3 females), fulfilled inclusion criteria of subgroup C. Symptoms reported were primarily heartburn, (85%), and regurgitation as well as chest pain. Four patients also complained of extraesophageal reflux symptoms. Patients suffered from severe esophageal acid exposure with an abnormal mean DeMeester score of 242, (range 26.4-319.5), and also abnormal Restech results with a mean RYAN score of 84.8, (range, 10.61-381), in upright position. Further details are depicted in Tables 3 and 4.
Group D (normal Eso-pH, normal Restech): A total of 4 patients, (2 females), fulfilled inclusion criteria of subgroup D. Symptoms reported were primarily dysphagia, (n = 2), and weight loss. Only one patient complained of extraesophageal reflux symptoms. Patients had a normal esophageal acid exposure with a mean DeMeester score of 0 and a normal Restech results with a mean RYAN score of 2.12 (range 2.12-2.17), in upright position. Further details are depicted in Tables 3 and 4.
Critical comparison of groups: Subgroup analysis of this study was based on our previous study[10], showing that different reflux scenarios exist and that those can be represented by four groups. Group A (abnormal Eso-pH, normal Restech) can physiologically be explained by reflux episodes that do not reach the oropharynx and therefore do not get detected by oropharyngeal pH testing. All patients in this group showed primarily typical reflux symptoms such as heartburn and regurgitation. Group B (normal Eso-pH, abnormal Restech) was previously described in patients with suspected GERD that underwent simultaneous esophageal and oropharyngeal pH testing. However, no patients fulfilled criteria for this group in this collective of heavy volume reflux patients (University of Cologne Human Reflux model). Group C (eso-pH abnormal, Restech abnormal) and group D (Eso-pH normal, Restech normal) show correlating results. Patients in group D showed a significantly lower symptom load than patients with an abnormal pH test. A correlation between extraesophageal reflux symptoms and group assignment could not be found. Endoscopic findings differed in our subgroups but were in alignment with pH test results. Endoscopy revealed reflux esophagitis in 78% of patients in group A, compared to 50% in group C and only 25% in group D. Severe esophagitis (LA Grade C or D) was present in 22% of patients in group A and 25% of group C compared to no patient in group D. Demographic factors did not differ in our group comparison and only trends could be found (P > 0.05). Only 12% (n = 4) showed normal test results for both pH tests validating the use of this selected cohort of patients as a human reflux model.
Measurement of gastric conduit acidity was available for 35 patients of our cohort. This depicts patients that underwent esophageal pH measurement using the conventional system. Mean gastric pH overall was 2.97 (range 1.2-6.7, median 2.5). Patients were further grouped according to length of follow up. Already shortly after surgery (group 1, follow up 3-6 mo) acidity of the gastric conduit almost normalized (mean pH 2.4; range 2-2.8). Mean pH in group 2 (Follow up 6-24 mo) was 3.4 (range 1.6-6.7). Group 3 (Follow up > 24 mo) showed a mean gastric pH of 2.4 (range 1.2-4.8). A comparison of groups 2 and 3 showed a clear trend of normalization of gastric pH in correlation with length of follow up (P = 0.0608). Further details are depicted in Table 5.
| Group 1 (3-6 mo follow up) | Group 2 (6-24 mo follow up) | Group 3 (> 24 mo follow up) | ||||
| n | % | n | % | n | % | |
| Total | 3 | 100 | 19 | 100 | 13 | 100 |
| Females | 2 | 67 | 5 | 26 | 0 | 0 |
| Esophagitis | ||||||
| LA Grade A | 0 | 0 | 5 | 26 | 1 | 8 |
| LA Grade B | 0 | 0 | 2 | 11 | 3 | 23 |
| LA Grade C | 0 | 0 | 1 | 5 | 3 | 23 |
| LA Grade D | 0 | 0 | 4 | 21 | 0 | 0 |
| Mean | Range | Mean | Range | Mean | Range | |
| Follow up time (d) | 127 | 106-167 | 414 | 209-652 | 1322 | 725-1966 |
| Gastric pH | 2.4 | 2 – 2.8 | 3.4 | 1.6-6.7 | 2.4 | 1.2-4.8 |
| DeMeester score | 159.5 | 0-273.5 | 197 | 0-313.4 | 281.3 | 116.5-319.5 |
Using the previously described subgroups A-D, a trend of correlation between pH test result and gastric conduit acidity can be seen between group C (abnormal Eso-pH, abnormal Restech) and group D (normal Eso-pH, normal Restech). Patients with an abnormal acid exposure showed a lower pH of their gastric conduit than patients with a normal test result (P = 0.073). Further details are depicted in Table 3 and 4.
As stated before, we believe that a variety of different reflux scenarios following Ivor-Lewis esophagectomy exist and that the 3 groups evaluated in our study explain these options in a logical way. It is in interesting to note that patients in all analyzed groups suffer from many different (including extraesophageal) reflux symptoms. Patients were informed upon inclusion into the study that only limited options for improvement of these symptoms exist, and that in contrast to patients that did not undergo esophagectomy for cancer, no surgical option such as fundoplication were possible. On the other hand, all included patients were happy to learn more about their altered anatomy and how to conservatively overcome reflux-related impairment of life.
Whereas we defined a group B (normal Eso-pH, abnormal Restech) in our previous study about the Restech device, no patients in this human reflux model fulfilled inclusion criteria for this group. Group B is not explainable from a pathophysiological standpoint and probably not valid for patients with volume reflux such as patients in this present collective. Patients without any anatomical alteration and mainly acidic gas vapor might be the ones that fall into this category.
Previous validation studies tried to prove corresponding results in oropharyngeal and esophageal pH-metry[24-26]. As stated with our four different groups from our previous study, we do not believe that Restech and eso-pH necessarily need to correspond. This thinking resulted from two groups with non-corresponding results: Group A, (abnormal Eso-pH, normal Restech), and Group B, (normal Eso-pH, abnormal Restech). Group A is physiologically explicable with reflux episodes that do not reach the oropharynx. Group B is more difficult to explain with esophageal reflux alone from a physiological standpoint. Results from our last paper indicated that acidic vapor or other factors may cause abnormal Restech results with simultaneous normal eso-pH results.
Previous studies showed only a weak correlation between esophageal and laryngopharyngeal pH measurement resulting in the conclusion that the Restech device adds no or little value as a diagnostic device in the evaluation of GERD and LPR[24-26]. However, only small numbers of patients were included in those studies (n = 10-36) and esophageal pH monitoring was either not performed simultaneously or not performed at all, leading to an insufficient comparison of results. In their critical report about laryngopharyngeal pH testing, Wilhelm at al. concluded from abnormal results of 6 out of 10 patients after gastrectomy that “pH values assessed by the Dx-pH device […] are obviously dissociated from gastric acid production”. Yet, no simultaneous esophageal pH testing for validation was performed. Another study focused on the correlation results of laryngopharyngeal pH testing and clinical findings during laryngoscopy. A significant correlation could not be found, however again only a small number of patients (n = 33) was included in the study and trends approaching statistical significance were noted[27]. In addition, Yadlapati et al[28] investigated the correlation between laryngopharyngeal pH testing and PPI response and found no significant correlation. Interestingly, only 35% of patients with atypical symptoms and a positive reflux symptom index showed a completed response to PPIs and 50% of patients showed no response at all. Another study of the same group showed that neither laryngopharyngeal pH testing nor salivary pepsin analysis are able to distinguish between reflux patients and healthy ones. Like many other studies, no esophageal pH testing was performed to validate the results[29]. In comparison, Vailati et al[30] have previously shown the Restech Dx-pH device to have a 69% sensitivity and 100% specificity for the responsiveness to medical therapy in patients with LPR, making it a valuable tool for those. The same group later showed a poor correlation between esophageal and laryngopharyngeal pH testing resulting in a currently rather low level of evidence for the use of the Restech device in patient evaluation[26]. The current normal values and discriminating pH thresholds of Restech were initially validated by a study group at University of Southern California in 2009 and 2010 using 55 and 81 normal subjects[7,8]. Later, the same institution published data suggesting that patients with abnormal results in pharyngeal pH monitoring might benefit from antireflux surgery[9]. The latter report is again limited by sample size, (n = 20), and the fact that that esophageal pH-metry and Restech were not performed simultaneously and may therefore not represent the same reflux scenario.
Another important issue addressed in this study focuses on the concern that current literature shows up to 50% of patients developing Neo-Barrett’s Esophagus above the anastomosis after Ivor-Lewis esophagectomy[31]. In addition, as we observed in a previous study, patients with known Barrett’s esophagus and adenocarcinoma have a higher risk of developing reflux-associated lesions in the remnant esophagus than patients with SCC. This group showed significantly less mucosal damage in the remnant esophagus given the same surgical approach[12]. Our established reflux model was based upon patients that underwent esophagectomy with gastric tube reconstruction resulting in limited esophageal motility and no reflux barrier being present. Hardly any studies examine the functional changes after esophagectomy with gastric tube reconstruction that can lead to mucosal damage in the remnant esophagus. Due to bilateral vagotomy during transthoracic resection, gastric acidity was thought to be reduced permanently. However, acidity of the gastric conduit can quickly recover even though bilateral vagotomy is performed[32]. Our data shows a normalization of gastric pH as early as 106 d after surgery. This phenomenon seems to heavily contribute to the occurrence of mucosal damage in the esophageal remnant and has important implications on the pathogenesis of Barrett’s esophagus and esophageal cancer.
Overall, the current level of evidence using the Restech device is limited by rather small case studies concluding that more research regarding the new reflux measurement device needs to be done. We have already added to the literature our large series of 101 patients with benign disease that were simultaneously measured by a standardized and validated system, and also by the Restech system. This present research using the University of Cologne Reflux model was needed and helped to better understand the different existing reflux scenarios as well as purely validate Restech in volume refluxers. In addition, one of our previous projects focused on the validation of the new software version DataView 4 for analysis of measured pH data with the Restech device, suggesting that improvements made to the new software version might increase quality of results and correlation with esophageal pH measurement[33].
Our study has some limitations that may be related to partly retrospective data analysis. Also, the limited sample size as a result of incomplete datasets might limit the conclusions that can be made. In addition, non-acid reflux episodes were not analyzed as no impedance pH-monitoring was performed. On the other hand, our collective of patients that undergo 24-h pH-monitoring with simultaneous 24-h Restech pH-monitoring after Ivor Lewis esophagectomy is to our knowledge quite unique in literature. Of significance, our study has several features and important implications on treatment of GERD patients with atypical reflux symptoms. Our study again emphasizes that important conclusions can be made from a Restech evaluation. Our key message as demonstrated by our 3 comparison groups in this human reflux model, representing different reflux scenarios, is that Eso-pH and Restech do not necessarily need to correspond. Nevertheless, all patients with an abnormal Restech evaluation also showed abnormal Eso-pH showing an evident relationship between both measurements. The Restech Dx-pH may therefore, in combination with upper gastrointestinal endoscopy, be a sufficient tool for evaluation of this patient group.
Patients following esophagectomy and reconstruction with gastric interposition can ideally serve as a human reflux model, as a large proportion suffers from severe postoperative GERD. Interestingly, laryngopharyngeal reflux phases occur mainly in the upright position, and acidity of the gastric conduit is already nearly normalized shortly after surgery.
In this human volume-reflux model, esophageal pH-metry correlated precisely with an abnormal laryngopharyngeal pH-metry (Restech).
There is no established correlation between 24-h esophageal pH-metry (Eso-pH) and the new laryngopharyngeal pH-monitoring system (Restech) as only small case series exist. Eso-pH was not designed to detect laryngopharyngeal reflux (LPR) and Restech may detect LPR better. We have previously published a dataset using the two techniques in a large patient collective with Gastroesophageal Reflux Disease. Anatomically, patients after esophagectomy were reported to represent an ideal human reflux model as no reflux barrier exists.
Patients after esophagectomy ideally serve as a human reflux model, as they show an impaired esophageal motility and no reflux barrier. This study aims to use this human reflux model to examine a previously established correlation between esophageal and laryngopharyngeal pH testing and to further validate laryngopharyngeal pH testing.
Previous validation studies tried to prove corresponding results in laryngopharyngeal and esophageal pH-metry. We, however, believe that a variety of different reflux scenarios exist and that those can be logically explained by our human reflux model in patients after Ivor-Lewis esophagectomy. Group A (abnormal Eso-pH, normal Restech) can easily be explained by reflux episodes that do not reach the oropharynx and are therefore not measured by laryngopharyngeal pH testing. Group B (normal Eso-pH, abnormal Restech) is not explainable from a pathophysiological standpoint. Results from our last paper indicated that acidic vapor or other factors may cause abnormal Restech results with simultaneous normal Eso-pH results. Previous studies showed only a weak correlation between esophageal and laryngopharyngeal pH measurement resulting in the conclusion that the Restech device adds no or little value as a diagnostic device in the evaluation of Gastroesophageal reflux disease (GERD) and LPR. However, only small numbers of patients were included in those studies and esophageal pH monitoring was either not performed simultaneously or not performed at all, leading to an insufficient comparison of results.
A prospective analysis of patient data during follow-up after hybrid minimally invasive Ivor Lewis esophagectomy for cancer was performed. To obtain a homogenous population, only patients at least 3 mo out of surgery and disease-free survival were included in this study to eliminate immediate postoperative effects. Demographics, endoscopic findings, biopsies at different follow-up time points, as well as tumor histology and stage were recorded in the prospective database. Additionally, symptoms were recorded at all times of follow-up. Only patients that presented with reflux-related symptoms or those showing endoscopic proof of mucosal damage in the esophageal remnant were offered inclusion in this study. Gastrointestinal function testing (simultaneous esophageal and laryngopharyngeal pH testing) as well as upper GI endoscopy was completed. No HRM or barium swallow was performed in this study. Subsequently, the relationship between the two techniques was evaluated.
A total of 43 patients from May 2016 - November 2018 were included. All patients presented with mainly typical reflux symptoms such as heartburn (74%), regurgitation (84%), chest pain (58%), and dysphagia (47%). Extraesophageal symptoms such as cough, hoarseness, asthma symptoms, and globus sensation were also present. Esophageal 24-hour pH-metry was abnormal in 88% of patients with a mean DeMeester Score of 229.45 [range 26.4-319.5]. Restech evaluation was abnormal in 61% of cases in this highly selective patient cohort. All patients with abnormal supine LPR were also abnormal for supine esophageal reflux measured by conventional eso-pH.
Patients following esophagectomy and reconstruction with gastric interposition can ideally serve as a human reflux model, as a large proportion suffers from severe postoperative GERD. Interestingly, laryngopharyngeal reflux phases occur mainly in the upright position, and acidity of the gastric conduit is already nearly normalized shortly after surgery. In this human volume-reflux model, esophageal pH-metry correlated precisely with an abnormal laryngopharyngeal pH-metry (Restech).
Overall, the current level of evidence using the Restech device is limited by rather small case studies concluding that more research regarding the new reflux measurement device needs to be done. In addition, patients after esophagectomy can ideally serve as a human reflux model for further investigations and validation studies.
Manuscript source: Invited manuscript
Specialty type: Research and experimental medicine
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salvatore S S-Editor: Gong ZM L-Editor: A P-Editor: Li JH
| 1. | Fuchs KH, Babic B, Breithaupt W, Dallemagne B, Fingerhut A, Furnee E, Granderath F, Horvath P, Kardos P, Pointner R, Savarino E, Van Herwaarden-Lindeboom M, Zaninotto G; European Association of Endoscopic Surgery (EAES). EAES recommendations for the management of gastroesophageal reflux disease. Surg Endosc. 2014;28:1753-1773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 2. | Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900-1920; quiz 1943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2368] [Cited by in F6Publishing: 2224] [Article Influence: 123.6] [Reference Citation Analysis (2)] |
| 3. | Hom C, Vaezi MF. Extra-esophageal manifestations of gastroesophageal reflux disease: diagnosis and treatment. Drugs. 2013;73:1281-1295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Naik RD, Vaezi MF. Extra-esophageal manifestations of GERD: who responds to GERD therapy? Curr Gastroenterol Rep. 2013;15:318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Schnatz PF, Castell JA, Castell DO. Pulmonary symptoms associated with gastroesophageal reflux: use of ambulatory pH monitoring to diagnose and to direct therapy. Am J Gastroenterol. 1996;91:1715-1718. [PubMed] [Cited in This Article: ] |
| 6. | Issing WJ, Karkos PD, Perreas K, Folwaczny C, Reichel O. Dual-probe 24-hour ambulatory pH monitoring for diagnosis of laryngopharyngeal reflux. J Laryngol Otol. 2004;118:845-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Ayazi S, Lipham JC, Hagen JA, Tang AL, Zehetner J, Leers JM, Oezcelik A, Abate E, Banki F, DeMeester SR, DeMeester TR. A new technique for measurement of pharyngeal pH: normal values and discriminating pH threshold. J Gastrointest Surg. 2009;13:1422-1429. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 112] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Ayazi S, Hagen JA, Zehetner J, Oezcelik A, Abate E, Kohn GP, Sohn HJ, Lipham JC, Demeester SR, Demeester TR. Proximal esophageal pH monitoring: improved definition of normal values and determination of a composite pH score. J Am Coll Surg. 2010;210:345-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Worrell SG, DeMeester SR, Greene CL, Oh DS, Hagen JA. Pharyngeal pH monitoring better predicts a successful outcome for extraesophageal reflux symptoms after antireflux surgery. Surg Endosc. 2013;27:4113-4118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Fuchs HF, Müller DT, Berlth F, Maus MK, Fuchs C, Dübbers M, Schröder W, Bruns CJ, Leers JM. Simultaneous laryngopharyngeal pH monitoring (Restech) and conventional esophageal pH monitoring-correlation using a large patient cohort of more than 100 patients with suspected gastroesophageal reflux disease. Dis Esophagus. 2018;31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Dresner SM, Griffin SM, Wayman J, Bennett MK, Hayes N, Raimes SA. Human model of duodenogastro-oesophageal reflux in the development of Barrett's metaplasia. Br J Surg. 2003;90:1120-1128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Fuchs HF, Schmidt HM, Meissner M, Brinkmann S, Maus M, Bludau M, Schröder W, Hölscher AH, Leers JM. Endoscopic and histopathologic reflux-associated mucosal damage in the remnant esophagus following transthoracic esophagectomy for cancer-5-year long-term follow-up. Dis Esophagus. 2018;31:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Moehler M, Al-Batran SE, Andus T, Anthuber M, Arends J, Arnold D, Aust D, Baier P, Baretton G, Bernhardt J, Boeing H, Böhle E, Bokemeyer C, Bornschein J, Budach W, Burmester E, Caca K, Diemer WA, Dietrich CF, Ebert M, Eickhoff A, Ell C, Fahlke J, Feussner H, Fietkau R, Fischbach W, Fleig W, Flentje M, Gabbert HE, Galle PR, Geissler M, Gockel I, Graeven U, Grenacher L, Gross S, Hartmann JT, Heike M, Heinemann V, Herbst B, Herrmann T, Höcht S, Hofheinz RD, Höfler H, Höhler T, Hölscher AH, Horneber M, Hübner J, Izbicki JR, Jakobs R, Jenssen C, Kanzler S, Keller M, Kiesslich R, Klautke G, Körber J, Krause BJ, Kuhn C, Kullmann F, Lang H, Link H, Lordick F, Ludwig K, Lutz M, Mahlberg R, Malfertheiner P, Merkel S, Messmann H, Meyer HJ, Mönig S, Piso P, Pistorius S, Porschen R, Rabenstein T, Reichardt P, Ridwelski K, Röcken C, Roetzer I, Rohr P, Schepp W, Schlag PM, Schmid RM, Schmidberger H, Schmiegel WH, Schmoll HJ, Schuch G, Schuhmacher C, Schütte K, Schwenk W, Selgrad M, Sendler A, Seraphin J, Seufferlein T, Stahl M, Stein H, Stoll C, Stuschke M, Tannapfel A, Tholen R, Thuss-Patience P, Treml K, Vanhoefer U, Vieth M, Vogelsang H, Wagner D, Wedding U, Weimann A, Wilke H, Wittekind C; AWMF; AWMF. [German S3-guideline "Diagnosis and treatment of esophagogastric cancer"]. Z Gastroenterol. 2011;49:461-531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 14. | Moehler M, Baltin CT, Ebert M, Fischbach W, Gockel I, Grenacher L, Hölscher AH, Lordick F, Malfertheiner P, Messmann H, Meyer HJ, Palmqvist A, Röcken C, Schuhmacher C, Stahl M, Stuschke M, Vieth M, Wittekind C, Wagner D, Mönig SP. International comparison of the German evidence-based S3-guidelines on the diagnosis and multimodal treatment of early and locally advanced gastric cancer, including adenocarcinoma of the lower esophagus. Gastric Cancer. 2015;18:550-563. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 15. | Hölscher AH, Stahl M, Messmann H, Stuschke M, Meyer HJ, Porschen R. [New S3 guideline for esophageal cancer : Important surgical aspects]. Chirurg. 2016;87:865-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Egberts JH, Biebl M, Perez DR, Mees ST, Grimminger PP, Müller-Stich BP, Stein H, Fuchs H, Bruns CJ, Hackert T, Lang H, Pratschke J, Izbicki J, Weitz J, Becker T. Robot-Assisted Oesophagectomy: Recommendations Towards a Standardised Ivor Lewis Procedure. J Gastrointest Surg. 2019;23:1485-1492. [PubMed] [Cited in This Article: ] |
| 17. | Straatman J, van der Wielen N, Cuesta MA, Daams F, Roig Garcia J, Bonavina L, Rosman C, van Berge Henegouwen MI, Gisbertz SS, van der Peet DL. Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial: the TIME Trial. Ann Surg. 2017;266:232-236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 327] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 18. | Briez N, Piessen G, Bonnetain F, Brigand C, Carrere N, Collet D, Doddoli C, Flamein R, Mabrut JY, Meunier B, Msika S, Perniceni T, Peschaud F, Prudhomme M, Triboulet JP, Mariette C. Open versus laparoscopically-assisted oesophagectomy for cancer: a multicentre randomised controlled phase III trial - the MIRO trial. BMC Cancer. 2011;11:310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Fuchs HF, Harnsberger CR, Broderick RC, Chang DC, Sandler BJ, Jacobsen GR, Bouvet M, Horgan S. Simple preoperative risk scale accurately predicts perioperative mortality following esophagectomy for malignancy. Dis Esophagus. 2017;30:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999;45:172-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1518] [Cited by in F6Publishing: 1521] [Article Influence: 60.8] [Reference Citation Analysis (1)] |
| 21. | Baretton GB, Aust DE. [Barrett's esophagus. An update]. Pathologe. 2012;33:5-16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Velanovich V. Comparison of generic (SF-36) vs. disease-specific (GERD-HRQL) quality-of-life scales for gastroesophageal reflux disease. J Gastrointest Surg. 1998;2:141-145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 149] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Eypasch E, Williams JI, Wood-Dauphinee S, Ure BM, Schmülling C, Neugebauer E, Troidl H. Gastrointestinal Quality of Life Index: development, validation and application of a new instrument. Br J Surg. 1995;82:216-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 852] [Cited by in F6Publishing: 841] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 24. | Becker V, Graf S, Schlag C, Schuster T, Feussner H, Schmid RM, Bajbouj M. First agreement analysis and day-to-day comparison of pharyngeal pH monitoring with pH/impedance monitoring in patients with suspected laryngopharyngeal reflux. J Gastrointest Surg. 2012;16:1096-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 25. | Wilhelm D, Jell A, Feussner H, Schmid RM, Bajbouj M, Becker V. Pharyngeal pH monitoring in gastrectomy patients - what do we really measure? United European Gastroenterol J. 2016;4:541-545. [PubMed] [Cited in This Article: ] |
| 26. | Mazzoleni G, Vailati C, Lisma DG, Testoni PA, Passaretti S. Correlation between oropharyngeal pH-monitoring and esophageal pH-impedance monitoring in patients with suspected GERD-related extra-esophageal symptoms. Neurogastroenterol Motil. 2014;26:1557-1564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Agrawal N, Yadlapati R, Shabeeb N, Price CP, Lidder A, Shintani-Smith S, Bové M, Pandolfino J, Tan B. Relationship between extralaryngeal endoscopic findings, proton pump inhibitor (PPI) response, and pH measures in suspected laryngopharyngeal reflux. Dis Esophagus. 2019;32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Yadlapati R, Pandolfino JE, Lidder AK, Shabeeb N, Jaiyeola DM, Adkins C, Agrawal N, Cooper A, Price CP, Ciolino JD, Gawron AJ, Smith SS, Bove M, Tan BK. Oropharyngeal pH Testing Does Not Predict Response to Proton Pump Inhibitor Therapy in Patients with Laryngeal Symptoms. Am J Gastroenterol. 2016;111:1517-1524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Yadlapati R, Adkins C, Jaiyeola DM, Lidder AK, Gawron AJ, Tan BK, Shabeeb N, Price CP, Agrawal N, Ellenbogen M, Smith SS, Bove M, Pandolfino JE. Abilities of Oropharyngeal pH Tests and Salivary Pepsin Analysis to Discriminate Between Asymptomatic Volunteers and Subjects With Symptoms of Laryngeal Irritation. Clin Gastroenterol Hepatol 2016; 14: 535-542. e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Vailati C, Mazzoleni G, Bondi S, Bussi M, Testoni PA, Passaretti S. Oropharyngeal pH monitoring for laryngopharyngeal reflux: is it a reliable test before therapy? J Voice. 2013;27:84-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Dunn LJ, Burt AD, Hayes N, Griffin SM. Columnar Metaplasia in the Esophageal Remnant After Esophagectomy: A Common Occurrence and a Valuable Insight Into the Development of Barrett Esophagus. Ann Surg. 2016;264:1016-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Gutschow C, Collard JM, Romagnoli R, Salizzoni M, Hölscher A. Denervated stomach as an esophageal substitute recovers intraluminal acidity with time. Ann Surg. 2001;233:509-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 79] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Müller DT, Schulte E, Babic B, Knepper L, Fuchs C, Schröder W, Bruns CJ, Leers JM, Fuchs HF. Software improvement for evaluation of laryngopharyngeal pH testing (Restech) - a comparison between DataView 3 and 4. World J Gastrointest Surg. 2020;12:236-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |