Published online Jan 15, 2021. doi: 10.4251/wjgo.v13.i1.24
Peer-review started: September 8, 2020
First decision: October 21, 2020
Revised: November 3, 2020
Accepted: November 29, 2020
Article in press: November 29, 2020
Published online: January 15, 2021
Positive peritoneal wash cytology with no peritoneal metastasis (CY1P0) is a special type of distant gastric cancer metastasis, which describes a patient with positive peritoneal lavage cytology, but no definitive peritoneal metastasis, and there are no widely accepted treatment guidelines. We enrolled 48 primary CY1P0 gastric cancer patients treated by radical gastrectomy in this study. Our study illustrated the efficacy of radical gastrectomy for CY1P0 gastric cancer patients, and suggested that the pathological N factor and vascular invasion were significant independent risk factors for overall survival (OS).
To assess the survival of CY1P0 gastric cancer patient post-radical gastrectomy, and to identify factors associated with long-term prognosis.
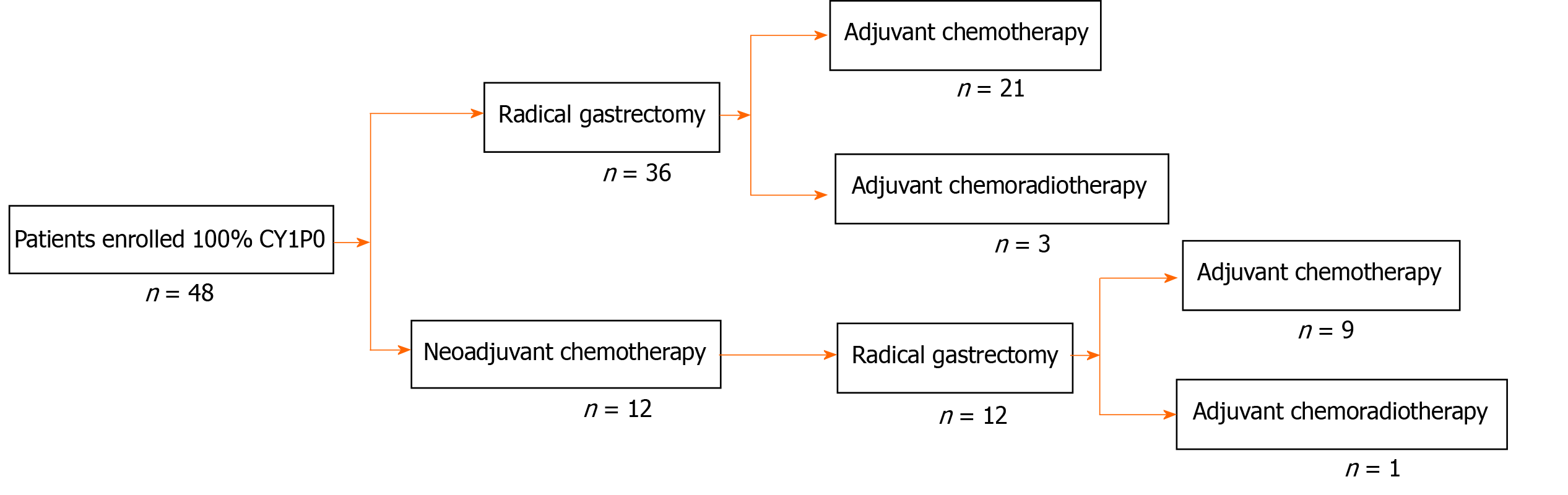
Our study included 48 patients with primary CY1P0 gastric cancer who had radical gastrectomies at the Cancer Hospital, Chinese Academy of Medical Sciences, Beijing, China between 2013 and 2018. R0 resection was achieved in all 48 patients. Twelve patients received neoadjuvant chemotherapy. Thirty patients received adjuvant chemotherapy and four received adjuvant chemoradiotherapy. OS statistics were available for 48 patients. Follow-up continued through March 2020. Univariate and multivariate analyses were performed using a Cox proportional hazards model to identify prognostic factors.
Median OS was 22.0 mo (95% confidence interval: 13.366-30.634 mo) post-surgery. Univariate analyses demonstrated that tumor site (P = 0.021), pathological N factor (P = 0.001), pathological T factor (P = 0.028), vascular invasion (P = 0.046), and the level of CA199 prior to initiating therapy (P = 0.002) were significant risk factors for OS. Multivariate analyses demonstrated that pathological N factor (P = 0.001) and vascular invasion (P = 0.031) were significant independent risk factors for OS.
This study suggested that radical gastrectomy may be efficient for CY1P0 gastric cancer patient post-radical gastrectomy and the pathological N factor and vascular invasion are significant independent risk factors for OS.
Core Tip: This is a retrospective study to investigate the survival of gastric cancer patients with positive peritoneal wash cytology but no peritoneal metastasis post-radical gastrectomy and to identify factors associated with long-term prognosis. Our study included 48 such patients and demonstrated that more effective treatment should be established for patients who are diagnosed with pN3b disease and vascular invasion.
- Citation: Kang WZ, Zhong YX, Ma FH, Xue LY, Xiong JP, Ma S, Li Y, Xie YB, Quan X, Tian YT. Survival outcomes and prognostic indicators for gastric cancer patients with positive peritoneal wash cytology but no peritoneal metastasis after radical gastrectomy. World J Gastrointest Oncol 2021; 13(1): 24-36
- URL: https://www.wjgnet.com/1948-5204/full/v13/i1/24.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i1.24
Gastric cancer is one of the most common malignant tumors worldwide. There are no specific symptoms in early-stage gastric cancer, and when patients are diagnosed, the disease is usually advanced, and may even have metastasized. Advanced gastric cancer often metastasizes to the peritoneum, and metastasis is the main cause of disease-related death[1]. Peritoneal lavage cytology has been widely used to stage gastric cancer[2]. Positive peritoneal wash cytology with no peritoneal metastasis (CY1P0) is a special type of distant gastric cancer metastasis, which describes a patient with positive peritoneal lavage cytology, but no definitive peritoneal metastasis. The Japanese Classifications of Gastric Carcinoma define this as stage IV disease[3]. Although the American Joint Committee on Cancer guidelines for gastric cancer (eighth edition) clearly state that CY1P0 is equivalent to M1 disease[4], it was still potentially treatable. Positive intraperitoneal free cancer cells are an important risk factor for postoperative intraperitoneal recurrence and metastasis in patients with gastric cancer[5]. Positive peritoneal lavage cytology is a predictor of peritoneal dissemination[6] and poor prognosis[7-10].
Currently, there are no widely accepted treatment guidelines for CY1P0 gastric cancer patients[11]. Some retrospective studies have demonstrated the efficacy of radical surgery combined with intraoperative chemotherapy and systemic chemotherapy; however, larger, randomized, controlled clinical studies are needed to standardize the treatment of CY1P0 patients and to develop relevant guidelines. The aim of this study was to evaluate the effect of radical gastrectomy on the survival of CY1P0 gastric cancer patients and to identify risk factors associated with prognosis.
This retrospective study included 48 patients with primary CY1P0 gastric cancer who had radical gastrectomy at the Cancer Hospital, Chinese Academy of Medical Sciences, Beijing, China between 2013 and 2018. All patients were diagnosed with gastric adenocarcinoma (with no peritoneal metastasis or distant metastasis). All patients underwent abdominal lavage before surgery, and in all cases, they had positive peritoneal lavage cytology, but no definitive peritoneal metastasis. R0 resection was achieved in all 48 patients. Patients who had undergone palliative surgery or received only chemoradiotherapy were excluded from the study.
All patients underwent gastroscopy and computed tomography (CT) examination to assess their condition. Because of advanced disease or suspected lymph node metastasis, 12 patients received neoadjuvant chemotherapy. All 48 patients had radical surgery and D2 lymph node dissection. The cytological examination of the peritoneal lavage samples was performed before surgery. Thirty patients received adjuvant chemotherapy and four received adjuvant chemoradiotherapy.
Patients reported for follow-up every 3 mo to the out-patient department. Follow-up included physical examination, routine blood work, blood biochemistry, and tumor biomarkers including CEA, CA724, CA242, AFP, and CA19-9. CT examination and endoscopy were performed every 6 mo. Hematological tests were performed at least every 2 wk during chemoradiotherapy. Disease progression, unacceptable adverse events, and patient death were recorded. We regularly followed the patients by telephone to ensure that we had up-to-date information for all patients. Follow-up continued through March 2020. Overall survival (OS) was measured from the time of surgery.
Cumulative survival rates were obtained using the Kaplan–Meier method and were compared using the log-rank test to evaluate statistically significant differences. Cox proportional hazard regression analysis was employed to evaluate factors affecting OS. A P value < 0.05 was considered statistically significant. Statistical analyses were performed with statistic package for social science for windows, version 22.0.
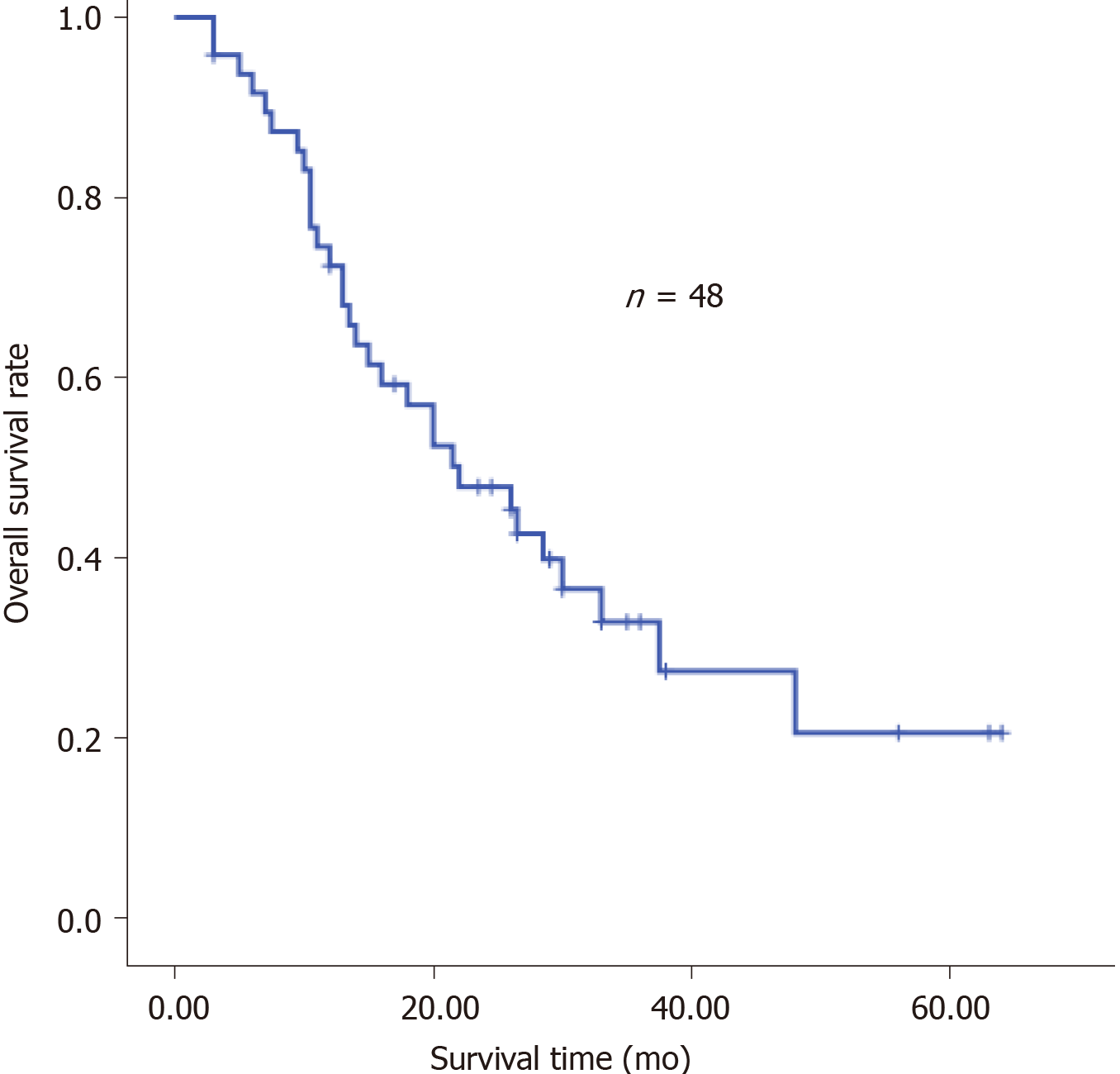
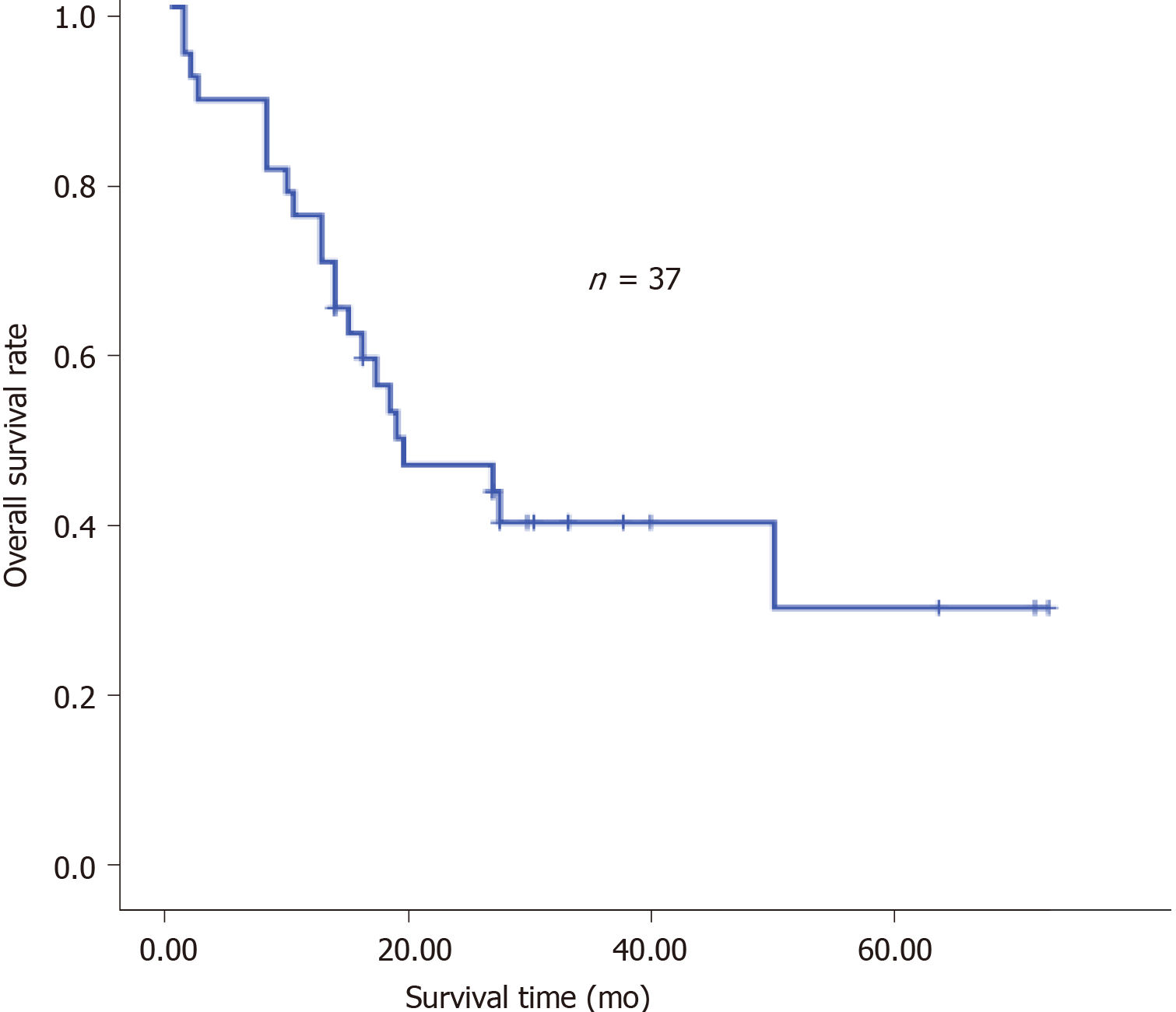
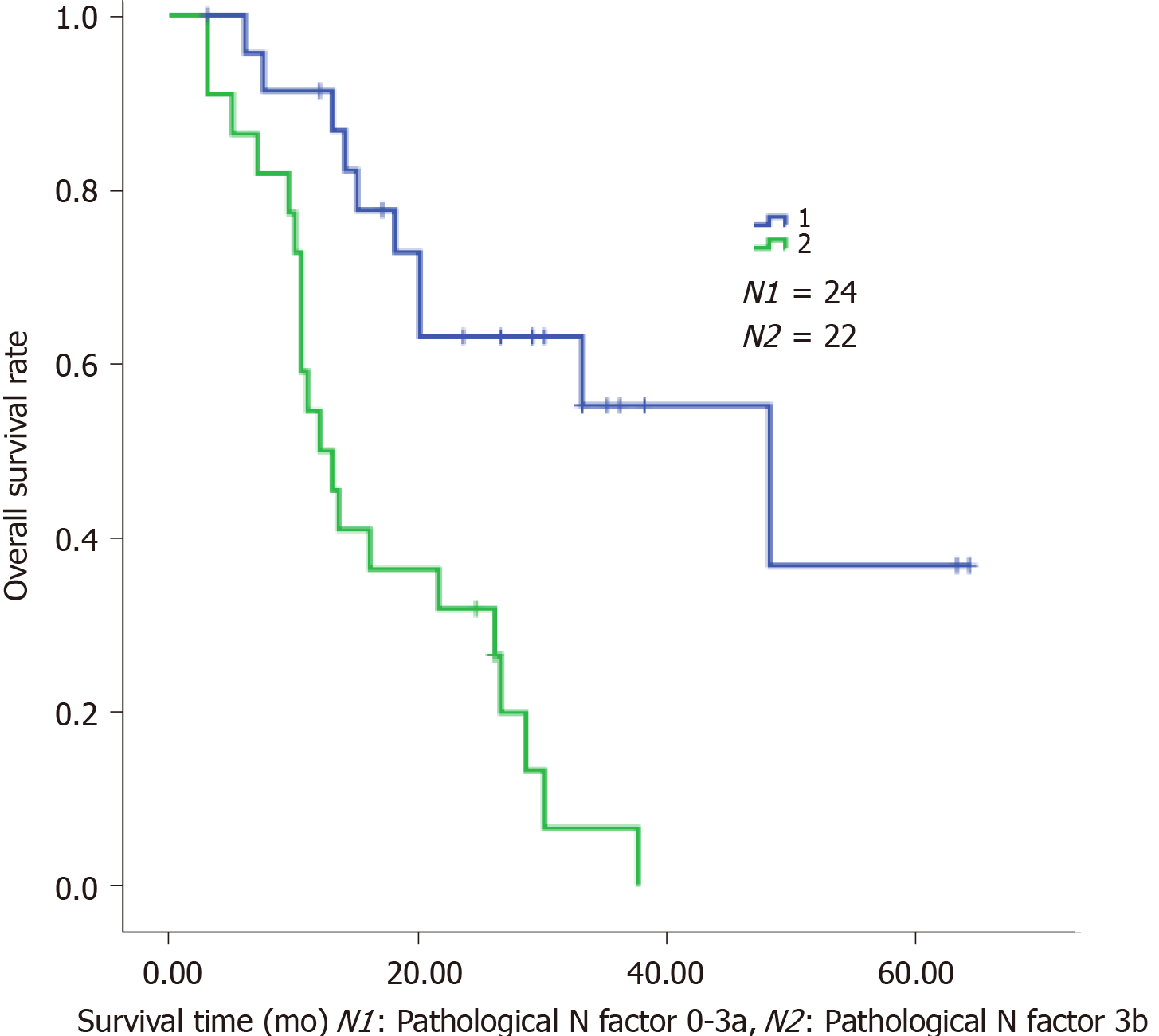
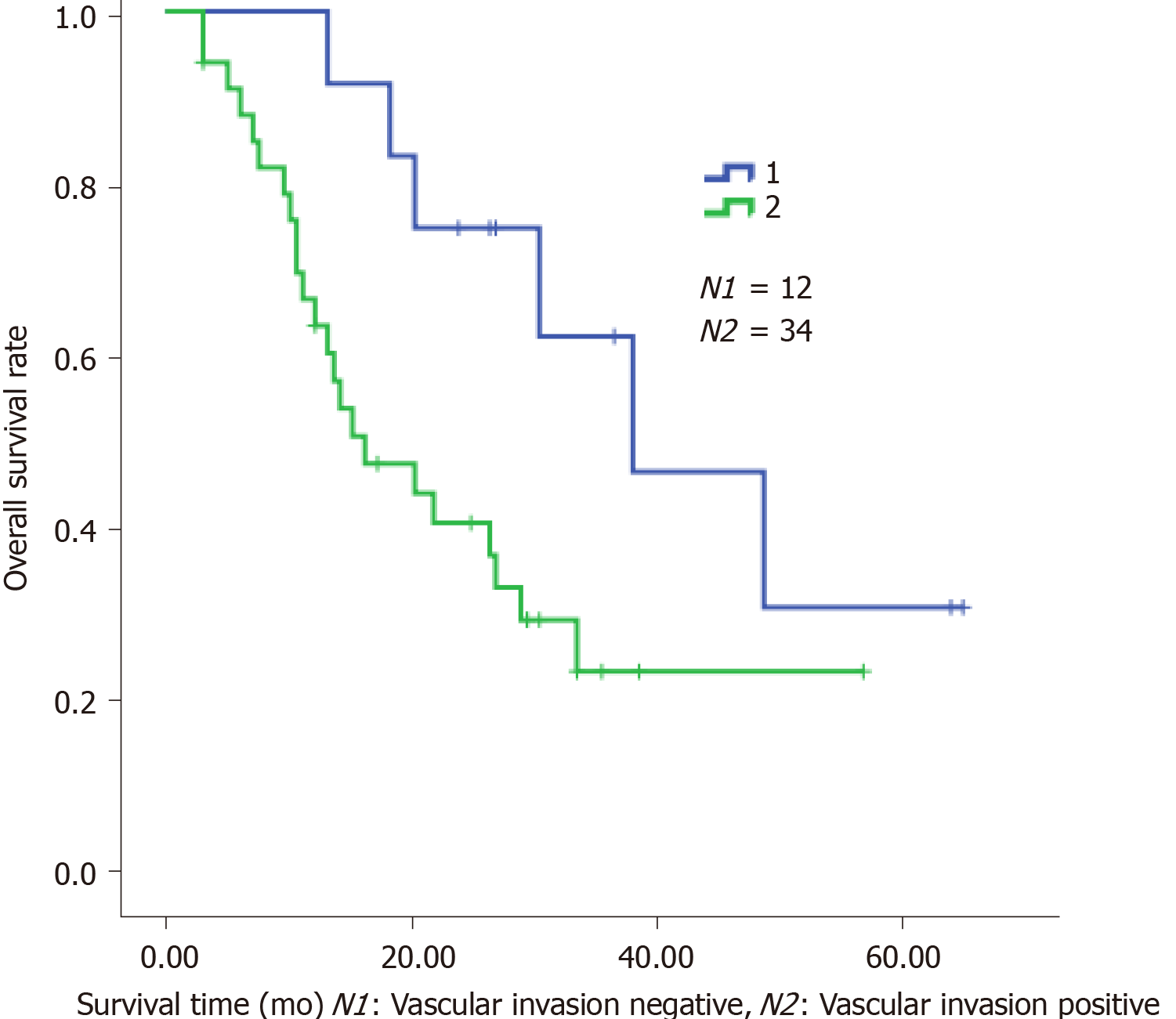
The clinical characteristics of the patients are shown in Table 1. Neoadjuvant chemotherapy was administered to 12 patients (Table 2) and the types of surgery are listed in Table 3. Thirty-four patients received adjuvant therapy; 30 patients received adjuvant chemotherapy and 4 received adjuvant chemoradiotherapy (Table 4). A flow diagram of the 48 patients who underwent radical gastrectomy is shown in Figure 1. OS was measured from the time of surgery. For the 48 CY1P0 patients, median OS was 22.0 mo [95% confidence interval (CI): 13.366–30.634 mo] (Figure 2). The 1-, 2-, 3-, and 5-year OS rates were 72.4%, 47.8%, 32.9%, and 20.5%, respectively. Median recurrence-free survival was 16.5 mo (95%CI: 5.141–27.859 mo) (Figure 3). Median follow-up was 35.0 mo. Univariate analysis showed that tumor site (P = 0.021), pathological N factor (P = 0.001), pathological T factor (P = 0.028), vascular invasion (P = 0.046), and the level of CA199 prior to initiating therapy (P = 0.002) were significant risk factors for OS (Table 5). Compared with gastric cardia cancer and gastric body cancer, gastric antrum tumors had better prognosis [odds ratio (OR): 0.427; 95%CI: 0.207-0.880; P = 0.021]. Pathological N factor in 3b (OR: 4.194; 95%CI: 1.870-9.406; P = 0.001) and T factor in 4a-4b (OR: 5.008; 95%CI: 1.190-21.072; P = 0.028) correlated with poor OS rate. Patients with vascular invasion had a poor prognosis (OR: 2.554; 95%CI: 1.017-6.413; P = 0.046). Patients with normal CA199 levels before treatment had a better prognosis (OR: 0.267; 95%CI: 0.118-0.604; P = 0.002). Multivariate analysis was performed based on factors with P < 0.1 in the univariate analysis (Table 6). Pathological N factor (P = 0.001) and vascular invasion (P = 0.031) were identified to be significant independent risk factors for OS (Figures 4 and 5).
| Characteristic | Patients |
| Age | |
| < 60 yr | 23 |
| ≥ 60 yr | 25 |
| Smoking history | |
| Yes | 26 |
| No | 22 |
| Drinking history | |
| Yes | 26 |
| No | 22 |
| Family history | |
| Yes | 18 |
| No | 30 |
| Treament | |
| Sugery | 48 |
| Neoadjuvant chemotherapy | 12 |
| Adjuvant chemotherapy | 30 |
| Adjuvant chemoradiotherapy | 4 |
| Site of tumor | |
| Upper | 5 |
| Middle | 16 |
| Lower | 27 |
| Bormann classification | |
| Type 1 | 3 |
| Type 2 | 9 |
| Type 3 | 20 |
| Type 4 | 12 |
| Lauren’s classification | |
| Type 1 | 9 |
| Type 2 | 22 |
| Type 3 | 13 |
| Pathological N factor | |
| 0-3a | 25 |
| 3b | 23 |
| Pathological T factor | |
| 0-3 | 8 |
| 4a-4b | 38 |
| Neoadjuvant chemotherapy regimen | Patients |
| SOX | 5 |
| DOS | 1 |
| XELOX | 1 |
| PTX + L-OHP + S-1 | 2 |
| Paclitaxel liposome + L-OHP + S-1 | 1 |
| PTX + DDP + S-1 | 1 |
| DXT + S-1/5-Fu + L-OHP + CPT-11 | 1 |
| Surgery type | Patients |
| Laparoscopic assist distal gastrectomy + D2 | 16 |
| Laparoscopic assist total gastrectomy + D2 | 9 |
| Traditional distal gastrectomy + D2 | 11 |
| Traditional total gastrectomy + D2 | 10 |
| Laparoscopic assisted proximal gastrectomy + splenectomy + D2 | 1 |
| Laparoscopic assisted proximal gastrectomy + D2 | 1 |
| Adjuvant chemotherapy regimen | Patients |
| SOX | 12 |
| SOX + radiotherapy | 4 |
| XELOX | 3 |
| Paclitaxel liposome + L-OHP + S- | 1 |
| S-1 | 2 |
| PTX + 5-Fu + L-OHP | 1 |
| DXT + S-1 | 1 |
| PTX + CAP | 1 |
| DXT + L-OHP + CAP | 1 |
| S-1 + DDP | 2 |
| Unknown | 6 |
| Patient characteristic | OR | 95%CI | P value | |
| Age | 0.588 | |||
| < 60 yr | 23 | 1.000 | ||
| ≥ 60 yr | 25 | 0.822 | 0.404-1.671 | |
| Smoking history | 0.935 | |||
| Yes | 26 | 1.000 | ||
| No | 22 | 0.971 | 0.476-1.979 | |
| Drinking history | 0.137 | |||
| Yes | 26 | 1.000 | ||
| No | 22 | 1.726 | 0.841-3.540 | |
| Site of tumor | 0.021 | |||
| Upper/Middle | 21 | 1.000 | ||
| Lower | 27 | 0.427 | 0.207-0.880 | |
| Signet-ring cell | 0.229 | |||
| Yes | 19 | 1.000 | ||
| No | 28 | 0.640 | 0.309-1.325 | |
| Bormann classification | 0.416 | |||
| Type1/2/3 | 32 | 1.000 | ||
| Type4 | 12 | 1.431 | 0.603-3.392 | |
| Lauren’s classification | 0.080 | |||
| Type 1 | 9 | 1.000 | ||
| Type 2/Type 3 | 35 | 2.588 | 0.892-7.508 | |
| Pathological N factor | 0.001 | |||
| 0-3a | 24 | 1.000 | ||
| 3b | 22 | 4.194 | 1.870-9.406 | |
| Pathological T factor | 0.028 | |||
| 0-3 | 9 | 1.000 | ||
| 4a-4b | 37 | 5.008 | 1.190-21.072 | |
| Vascular invasion | 0.046 | |||
| Negative | 12 | 1.000 | ||
| Positive | 34 | 2.554 | 1.017-6.413 | |
| CA199 | 0.002 | |||
| Elevate | 13 | 1.000 | ||
| Normal | 31 | 0.267 | 0.118-0.604 | |
| CEA | 0.837 | |||
| Elevated | 13 | |||
| Normal | 32 | 0.917 | 0.403-2.089 | |
| Therapy | 0.112 | |||
| Surgery along | 12 | |||
| Combined therapy | 36 | 0.540 | 0.252-1.154 |
| Patient characteristic | OR | 95%CI | P value | |
| Site of tumor | 0.105 | |||
| Upper/Middle | 18 | |||
| Lower | 21 | |||
| Lauren’s classification | 0.476 | |||
| Type 1 | 8 | |||
| Type 2/Type 3 | 31 | |||
| Pathological N factor | 0.001 | |||
| 0-3a | 19 | 1.000 | ||
| 3b | 20 | 5.365 | 1.971-14.609 | |
| Pathological T factor | 0.146 | |||
| 0-3 | 9 | |||
| 4a-4b | 30 | |||
| Vascular invasion | 0.031 | |||
| Negative | 10 | 1.000 | ||
| Positive | 29 | 3.660 | 1.124-11.917 | |
| CA199 | ||||
| Elevated | 12 | 0.789 | ||
| Normal | 27 |
CY1P0 is a special type of distant gastric cancer metastasis, which describes a patient with positive peritoneal lavage cytology, but no definitive peritoneal metastasis. Currently, there are no widely accepted treatment guidelines for CY1P0 gastric cancer patients[11].
The positive rate of peritoneal lavage cytology of Japanese patients with gastric carcinoma is approximately 5%–20%[12,13]. In this study, we assessed the survival of CY1P0 gastric cancer patients and sought to identify prognostic risk factors. We performed surgery on 48 patients with positive peritoneal lavage cytology but without peritoneal metastases. Median OS was 22.0 mo. The 1-, 2-, 3-, and 5-year OS rates were 72.4%, 47.8%, 32.9%, and 20.5%, respectively. It was reported in another study that the 5-year OS was 17.6% for CY1 gastric cancer patients[11], while the OS of patients who received chemotherapy alone was 9.9-12.6 mo[7,14]. These results suggest that radical gastrectomy is effective, and surgery is the most crucial component of this conversion therapy[15]. However, OS of the 36 patients who received neoadjuvant or adjuvant therapy combined with surgery was no better than that of the 12 patients who had surgery alone (P = 0.112). We hypothesize that this may be because the disease had progressed further in patients who received combined therapy; however, since CY1 represents peritoneal seeding, we believe that chemotherapy after surgery is warranted. The univariate and multivariate Cox proportional hazard analyses of the clinicopathological factors associated with OS showed that the lymph node metastasis status affected the OS of CY1-only gastric cancer patients who underwent radical gastrectomy. In addition, pathological pN3b is an indicator of distant nodal metastasis[6]. A more effective treatment should be established for patients who are diagnosed with CY1 and pN3b disease. Similarly, vascular invasion is an important prognostic factor, and these patients require further treatment and regular review. Several previous publications demonstrated that Borrmann type-4 tumors are negatively associated with survival and prognosis of this population. Noda et al[16] evaluated the survival of 91 CY1P0 patients with Borrmann type-4 tumors. They found that the 5-year OS rate of these patients was 6.3%, while that of patients with other types of tumors was 27.7%. In another study, researchers assessed clinicopathological features associated with prognosis in 37 CY1P0 gastric cancer patients[10]. A multiple linear regression analysis revealed that Borrmann type-4 tumors were an independent predictor of poor prognosis; however, Borrmann type-4 tumors were not prognostic in the current study (P = 0.416). In the univariate analysis, tumor site (P = 0.021) and the level of CA199 before therapy (P = 0.002) were risk factors for OS. These two factors were not statistically significant in the multivariate analysis. In general, cardia cancer and gastric body cancer have worse prognoses and require more difficult surgical procedures, and postoperative tumor marker levels are useful during follow-up. Changes in the levels of tumor markers may be associated with tumor recurrence. At present, radical gastrectomy, regional radiotherapy, and adjuvant antitumor chemotherapy have been proven effective for the treatment of advanced gastric cancer[17]. Standard treatment for gastric cancer patients with distant metastasis is systemic chemotherapy. Conversion therapy provides a new approach for the treatment of patients with advanced gastric cancer[18]. In one study, Japanese researchers treated 41 patients with peritoneal metastasis (30 of whom were positive for free abdominal cancer cells) with S-1 combined with cisplatin. The treatment eradicated peritoneal metastasis in 19 patients. After radical surgery, median survival of these patients increased from 12.6 mo to 43.2 mo[7]. Patients with good therapeutic effect may selectively benefit from radical surgery. Another study came to the same conclusion. Patients received systemic chemotherapy combined with S-1+ paclitaxel intraperitoneal infusion chemotherapy. Then, if free tumor cells were not detected in the abdominal cavity, the patients had radical surgery. The safety of surgeries was acceptable and postoperative prognosis of the patients improved[15]. Radical surgery combined with postoperative adjuvant chemotherapy has been widely used in patients with advanced gastric cancer for some time. Some clinical studies have confirmed the significance of S-1 adjuvant chemotherapy in CY1P0 patients after radical surgery. Kano et al[2] found that the median OS survival of 36 CY1P0 patients who underwent radical surgery and postoperative S1 monotherapy was 22.3 mo. In another study, long-term follow-up of CY1P0 patients who underwent D2 radical surgery and postoperative S1 monotherapy demonstrated a 2-year survival rate of 46%, a 5-year OS rate of 26%, and a relapse-free survival rate of 21%, which exceeded the researchers' expectations[19]. Therefore, further studies were conducted. The effect of preoperative neoadjuvant chemotherapy was assessed in CY1P0 patients who had radical surgery and S1 single-drug adjuvant chemotherapy after surgery. The 5-year survival rate was 15%, with or without preoperative neoadjuvant chemotherapy. This study also showed that preoperative chemotherapy efficacy and lymph node involvement significantly impacted patient prognosis[20]. As researchers explore more aggressive treatments, hyperthermic intraperitoneal chemotherapy (HIPEC) shows unique application prospects, and multiple basic and clinical studies have confirmed the safety and effectiveness of HIPEC. HIPEC is a highly selective regional chemotherapy, characterized by high local drug concentrations, long duration of action, direct effects on tumor cells, synergy of chemotherapy and thermal effect, and negligible systemic toxicity and side effects, which has obvious advantages over traditional, peripheral venous chemotherapy. Results of a meta-analysis also showed that surgery combined with intraperitoneal chemotherapy increased the 5-year survival rate of CY1P0 patients and reduced the risk of recurrence compared to surgery alone. These benefits could be further increased when combined with extensive intraoperative peritoneal lavage therapy[21]. Extensive intraoperative peritoneal lavage therapy is another effective means to reduce the number of free cancer cells in the abdomen, which can significantly improve the postoperative survival rate of CY1P0 gastric cancer patients[22]. A study of 37 CY1P0 patients showed a 5-year survival rate of 46.5% after radical surgery and extensive intraoperative peritoneal lavage. This prognosis is similar to that of gastric cancer patients receiving the same treatment at stage III B and III C, which means that these patients achieved a reduction in tumor staging[23]. Phase II clinical studies have shown that the combination of intravenous and abdominal injections of paclitaxel and S-1 to treat gastric cancer patients with peritoneal metastasis is effective, providing a new idea for the treatment of CY1P0 patients[24].
Our study has several potential limitations. First, different types of surgery may result in different OS; however, we did not investigate the effect of different surgical procedures on prognosis. Second, we did not assess the effects of postoperative complications. The safety of such procedures and the incidence of complications associated with these procedures should be evaluated. Third, since this was a retrospective study, there were no data available that recorded the effect of neoadjuvant chemotherapy prior to surgery. Some studies report that surgery after response to systemic chemotherapy is safe and may prolong the survival of gastric cancer patients[15]. A prospective, randomized controlled trial or a large cohort study should be conducted to verify the efficacy of surgery for gastric cancer patients with positive peritoneal cytology findings. Yamashita et al[11] reported that preoperative serum albumin levels may be a predictive factor for CY1 gastric cancer patients. Finally, in this study, we did not consider the effects of preoperative nutritional status, biochemical indicators, and complications on prognosis. Currently, most studies evaluating the treatment of CY1P0 patients are retrospective and have small sample sizes. Some guidelines recommend treating these patients using guidelines for patients with recurrent or metastatic gastric cancer. We found that for eligible CY1P0 gastric cancer patients, radical surgery combined with intraoperative chemotherapy and systemic chemotherapy is effective; however, the precise timing, indications, and surgical methods for patients undergoing translational therapy have not been determined. Usually, abdominal lavage fluid is assessed for the presence of free tumor cells after neoadjuvant chemotherapy. The absence of tumor cells in the lavage fluid reflects effective conversion therapy, and patients with this result should be considered for radical surgical resection. For eligible CY1P0 gastric cancer patients, we recommend multidisciplinary MDT discussions, the development of individualized treatment regimens, and participation in clinical studies. With a growing list of new drugs and the maturation of HIPEC and other technologies, cancer cells found during abdominal lavage may not always prognose disease-related mortality for CY1P0 gastric cancer patients.
In conclusion, this study illustrated the efficacy of radical gastrectomy for CY1P0 gastric cancer patients. More effective treatment should be established for patients who are diagnosed with pN3b disease and vascular invasion. We look forward to the insights offered by future, prospective, randomized, controlled clinical studies with larger sample sizes to verify the efficacy of radical surgery and to standardize the recommendations for the treatment of patients with CY1P0 gastric cancer.
Positive peritoneal wash cytology with no peritoneal metastasis (CY1P0) is a special distant metastasis of gastric cancer, and currently there are no extensive treatment guidelines for patients with CY1P0 gastric cancer.
To assess survival after radical gastrectomy for CY1P0 gastric cancer and to identify factors associated with long-term prognosis.
To evaluate the effect of radical gastrectomy on survival in patients with CY1P0 gastric cancer, and to identify prognostic risk factors.
Our study included 48 patients with primary CY1P0 gastric cancer who had radical gastrectomies. Overall survival (OS) statistics were available for 48 patients. Follow-up continued through March 2020. Univariate and multivariate analyses were performed using a Cox proportional hazards model to identify prognostic factors.
For the 48 CY1P0 patients, median OS was 22.0 mo, while the OS of patients who received chemotherapy alone was 9.9-12.6 mo. Pathological N factor (P = 0.001) and vascular invasion (P = 0.031) were significant independent risk factors for OS.
This study illustrated the efficacy of radical gastrectomy for CY1P0 gastric cancer patients. More effective treatment should be established for patients who are diagnosed with pN3b disease and vascular invasion.
To formulate the standard treatment plan for CY1P0 gastric cancer.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Steenholdt C, Theiss AL, Wittkopf N S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, Porcheron J, Peix JL, François Y, Vignal J, Gilly FN. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358-363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 15] [Reference Citation Analysis (0)] |
| 2. | Kano K, Aoyama T, Maezawa Y, Nakajima T, Ikeda K, Yamada T, Sato T, Oshima T, Rino Y, Masuda M, Ogata T, Cho H, Yoshikawa T. The survival and prognosticators of peritoneal cytology-positive gastric cancer patients who received upfront gastrectomy and subsequent S-1 chemotherapy. Int J Clin Oncol. 2017;22:887-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2390] [Cited by in F6Publishing: 2656] [Article Influence: 204.3] [Reference Citation Analysis (0)] |
| 4. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. AJCC Cancer Staging Manual. 8th ed. New York: Springer. [Cited in This Article: ] |
| 5. | Mezhir JJ, Posner MC, Roggin KK. Prospective clinical trial of diagnostic peritoneal lavage to detect positive peritoneal cytology in patients with gastric cancer. J Surg Oncol. 2013;107:794-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Chuwa EW, Khin LW, Chan WH, Ong HS, Wong WK. Prognostic significance of peritoneal lavage cytology in gastric cancer in Singapore. Gastric Cancer. 2005;8:228-237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Okabe H, Ueda S, Obama K, Hosogi H, Sakai Y. Induction chemotherapy with S-1 plus cisplatin followed by surgery for treatment of gastric cancer with peritoneal dissemination. Ann Surg Oncol. 2009;16:3227-3236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Mezhir JJ, Shah MA, Jacks LM, Brennan MF, Coit DG, Strong VE. Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol. 2010;17:3173-3180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 9. | Chiu CC, Lin MH, Huang WT. Prognostic significance of peritoneal washing cytology in patients with gastric cancer (Br J Surg 2012; 99: 397-403). Br J Surg. 2012;99:1166; author reply 1166-1166; author reply 1167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Oh CA, Bae JM, Oh SJ, Choi MG, Noh JH, Sohn TS, Kim S. Long-term results and prognostic factors of gastric cancer patients with only positive peritoneal lavage cytology. J Surg Oncol. 2012;105:393-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Yamashita K, Ushiku H, Katada N, Hosoda K, Moriya H, Mieno H, Kikuchi S, Hoshi K, Watanabe M. Reduced preoperative serum albumin and absence of peritoneal dissemination may be predictive factors for long-term survival with advanced gastric cancer with positive cytology test. Eur J Surg Oncol. 2015;41:1324-1332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Miyashiro I, Takachi K, Doki Y, Ishikawa O, Ohigashi H, Murata K, Sasaki Y, Imaoka S, Nakaizumi A, Takenaka A, Furukawa H, Hiratsuka M. When is curative gastrectomy justified for gastric cancer with positive peritoneal lavage cytology but negative macroscopic peritoneal implant? World J Surg. 2005;29:1131-1134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Akaza K, Motoori T, Nakamura S, Koshikawa T, Kitoh K, Futamura N, Nakamura T, Kojima M, Kuroda M, Kasahara M. Clinicopathologic study of primary gastric lymphoma of B cell phenotype with special reference to low-grade B cell lymphoma of mucosa-associated lymphoid tissue among the Japanese. Pathol Int. 1995;45:832-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Yamaguchi T, Takashima A, Nagashima K, Makuuchi R, Aizawa M, Ohashi M, Tashiro K, Yamada T, Kinoshita T, Hata H, Kawachi Y, Kawabata R, Tsuji T, Hihara J, Sakamoto T, Fukagawa T, Katai H, Higuchi K, Boku N. Efficacy of Postoperative Chemotherapy After Resection that Leaves No Macroscopically Visible Disease of Gastric Cancer with Positive Peritoneal Lavage Cytology (CY1) or Localized Peritoneum Metastasis (P1a): A Multicenter Retrospective Study. Ann Surg Oncol. 2020;27:284-292. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Ishigami H, Yamaguchi H, Yamashita H, Asakage M, Kitayama J. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer. 2017;20:128-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Noda S, Yashiro M, Toyokawa T, Morimoto J, Shinto O, Muguruma K, Sawada T, Hirakawa K. Borrmann's macroscopic criteria and p-Smad2 expression are useful predictive prognostic markers for cytology-positive gastric cancer patients without overt peritoneal metastasis. Ann Surg Oncol. 2011;18:3718-3725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Yang XJ, Huang CQ, Suo T, Mei LJ, Yang GL, Cheng FL, Zhou YF, Xiong B, Yonemura Y, Li Y. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy improves survival of patients with peritoneal carcinomatosis from gastric cancer: final results of a phase III randomized clinical trial. Ann Surg Oncol. 2011;18:1575-1581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 398] [Cited by in F6Publishing: 449] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 18. | Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, Iwasaki Y, Hyung WJ, Takagane A, Park DJ, Yoshikawa T, Hahn S, Nakamura K, Park CH, Kurokawa Y, Bang YJ, Park BJ, Sasako M, Tsujinaka T; REGATTA study investigators. Gastrectomy plus chemotherapy vs chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol. 2016;17:309-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 365] [Cited by in F6Publishing: 446] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 19. | Kodera Y, Ito S, Mochizuki Y, Ohashi N, Tanaka C, Kobayashi D, Kojima H, Matsui T, Kondo K, Fujiwara M. Long-term follow up of patients who were positive for peritoneal lavage cytology: final report from the CCOG0301 study. Gastric Cancer. 2012;15:335-337. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Yamamoto M, Kawano H, Yamaguchi S, Egashira A, Minami K, Taguchi K, Ikeda Y, Morita M, Toh Y, Okamura T. Comparison of Neoadjuvant Chemotherapy to Surgery Followed by Adjuvant Chemotherapy in Japanese Patients with Peritoneal Lavage Cytology Positive for Gastric Carcinoma. Anticancer Res. 2015;35:4859-4863. [PubMed] [Cited in This Article: ] |
| 21. | Coccolini F, Catena F, Glehen O, Yonemura Y, Sugarbaker PH, Piso P, Ceresoli M, Montori G, Ansaloni L. Effect of intraperitoneal chemotherapy and peritoneal lavage in positive peritoneal cytology in gastric cancer. Systematic review and meta-analysis. Eur J Surg Oncol. 2016;42:1261-1267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Kuramoto M, Shimada S, Ikeshima S, Matsuo A, Yagi Y, Matsuda M, Yonemura Y, Baba H. Extensive intraoperative peritoneal lavage as a standard prophylactic strategy for peritoneal recurrence in patients with gastric carcinoma. Ann Surg. 2009;250:242-246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 171] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 23. | Masuda T, Kuramoto M, Shimada S, Ikeshima S, Yamamoto K, Nakamura K, Yoshimatsu S, Urata M, Baba H. The effect of extensive intraoperative peritoneal lavage therapy (EIPL) on stage III B + C and cytology-positive gastric cancer patients. Int J Clin Oncol. 2016;21:289-294. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, Otani K, Kamei T, Soma D, Miyato H, Yamashita H, Nagawa H. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 173] [Article Influence: 11.5] [Reference Citation Analysis (0)] |