Copyright
©The Author(s) 2020.
World J Gastrointest Oncol. Feb 15, 2020; 12(2): 149-172
Published online Feb 15, 2020. doi: 10.4251/wjgo.v12.i2.149
Published online Feb 15, 2020. doi: 10.4251/wjgo.v12.i2.149
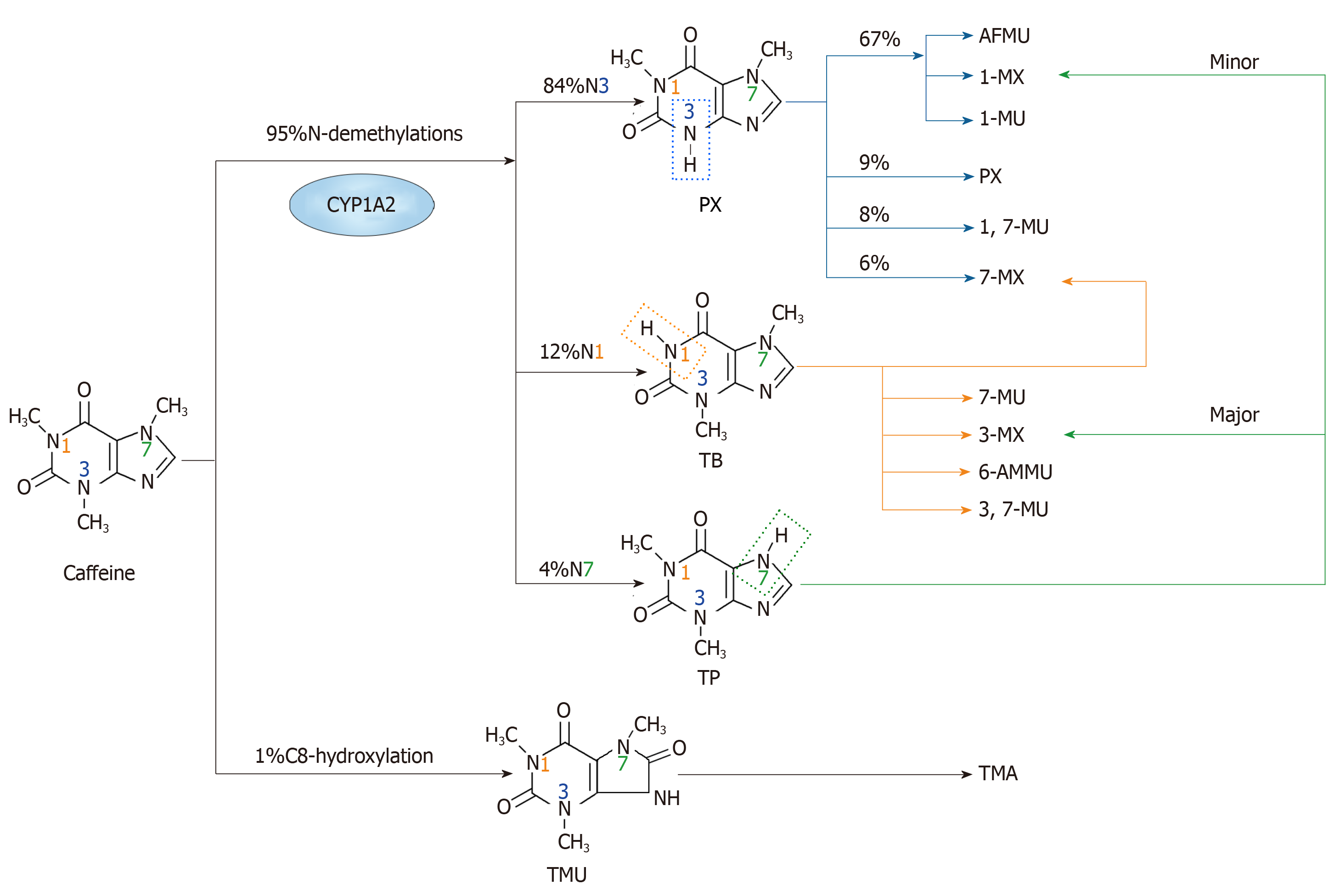
Figure 1 The major metabolism pathways of caffeine.
The primary metabolic action of caffeine involves two main pathways. One is N-demethylation, which includes N1, N3 and N7-demethylations. Through N3-demethylation, caffeine can be degraded to form paraxanthine, which may be further degraded to 5-acetyl-amino-6-formylamino-3-methyluracil, 1-methylxanthine, 1-methyluric acid, 1,7-methyluric acid and 7-methylxanthine. A small amount of paraxanthine can be cleared in its unchanged form. Theobromine can be formed through N1-demethylation of caffeine, and it can be metabolized to form 7-methylxanthine, 7-methyluric acid, 3-methylxanthine, 6-amino-5[N-methylformylamino]-1-methyluracil and 3,7-methyluric acid. Moreover, caffeine can be metabolized to theophylline through N7-demethylation, and then theophylline can be further degraded to 1-methylxanthine and 3-methylxanthine. The other pathway is C8-hydroxylation in which caffeine is metabolized to trimethyluric acid and then 3,6,8-trimethylallantoin. PX: Paraxanthine; AFMU: 5-acetyl-amino-6-formylamino-3-methyluracil; 1-MX: 1-methylxanthine; 1-MU: 1-methyluric acid; 7-MU: 7-methyluric acid; 1,7-MU: 1,7-methyluric acid; 7-MX: 7-methylxanthine; TB: Theobromine; 3-MX: 3-methylxanthine; 6-AMMU: 6-amino-5[N-methylformylamino]-1-methyluracil; 3,7-MU: 3,7-methyluric acid; TP: Theophylline; TMU: Trimethyluric acid; TMA: 3,6,8-trimethylallantoin.
- Citation: Cui WQ, Wang ST, Pan D, Chang B, Sang LX. Caffeine and its main targets of colorectal cancer. World J Gastrointest Oncol 2020; 12(2): 149-172
- URL: https://www.wjgnet.com/1948-5204/full/v12/i2/149.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i2.149