Published online Jan 15, 2021. doi: 10.4251/wjgo.v13.i1.58
Peer-review started: October 31, 2020
First decision: November 30, 2020
Revised: December 5, 2020
Accepted: December 17, 2020
Article in press: December 17, 2020
Published online: January 15, 2021
In recent years, two new narrow-band imaging (NBI) classifications have been proposed: The NBI international colorectal endoscopic (NICE) classification and Japanese NBI expert team (JNET) classification. Most validation studies of the two new NBI classifications were conducted in classification setting units by experienced endoscopists, and the application of use in different centers among endoscopists with different endoscopy skills remains unknown.
To evaluate clinical application and possible problems of NICE and JNET classification for the differential diagnosis of colorectal cancer and precancerous lesions.
Six endoscopists with varying levels of experience participated in this study. Eighty-seven consecutive patients with a total of 125 lesions were photographed during non-magnifying conventional white-light colonoscopy, non-magnifying NBI, and magnifying NBI. The three groups of endoscopic pictures of each lesion were evaluated by the six endoscopists in randomized order using the NICE and JENT classifications separately. Then we calculated the six endoscopists’ sensitivity, specificity, accuracy, positive predictive value, and negative predictive value for each category of the two classifications.
The sensitivity, specificity, and accuracy of JNET classification type 1 and 3 were similar to NICE classification type 1 and 3 in both the highly experienced endoscopist (HEE) and less-experienced endoscopist (LEE) groups. The specificity of JNET classification type 1 and 3 and NICE classification type 3 in both the HEE and LEE groups was > 95%, and the overall interobserver agreement was good in both groups. The sensitivity of NICE classification type 3 lesions for diagnosis of SM-d carcinoma in the HEE group was significantly superior to that in the LEE group (91.7% vs 83.3%; P = 0.042). The sensitivity of JNET classification type 2B lesions for the diagnosis of high-grade dysplasia or superficial submucosal invasive carcinoma in the HEE and LEE groups was 53.8% and 51.3%, respectively. Compared with other types of JNET classification, the diagnostic ability of type 2B was the weakest.
The treatment strategy of the two classification type 1 and 3 lesions can be based on the results of endoscopic examination. JNET type 2B lesions need further examination.
Core Tip: We evaluated the clinical application and possible problems of the narrow-band imaging international colorectal endoscopic (NICE) classification and Japanese NBI expert team (JNET) classification in our unit, which is a tertiary hospital in China. We found that the treatment strategy of NICE type 1 and 3 and JNET type 1, 2A and 3 lesions can be determined based on the results of endoscopic examination. Compared with other types of JNET classification, the diagnostic ability of type 2B is the weakest. The JNET type 2B lesions still needs further examinations, such as magnifying chromoendoscopy or endoscopic ultrasonography.
- Citation: Wang Y, Li WK, Wang YD, Liu KL, Wu J. Diagnostic performance of narrow-band imaging international colorectal endoscopic and Japanese narrow-band imaging expert team classification systems for colorectal cancer and precancerous lesions. World J Gastrointest Oncol 2021; 13(1): 58-68
- URL: https://www.wjgnet.com/1948-5204/full/v13/i1/58.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i1.58
Colorectal cancer (CRC) was the third most common malignancy and the second leading cause of cancer-related death globally in 2018[1]. The morbidity and mortality of CRC are still rising rapidly in many low- and middle-income countries[2]. The outcome and prognosis of patients with CRC are closely related to the stage of the disease. Miller et al[3] reported that the 5- and 10-year relative survival rates in CRC patients were 65% and 58%, respectively, but the 5-year relative survival rate was 90% when CRC was detected at a localized stage[3]. Therefore, it is important to improve the detection rate of early stage CRC and precancerous lesions.
Colorectal endoscopy can directly observe intestinal lesions, so it is irreplaceable in the examination of intestinal disease, especially CRC. To improve the detection rate of early-stage CRC and precancerous lesions, many new assistive techniques have been used in clinical practice such as chromoendoscopy, magnifying endoscopy, fluorescence endoscopy, confocal laser endoscopy, and electronic staining endoscopy. However, the process of chromoendoscopy is complicated and time-consuming, fluorescence endoscopy and confocal laser endoscopy are expensive, and these disadvantages limit the application of these new techniques.
Compared with these new techniques, electronic staining endoscopy is more convenient and practical, and its sensitivity and specificity in distinguishing colorectal neoplastic lesions from non-neoplastic lesions are about 90% and 85%, respectively[4]. Electronic staining endoscopy includes narrow-band imaging (NBI), flexile spectral imaging color enhancement, and i-scan, of which NBI is the most widely used. Since the emergence of NBI in 1999, it has been a reliable tool that has contributed to improving diagnostic accuracy, such as differentiation of neoplastic from non-neoplastic lesions and characterization of colorectal neoplasia[4,5]. Through the analysis of capillary vessel structure, surface structure and lesion color under NBI, researchers have proposed a variety of classifications to judge the nature of lesions accurately and select treatment strategy appropriately. In recent years, colorectal NBI magnifying classifications such as Hiroshima, Sano, Showa and Jikei classifications have been widely used in clinical practice and play an important role clinically[6,7]. However, magnifying endoscopy has not yet been widely applied outside of Japan.
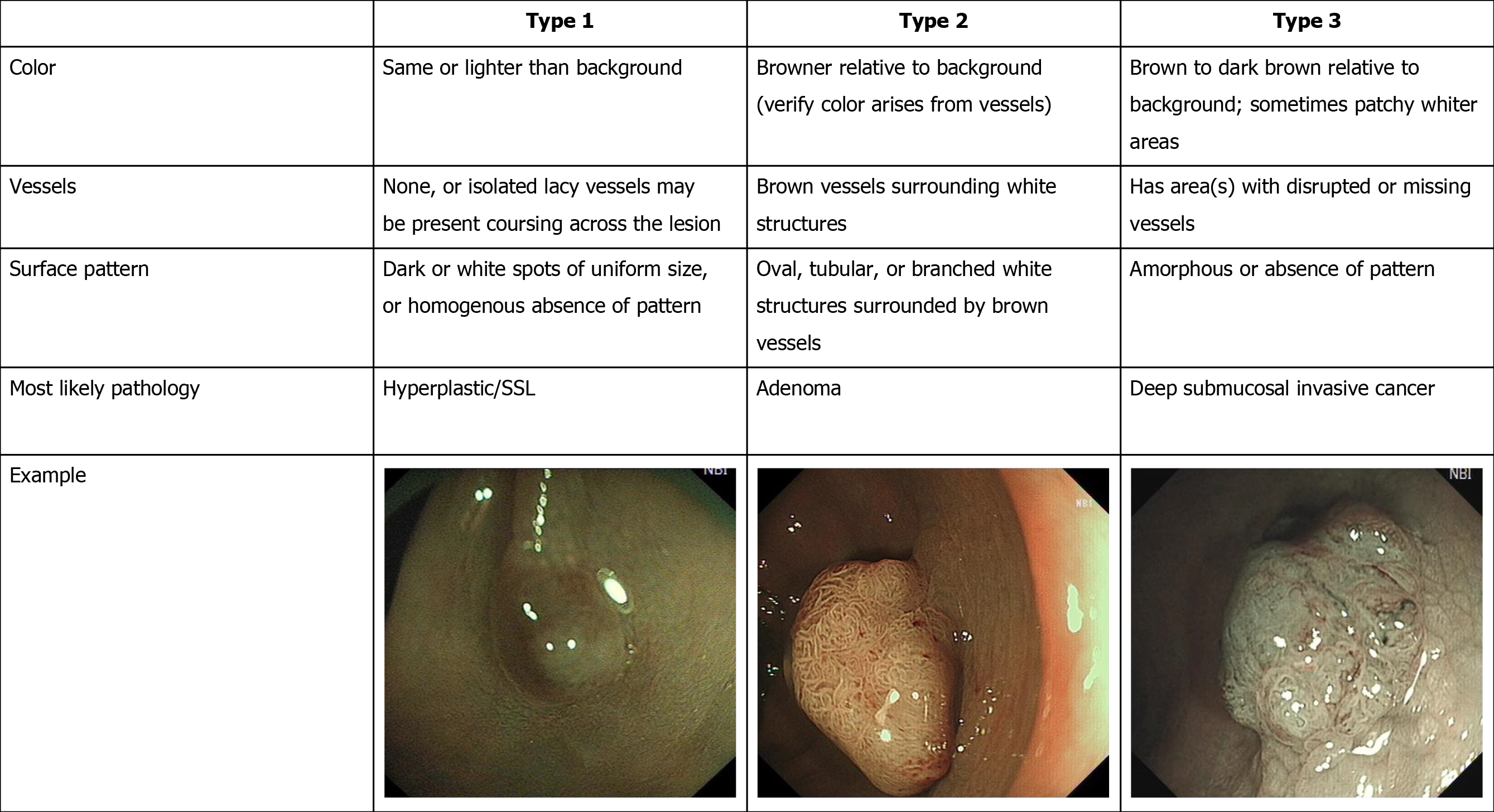
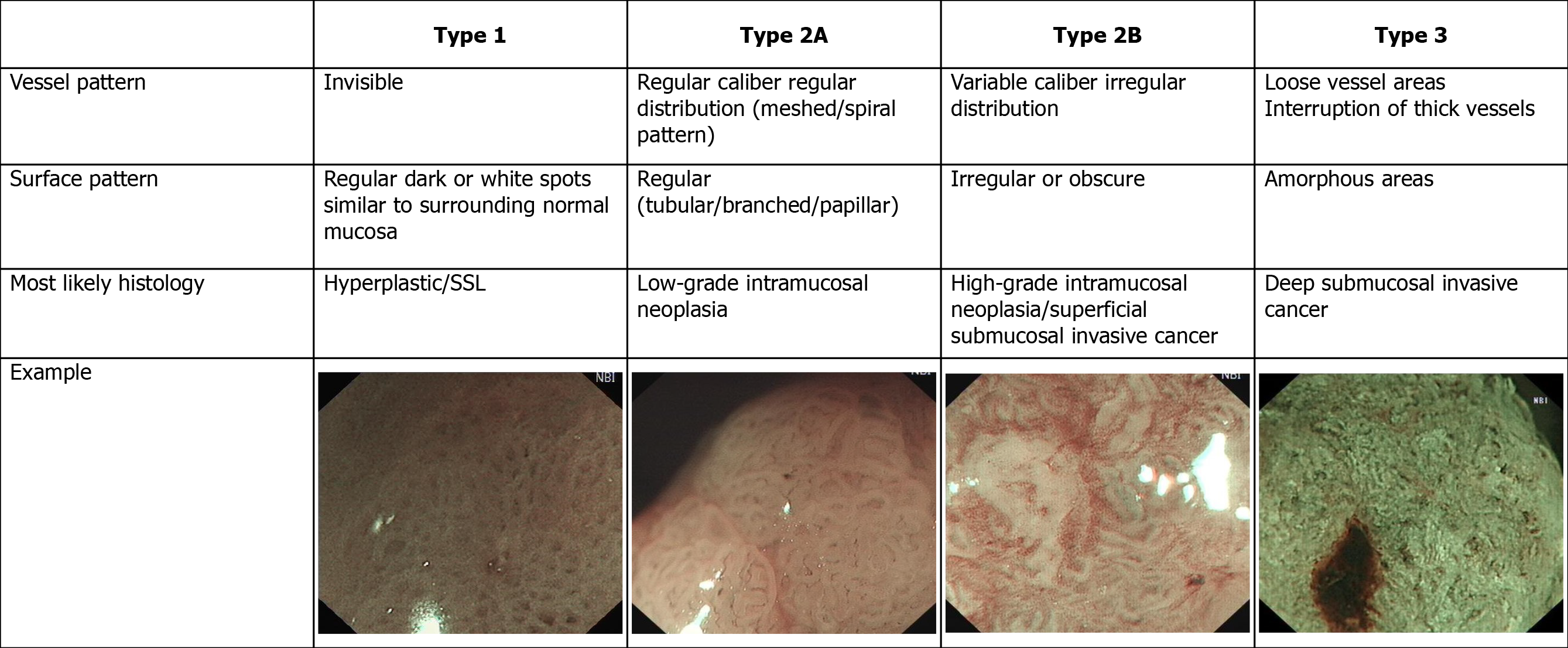
The Colon Tumor NBI Interest Group put forward a new NBI classification called the NBI international colorectal endoscopic (NICE) classification in 2009[8], and validation studies of this new NBI classification were conducted in 2012[9,10]. It is the first NBI classification that can be used for both non-magnifying and magnifying NBI endoscopy[8,11]. The NICE classification has a high diagnostic accuracy in detecting non-neoplastic lesions that do not require resection and deep submucosal invasive (SM-d) carcinoma that needs to be treated surgically[12,13]. However, it is difficult to differentiate high-grade dysplasia (HGD) or superficial submucosal invasive (SM-s) carcinoma from low-grade dysplasia (LGD)[8,14] using NICE classification. To solve this problem, the Japanese NBI expert team (JNET) composed of Japanese magnifying colonoscopists was organized in 2011, and a new NBI colorectal magnifying classification, the JNET classification was put forward in 2014[15].
To the best of our knowledge, most validation studies of the two new NBI classifications were conducted in originating centers by experienced endoscopists, but application in different centers among endoscopists with varying endoscopic skills remains unknown. To achieve external validity, in our study, we evaluated the clinical application and possible problems of NICE and JNET classifications in our unit, which is a tertiary hospital in China, and six endoscopists with varying levels of experience participated in this study.
From September 2014 to December 2019, we enrolled consecutive patients who received white-light colonoscopy, NBI colonoscopy, and magnifying NBI colonoscopy at the same time in Beijing Shijitan Hospital (Beijing, China). Informed consent was obtained from all patients before their examinations. Patients with inflammatory bowel disease, familial adenomatous polyposis, or incomplete clinical data were excluded from this study.
Patients drank 4 L of polyethylene glycol solution for their bowel preparation. A complete colonoscopy was performed by two experienced endoscopists, each of whom had previously performed > 1000 colonoscopies annually. All examinations were performed using magnifying colonoscopy (CF-H260AZI; Olympus Optical, Tokyo, Japan) and a standard videoendoscopic system (EVIS LUCERA; Olympus Optical), and magnifying images were taken with moderate-to-high-level power zoom. When a lesion was detected, the mucus and liquid feces on the surface of the lesion were washed away with lukewarm water. Endoscopic images of each lesion were taken in the following order: Non-magnifying conventional white-light colonoscopy, non-magnifying NBI, and magnifying NBI. The size of each lesion was estimated using the open-biopsy forceps method, with an open diameter of 7 mm (Radial Jaw 3; Boston Scientific Corp., Natick, MA, United States). The locations of the lesions were divided into three groups (proximal colon, distal colon, and rectum). Lesions were resected by biopsy, endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD), and the biopsy was analyzed histopathologically. The histopathological diagnosis was based on World Health Organization criteria.
The NICE classification[9,10] and JNET classification[15], and typical examples of the endoscopic images used in our study are shown in Figures 1 and 2.
Six endoscopists with varying levels of experience participated in the present study. The endoscopists were divided into two groups: A group of less-experienced endoscopists (LEE group) who had carried out colonoscopies for > 5 years but not with magnifying NBI, and a group of highly experienced endoscopists (HEE group) who had routinely used magnifying NBI for > 5 years[16]. The three groups of endoscopic pictures of each lesion (non-magnifying white-light colonoscopy, non-magnifying NBI, and magnifying NBI) were evaluated by the six endoscopists in a randomized order using the NICE and JENT classifications separately. The non-magnifying white-light colonoscopy and non-magnifying NBI images demonstrated an overview of each lesion in order to mimic real-time endoscopic examination, whereas the magnifying NBI images showed crucial findings to evaluate the histopathological features. Patients information such as age, sex, clinical diagnosis, and histopathological results was not disclosed to any of the evaluators, and discussions were not permitted among the endoscopists individually or in groups.
We calculated sensitivity, specificity, accuracy, positive predictive value (PPV), and negative predictive value (NPV) for each category of the two classifications. We received histology of colorectal lesions as the gold standard. Sensitivity, specificity, accuracy, PPV, and NPV of each category of the two classifications were compared between the two groups by using the Mann-Whitney U test. P < 0.05 was considered statistically significant. In addition, the interobserver agreement in each group was assessed using k values as follows: < 0.4, poor agreement; 0.41-0.60, fair agreement; 0.61-0.80, good agreement; and > 0.80, excellent agreement. All statistical analyses were conducted using SPSS Statistics for Windows version 22.0 (IBM Corp., Armonk, NY, United States). The statistical methods of this study were reviewed by Qing-Kun Song from Beijing Shijitan Hospital, Capital Medical University.
Ninety-six patients received white-light colonoscopy, NBI colonoscopy and magnifying NBI colonoscopy at the same time between September 2014 and December 2019, and 137 lesions were resected. Nine patients with inflammatory bowel disease, familial adenomatous polyposis, or incomplete clinical data were excluded. Finally, 87 consecutive patients were enrolled for endoscopic evaluation, and 125 lesions were photographed during non-magnifying conventional white-light colonoscopy, non-magnifying NBI, and magnifying NBI. Bowel preparation was achieved perfectly and complete colonoscopy was performed to the cecum in every patient. Demographic data and characteristics of the lesions such as size, location and pathology are shown in Table 1.
| Variables | Number |
| Patients | 87 |
| Lesions | 125 |
| Sex, male/female | 61; 26 |
| Age in yr, mean ± SD; range | 59.9 ± 10.6; 34-89 |
| Location | |
| Proximal colon | 42 |
| Distal colon | 61 |
| Rectum | 22 |
| Size in mm, mean ± SD; range | 14.3 ± 0.7; 4-45 |
| 5 | 9 |
| 6-10 | 44 |
| 11-20 | 58 |
| ≥ 20 | 14 |
| Morphology | |
| Ip | 22 |
| Isp | 39 |
| Is | 30 |
| IIa | 31 |
| IIb | 3 |
| Pathology | |
| Hyperplastic or sessile serrated lesion | 13 |
| Tubular adenoma | 67 |
| Tubulovillous adenoma | 27 |
| Low-grade intramucosal neoplasia | 1 |
| High-grade intramucosal neoplasia | 3 |
| Superficial submucosal invasive cancer | 10 |
| Deep submucosal invasive cancer | 4 |
The diagnostic characteristics of each category among the two groups are shown in Table 2. The sensitivity, specificity, accuracy, PPV and NPV of type 1 lesions for the diagnosis of hyperplastic lesions (HPLs) and sessile serrated lesions (SSLs) in the HEE group were 84.6%, 94.9%, 93.9%, 65.9%, and 98.2%, respectively, and 82.1%, 93.8%, 92.5%, 60.4%, and 97.8%, respectively, in the LEE group. The sensitivity, specificity, accuracy, PPV and NPV of type 2 lesions for the diagnosis of adenoma in the HEE group were 91.4%, 86.3%, 90.7%, 97.7%, and 61.2%, respectively, and 89.8%, 84.3%, 89.1%, 97.3%, and 56.6%, respectively, in the LEE group. The sensitivity, specificity, accuracy, PPV and NPV of type 3 lesions for the diagnosis of SM-d carcinoma in the HEE group were 91.7%, 97.0%, 96.8%, 54.0%, and 99.7%, respectively, and 83.3%, 96.4%, 96.0%, 45.8%, and 99.4%, respectively, in the LEE group. Except for sensitivity of type 3 lesions for diagnosis of SM-d carcinoma in the HEE group was significantly superior to that in the LEE group (91.7% vs 83.3%; P = 0.042), the diagnostic characteristic of each category of the NICE classification was comparable, and there were no significant differences between the two groups. The overall interobserver agreement was good in both groups (κ = 0.751 in HEE group, and κ = 0.744 in LEE group).
| NICE | Group1 | Sensitivity, % (95%CI) | Specificity, % (95%CI) | Accuracy, % (95%CI) | PPV, % (95%CI) | NPV, % (95%CI) |
| Type 1 | HEE | 84.6 (65.5-100.0) | 94.9 (93.7-96.2) | 93.9 (90.8-96.9) | 65.9 (55.6-76.3) | 98.2 (95.9-100.0) |
| LEE | 82.1 (60.0-100.0) | 93.8 (91.5-96.0) | 92.5 (88.4-96.7) | 60.4 (46.2-74.5) | 97.8 (95.2-100.0) | |
| P = 0.637 | P = 0.105 | P = 0.275 | P = 0.275 | P = 0.376 | ||
| Type 2 | HEE | 91.4 (88.7-94.0) | 86.3 (77.8-94.7) | 90.7 (89.5-91.8) | 97.7 (96.4-99.0) | 61.2 (56.0-66.4) |
| LEE | 89.8 (87.5-92.1) | 84.3 (75.9-92.8) | 89.1 (86.8-91.4) | 97.3 (96.0-98.7) | 56.6 (50.6-62.6) | |
| P = 0.105 | P = 0.456 | P = 0.043 0.1 | P = 0.121 | P = 0.100 | ||
| Type 3 | HEE | 91.7 (55.8-100.0) | 97.0 (92.2-100.0) | 96.8 (91.5-100.0) | 54.0 (-2.7-100.0) | 99.7 (99.5-100.0) |
| LEE | 83.3 (47.5-100.0) | 96.4 (92.1-100.0) | 96.0 (90.7-100.0) | 45.8 (0.7-91.0) | 99.4 (98.2-100.0) | |
| P = 0.042 | P = 0.487 | P = 0.367 | P = 0.376 | P = 0.346 |
The diagnostic characteristics of each category between the two groups are shown in Table 3. The sensitivity, specificity, accuracy, PPV and NPV of type 1 lesions for the diagnosis of HPLs and SSLs in the HEE group were 87.1%, 97.3%, 95.5%, 74.1%, and 98.5%, respectively, and 84.6%, 96.4%, 95.2%, 73.4%, and 98.2%, respectively, in the LEE group. The sensitivity, specificity, accuracy, PPV and NPV of type 2A lesions for the diagnosis of LGD in the HEE group were 82.5%, 90.0%, 81.9%, 93.3%, and 58.5%, respectively, and 82.5%, 91.1%, 84.5%, 96.7%, and 62.1%, respectively, in the LEE group. The sensitivity, specificity, accuracy, PPV and NPV of type 2B lesions for the diagnosis of HGD-SM-s carcinoma in the HEE group were 53.8%, 84.2%, 81.4%, 31.5%, and 92.2%, respectively, and 51.3%, 84.8%, 81.3%, 28.3%, and 93.8%, respectively, in the LEE group. The sensitivity, specificity, accuracy, PPV and NPV of type 3 lesions for the diagnosis of SM-d carcinoma in the HEE group were 91.7%, 98.1%, 97.9%, 63.2%, and 99.7%, respectively, and 83.3%, 98.4%, 97.9%, 63.3%, and 99.4%, respectively, in the LEE group. The overall interobserver agreement was good in both groups (κ = 0.747 in HEE group, κ = 0.759 in LEE group).
| JNET | Group1 | Sensitivity, % (95%CI) | Specificity, % (95%CI) | Accuracy, % (95%CI) | PPV, % (95%CI) | NPV, % (95%CI) |
| Type 1 | HEE | 87.1 (76.2-98.2) | 97.3 (95.1-99.5) | 95.5 (92.4-98.5) | 74.1 (60.0-88.1) | 98.5 (97.2-99.8) |
| LEE | 84.6 (65.5-100.0) | 96.4 (94.2-98.6) | 95.2 (91.8-98.6) | 73.4 (58.8-88.0) | 98.2 (96.0-100.0) | |
| P = 0.369 | P = 0.261 | P = 0.637 | P = 0.822 | P = 0.500 | ||
| Type2A | HEE | 82.5 (78.5-86.5) | 90.0 (81.7-98.3) | 81.9 (72.7-91.1) | 93.3 (77.6-108.9) | 58.5 (47.0-69.9) |
| LEE | 82.5 (81.0-84.0) | 91.1 (86.3-95.9) | 84.5 (83.4-85.7) | 96.7 (95.0-98.4) | 62.1 (60.3-63.9) | |
| P = 0.817 | P = 0.637 | P = 0.099 | P = 0.376 | P = 0.077 | ||
| Type 2B | HEE | 53.8 (34.7-73.0) | 84.2 (73.1-95.4) | 81.4 (69.2-93.5) | 31.5 (16.4-46.6) | 92.2 (81.5-100.0) |
| LEE | 51.3 (29.2-73.4) | 84.8 (81.0-88.7) | 81.3 (75.6-87.1) | 28.3 (14.2-42.3) | 93.8 (90.8-96.7) | |
| P = 0.817 | P = 0.825 | P = 0.825 | P = 0.268 | P = 0.825 | ||
| Type 3 | HEE | 91.7 (55.8-100.0) | 98.1 (94.9-100.0) | 97.9 (93.7-100.0) | 63.2 (16.4-100.0) | 99.7 (98.5-100.0) |
| LEE | 83.3 (47.5-100.0) | 98.4 (96.3-100.0) | 97.9 (94.8-100.0) | 63.3 (25.4-100.0) | 99.4 (98.3-100.0) | |
| P = 0.456 | P = 0.822 | P = 0.856 | P = 0.891 | P = 0.817 |
Colorectal adenoma is a precancerous lesion of CRC, and its resection can reduce the incidence and mortality of CRC; therefore, in western countries, removal of all adenomatous polyps has been standardized[17,18]. In clinical practice, the pathological diagnosis of all resected polyps is routinely performed, and the final pathological result determines the intervention of endoscopic follow-up[19]. However, the removal of all polyps and routine pathological diagnosis not only increase the risks associated with the resection process, but also the cost of both the operation and the pathological diagnosis. Therefore, the resect and discard policy has been proposed[20-22]. The policy states that the HPL do not need treatment, and the treatment of these lesions may increase the adverse events of polypectomy and cost of medical care[19,23-25]. As reported previously, the NICE classification is simple and practical in identifying HLP that should be left[8,26]. In our study, the sensitivity, specificity and accuracy of NICE classification type 1 lesions for the diagnosis of HPLs and SSLs in both the HEE and LEE groups were > 80%, with specificity and accuracy > 90%, with no significant difference between the two groups. This result shows that endoscopists can choose the treatment plan based on the NICE classification, which may improve the resect and discard strategy better promote.
The HEE group still had high specificity and accuracy > 95% when using NICE type 3 to diagnose SM-d carcinoma, and the sensitivity was 91.7%, but in the LEE group the sensitivity was only 83.3%. The P value of the sensitivity between the two groups was 0.042 by the Mann-Whitney U test, and the difference between the two groups for diagnosis of SM-d carcinoma was significant. Hayashi et al[10] found that the sensitivity of NICE type 3 for the diagnosis of SM-d carcinoma was 94.9%[10]. Compared with the study above, the sensitivity of the LEE group in the diagnosis of SM-d carcinoma was still low. This result may be related to the lack of experience in the diagnosis of SM-d carcinoma in the LEE group. Therefore, endoscopists in the LEE group should receive more training to avoid missed diagnosis of SM-d carcinoma.
To obtain a precise histological diagnosis, HGD or SM-s carcinoma should be resected by en bloc EMR/ESD or surgery rather than piecemeal EMR (pEMR). However, in clinical practice, we cannot determine the strategy of endoscopic treatment, such as pEMR, en bloc EMR/ESD or surgery, because NICE type 2 is difficult to differentiate HGD or SM-s carcinoma from LGD[8,14]. To solve this problem and unify the current NBI classifications, the JNET classification with magnification was proposed[15]. The principles and characteristics of the JNET classification are as follows: Mmagnification is essential and the basis is the NICE classification; NICE type 2 is divided into 2A and 2B subtypes using magnifying findings; Because magnifi-cation does not need estimation of color, the classification does not include the finding of color; and basic findings are composed of both vessel and surface patterns[27].
Our results suggested that the sensitivity, specificity and accuracy of JNET classification types 1 and 3 were similar to NICE classification types 1 and 3 in both the HEE and LEE groups, and the specificity of JNET classification types 1 and 3 and NICE classification type 3 in both the HEE and LEE groups was > 95%. The sensitivity, specificity, accuracy, PPV and NPV of JNET classification type 2A lesions for the diagnosis of LGD in the HEE group were 82.5%, 90.0%, 81.9%, 93.3%, and 58.5%, respectively, and 82.5%, 91.1%, 84.5%, 96.7%, and 62.1%, respectively, in the LEE group. The results are similar to those of Sumimoto et al[27]. In order to avoid missed diagnosis of lesions, the sensitivity of the classification is important. However, before treatment of the lesion, the specificity of the classification is more important, because only by accurately determining the nature of the lesion can the appropriate treatment strategy be selected. In our study, the specificity of NICE types 1 and 3 and JNET types 1, 2A and 3 in both the HEE and LEE groups was > 90%. So, when the endoscopist’s diagnostic confidence level is high (> 95%)[27], the treatment strategy for NICE types 1 and 3 and JNET types 1, 2A and 3 lesions can be determined based on the results of endoscopic examination. Of course, if the confidence level is low, an additional examination should be performed.
The JNET type 2B lesions are the most important for curation and the most difficult to be diagnosed endoscopically. In our study, the sensitivity of JNET classification type 2B lesions for the diagnosis of HGD-SM-s carcinoma in the HEE group was 53.8% and 51.3% in the LEE group. As our result, even in the HEE group the sensitivity was not more than 60%. Previous studies showed that the sensitivity of JNET classification Type 2B lesions for diagnosis of HGD-SM-s carcinoma was 44.9%-61.9%[27,28]. Compared with other types of JNET classification, the diagnostic ability of type 2B is the weakest. Although Sumimoto et al[29] further divided JNET type 2B into 2B-low and 2B-high[29], the ability to diagnose HGD-SM-s carcinoma has not been significantly improved. The original intention of the JNET classification introduce the type 2A and 2B lesion was to distinguish LGD and SM-d carcinoma from HGD-SM-s carcinoma, and then to choose an appropriate treatment strategy, such as pEMR, en bloc EMR/ESD or surgery. However, due to poor diagnostic capabilities of type 2B, this goal cannot be achieved, and the type 2B lesions is still the biggest challenge for the endoscopists. So, lesions of type 2B need a further pit pattern diagnosis using magnifying chromoendoscopy or endoscopic ultrasound[29-31].
Our study had some limitations. First, although six endoscopists with varying levels of experience participated in the study, they all belonged to the same institution and would be following similar guidelines, which may produce high interobserver agreement and threaten the external validity of the results. These results might be different when endoscopists belong to different units. Second, we initially presented non-magnifying conventional white-light overview images of entire lesion in order to mimic real-time endoscopic examination. For some endoscopists, the first observation of an entire lesion may affect their diagnosis of the lesion, especially when evaluating the lesion using the JNET classification. It is necessary for us to conduct a further study where we evaluate JNET classification using only magnifying NBI images of the lesions. Third, as a retrospective study, we enrolled as many cases as possible. However, in clinical practice, most SM-d carcinoma can be correctly diagnosed under white-light colonoscopy without further magnifying examination, so there were only four cases of deep-submucosal invasive cancer. The diagnostic accuracy and reliability of NICE and JNET classifications should be validated in a multicenter prospective study.
In conclusion, NICE types 1 and 3 and JNET types 1, 2A and 3 lesions showed excellent diagnostic ability in both the HEE and LEE groups. When the confidence level is high, the treatment strategy of the NICE types 1 and 3 and JNET types 1, 2A and 3 lesions can be determined based on the results of endoscopic examination. JNET type 2B lesions require extra examination, such as magnifying chromoendoscopy or endoscopic ultrasound, to make an accurate assessment of the invasion depth for selecting an appropriate treatment strategy.
Detecting and treating early stage colorectal cancer (CRC) and precancerous lesions is the most effective method to reduce the morbidity and mortality of CRC. Narrow-band imaging (NBI) endoscopy has been a very useful technique that has contributed to improving the detection rate of early stage CRC and precancerous lesions. Researchers have proposed a variety of NBI classifications to judge the nature of lesions accurately and select treatment strategy appropriately.
For the past few years, two new NBI classifications have been proposed: The NBI international colorectal endoscopic (NICE) classification and Japanese NBI expert team (JNET) classification. Most validation studies of the two new NBI classifications were conducted in originating centers by experienced endoscopists, but application in different centers among endoscopists with varying endoscopic skills remains unknown.
To achieve external validity, we evaluated the clinical application and possible problems of the NICE and JNET classifications for differential diagnosis of colorectal cancer and precancerous lesions.
Six endoscopists with varying levels of experience were divided into two groups: Highly experienced endoscopists (HEEs) and less-experienced endoscopists (LEE). Eighty-seven consecutive patients with a total of 125 lesions were photographed during non-magnifying conventional white-light colonoscopy, non-magnifying NBI, and magnifying NBI. The three groups of endoscopic pictures of each lesion were evaluated by the six endoscopists in a randomized order using the NICE and JENT classifications separately. We calculated sensitivity, specificity, accuracy, positive and negative predictive value for each category of the two classifications.
In both the HEE and LEE groups, the specificity of JNET classification type 1 and 3 and NICE classification type 3 was > 95%, and the overall interobserver agreement was good in both groups. However, the sensitivity of JNET classification type 2B lesions for the diagnosis of high-grade dysplasia or superficial submucosal invasive carcinoma in both the HEE and LEE groups was < 55%. Compared with other types of NICE and JNET classification, the diagnostic ability of JNET type 2B was the weakest.
Due to the poor diagnostic capabilities of JNET type 2B, the type 2B lesions is still the biggest challenge for the endoscopists. So, lesions of type 2B need an additional examination to choose an appropriate treatment strategy.
The JNET type 2B lesions are the most important for curation and the most difficult to be diagnosed endoscopically, and accurate diagnosis of JNET 2B lesions still requires further efforts.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eleftheriadis N, Zaniboni A S-Editor: Fan JR L-Editor: Filipodia P-Editor: Li JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Wanders LK, East JE, Uitentuis SE, Leeflang MM, Dekker E. Diagnostic performance of narrowed spectrum endoscopy, autofluorescence imaging, and confocal laser endomicroscopy for optical diagnosis of colonic polyps: a meta-analysis. Lancet Oncol. 2013;14:1337-1347. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | McGill SK, Evangelou E, Ioannidis JP, Soetikno RM, Kaltenbach T. Narrow band imaging to differentiate neoplastic and non-neoplastic colorectal polyps in real time: a meta-analysis of diagnostic operating characteristics. Gut. 2013;62:1704-1713. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Oka S, Tanaka S, Takata S, Kanao H, Chayama K. Clinical usefulness of narrow band imaging magnifying classification for colorectal tumors based on both surface pattern and microvessel features. Dig Endosc. 2011;23 Suppl 1:101-105. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Hirata I, Nakagawa Y, Ohkubo M, Yahagi N, Yao K. Usefulness of magnifying narrow-band imaging endoscopy for the diagnosis of gastric and colorectal lesions. Digestion. 2012;85:74-79. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Tanaka S, Sano Y. Aim to unify the narrow band imaging (NBI) magnifying classification for colorectal tumors: current status in Japan from a summary of the consensus symposium in the 79th Annual Meeting of the Japan Gastroenterological Endoscopy Society. Dig Endosc. 2011;23 Suppl 1:131-139. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Hewett DG, Kaltenbach T, Sano Y, Tanaka S, Saunders BP, Ponchon T, Soetikno R, Rex DK. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology 2012; 143: 599-607. e1. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Hayashi N, Tanaka S, Hewett DG, Kaltenbach TR, Sano Y, Ponchon T, Saunders BP, Rex DK, Soetikno RM. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc. 2013;78:625-632. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Hattori S, Iwatate M, Sano W, Hasuike N, Kosaka H, Ikumoto T, Kotaka M, Ichiyanagi A, Ebisutani C, Hisano Y, Fujimori T, Sano Y. Narrow-band imaging observation of colorectal lesions using NICE classification to avoid discarding significant lesions. World J Gastrointest Endosc. 2014;6:600-605. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Oba S, Tanaka S, Oka S, Kanao H, Yoshida S, Shimamoto F, Chayama K. Characterization of colorectal tumors using narrow-band imaging magnification: combined diagnosis with both pit pattern and microvessel features. Scand J Gastroenterol. 2010;45:1084-1092. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Wani S, Rastogi A. Narrow-band imaging in the prediction of submucosal invasive colon cancer: how "NICE" is it? Gastrointest Endosc. 2013;78:633-636. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Tanaka S, Hayashi N, Oka S, Chayama K. Endoscopic assessment of colorectal cancer with superficial or deep submucosal invasion using magnifying colonoscopy. Clin Endosc. 2013;46:138-146. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Kaneko K, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Iwatate M, Ishikawa H, Murakami Y, Yoshida S, Saito Y. Narrow-band imaging (NBI) magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Dig Endosc. 2016;28:526-533. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Higashi R, Uraoka T, Kato J, Kuwaki K, Ishikawa S, Saito Y, Matsuda T, Ikematsu H, Sano Y, Suzuki S, Murakami Y, Yamamoto K. Diagnostic accuracy of narrow-band imaging and pit pattern analysis significantly improved for less-experienced endoscopists after an expanded training program. Gastrointest Endosc. 2010;72:127-135. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Pohl H, Robertson DJ. Confidence with narrow band imaging: will it change our practice of polyp resection? Gastroenterology. 2009;136:1149-1151. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Rex DK, Kahi C, O'Brien M, Levin TR, Pohl H, Rastogi A, Burgart L, Imperiale T, Ladabaum U, Cohen J, Lieberman DA. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419-422. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844-857. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Ignjatovic A, East JE, Suzuki N, Vance M, Guenther T, Saunders BP. Optical diagnosis of small colorectal polyps at routine colonoscopy (Detect InSpect ChAracterise Resect and Discard; DISCARD trial): a prospective cohort study. Lancet Oncol. 2009;10:1171-1178. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Hassan C, Pickhardt PJ, Rex DK. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol 2010; 8: 865-869, 869.e1-869. e3. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Takeuchi Y, Hanafusa M, Kanzaki H, Ohta T, Hanaoka N, Yamamoto S, Higashino K, Tomita Y, Uedo N, Ishihara R, Iishi H. An alternative option for "resect and discard" strategy, using magnifying narrow-band imaging: a prospective "proof-of-principle" study. J Gastroenterol. 2015;50:1017-1026. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology. 2009;136:1174-1181. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Hewett DG, Huffman ME, Rex DK. Leaving distal colorectal hyperplastic polyps in place can be achieved with high accuracy by using narrow-band imaging: an observational study. Gastrointest Endosc. 2012;76:374-380. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Rex DK, Clodfelter R, Rahmani F, Fatima H, James-Stevenson TN, Tang JC, Kim HN, McHenry L, Kahi CJ, Rogers NA, Helper DJ, Sagi SV, Kessler WR, Wo JM, Fischer M, Kwo PY. Narrow-band imaging versus white light for the detection of proximal colon serrated lesions: a randomized, controlled trial. Gastrointest Endosc. 2016;83:166-171. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Raghavendra M, Hewett DG, Rex DK. Differentiating adenomas from hyperplastic colorectal polyps: narrow-band imaging can be learned in 20 minutes. Gastrointest Endosc. 2010;72:572-576. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Sumimoto K, Tanaka S, Shigita K, Hirano D, Tamaru Y, Ninomiya Y, Asayama N, Hayashi N, Oka S, Arihiro K, Yoshihara M, Chayama K. Clinical impact and characteristics of the narrow-band imaging magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Gastrointest Endosc. 2017;85:816-821. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Iwatate M, Sano Y, Tanaka S, Kudo SE, Saito S, Matsuda T, Wada Y, Fujii T, Ikematsu H, Uraoka T, Kobayashi N, Nakamura H, Hotta K, Horimatsu T, Sakamoto N, Fu KI, Tsuruta O, Kawano H, Kashida H, Takeuchi Y, Machida H, Kusaka T, Yoshida N, Hirata I, Terai T, Yamano HO, Nakajima T, Sakamoto T, Yamaguchi Y, Tamai N, Nakano N, Hayashi N, Oka S, Ishikawa H, Murakami Y, Yoshida S, Saito Y; Japan NBI Expert Team (JNET). Validation study for development of the Japan NBI Expert Team classification of colorectal lesions. Dig Endosc. 2018;30:642-651. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Sumimoto K, Tanaka S, Shigita K, Hayashi N, Hirano D, Tamaru Y, Ninomiya Y, Oka S, Arihiro K, Shimamoto F, Yoshihara M, Chayama K. Diagnostic performance of Japan NBI Expert Team classification for differentiation among noninvasive, superficially invasive, and deeply invasive colorectal neoplasia. Gastrointest Endosc. 2017;86:700-709. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Komeda Y, Kashida H, Sakurai T, Asakuma Y, Tribonias G, Nagai T, Kono M, Minaga K, Takenaka M, Arizumi T, Hagiwara S, Matsui S, Watanabe T, Nishida N, Chikugo T, Chiba Y, Kudo M. Magnifying Narrow Band Imaging (NBI) for the Diagnosis of Localized Colorectal Lesions Using the Japan NBI Expert Team (JNET) Classification. Oncology. 2017;93 Suppl 1:49-54. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Mukae M, Kobayashi K, Sada M, Yokoyama K, Koizumi W, Saegusa M. Diagnostic performance of EUS for evaluating the invasion depth of early colorectal cancers. Gastrointest Endosc. 2015;81:682-690. [PubMed] [DOI] [Cited in This Article: ] |