Published online Jun 15, 2018. doi: 10.4251/wjgo.v10.i6.124
Peer-review started: February 23, 2018
First decision: March 23, 2018
Revised: March 23, 2018
Accepted: April 19, 2018
Article in press: April 19, 2018
Published online: June 15, 2018
Gastric cancer (GC) is one of the most frequently diagnosed cancers in the world. Most GC patients are diagnosed when the cancer is in an advanced stage, and consequently, some develop metastatic lesions that generally cause cancer-related death. Metastasis establishment is affected by various conditions, such as tumor location, hemodynamics and organotropism. While digestive cancers may share a primary site, certain cases develop hematogenous metastasis with the absence of peritoneal metastasis, and vice versa. Numerous studies have revealed the clinicopathological risk factors for hematogenous metastasis from GC, such as vascular invasion, advanced age, differentiation, Borrmann type 1 or 2 and expansive growth. Recently, molecular mechanisms that contribute to metastatic site determination have been elucidated by advanced molecular biological techniques. Investigating the molecules that specifically participate in metastasis establishment in distinct secondary organs will lead to the development of novel biomarkers for patient stratification according to their metastatic risk and strategies for preventing and treating distinct metastases. We reviewed articles related to the molecular landscape of hematogenous metastasis from GC.
Core tip: Gastric cancer (GC) has high cancer-related mortality, which is mainly caused by distant metastasis including hematogenous metastasis. Numerous steps are required to establish a metastatic focus, and understanding the molecular mechanisms of each step is necessary to conquer metastasis. Development and dissemination of sequencing technology have elucidated some of the molecular biological mechanisms associated with cancer metastasis. This review aims to summarize the molecules reportedly contributing to hematogenous metastasis from GC and to become the groundwork for the further development of novel biomarkers and molecular targets.
- Citation: Shimizu D, Kanda M, Kodera Y. Emerging evidence of the molecular landscape specific for hematogenous metastasis from gastric cancer. World J Gastrointest Oncol 2018; 10(6): 124-136
- URL: https://www.wjgnet.com/1948-5204/full/v10/i6/124.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i6.124
Malignant tumor cells have characteristics unlike noncancerous cells, such as autonomous growth, immortalization, invasion and metastasis. Among these characteristics, metastasis greatly affects the quality of life of patients and is the main cause of cancer-related mortality. Understanding the mechanism and management of metastasis is urgently required to improve the prognosis of cancer patients.
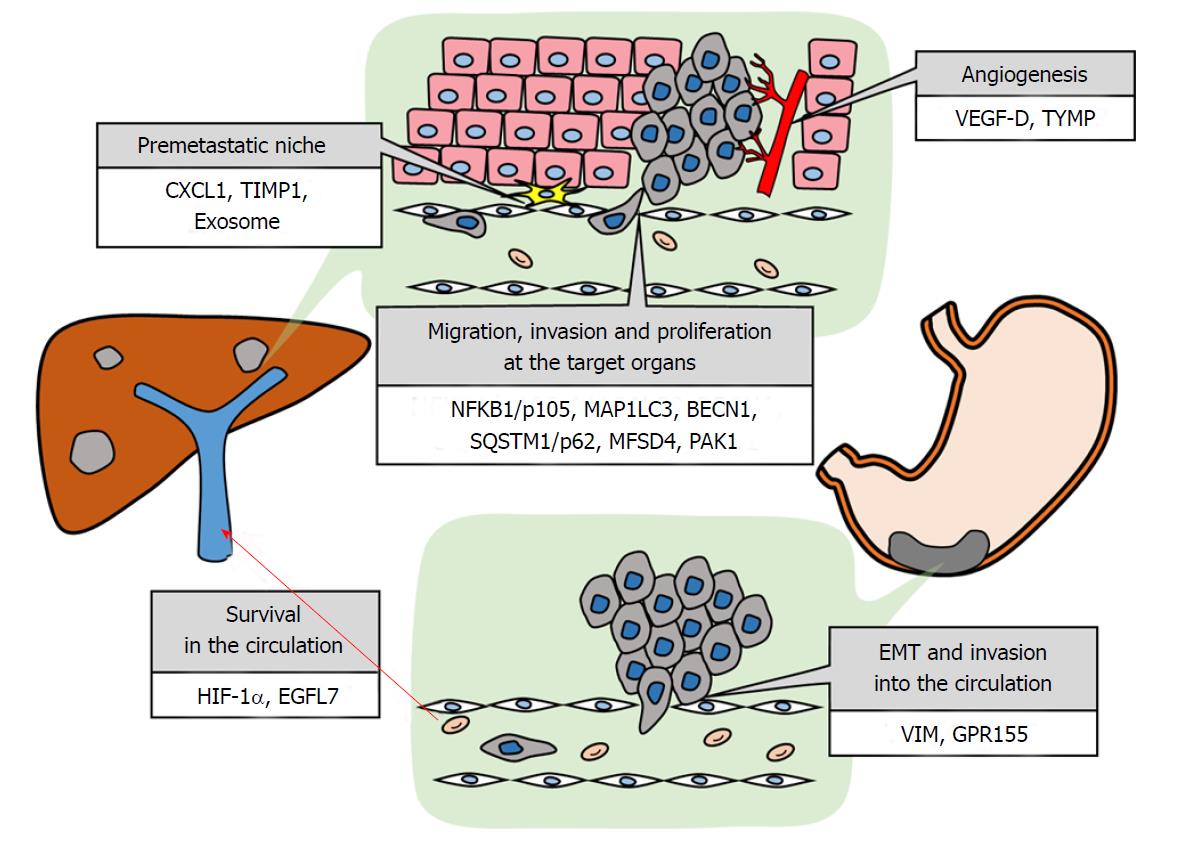
The establishment of metastasis is affected by various conditions. Metastatic sites depend on anatomical and hemodynamic structures of the vascular system[1]. Digestive cancers have a higher incidence of hepatic metastasis than other malignancies, and colon cancer tends to metastasize to the liver more frequently than rectal cancer because of the portal vein reflux. Alternatively, the frequency of hepatic metastasis is lower in gastric cancer (GC) than that in colon cancer despite the similar portal vein reflux in both cancers[2]. Cancers have respective organotropism that cannot be illustrated by only the anatomical viewpoint. A compatibility between circulating tumor cells and a premetastatic niche is required, which is referred to as the seed and soil hypothesis[3]. This hypothesis has pioneered the molecular biological understanding of the mechanism of tumor metastasis. Recently, improved sequencing technology has provided new insight into the steps required for tumor metastasis, such as vascular invasion, detachment, survival in hypoxic or non-anchored environments, immune evasion, tissue engraftment, and colonization[4,5]. In addition to metastatic organotropism due to the primary organ, intratumor and intertumor heterogeneity contributes to metastatic target organ determination[6,7]. Only an appropriate subclone with suitable attributes for a certain microenvironment can form a metastatic focus in a corresponding organ. In this article, we review the molecules associated with hematogenous metastasis from GC and microenvironment establishment for hepatic metastasis that is representative of hematogenous metastasis and list the molecules in Table 1 and Figure 1.
| Molecule | Full name | Biological function | Specimens | Detection methods | In vivo | Associating molecules and cells | Ref. |
| EMT and invasion into the circulation | |||||||
| VIM | Vimentin | Type III intermediate filament | GC tissue | IHC | - | HER2 | [16] |
| GPR155 | G protein-coupled receptor 155 | Seven-pass transmembrane receptor | GC tissue, GC cell line | qPCR, IHC | - | TWIST1, WNT5B, p-ERK1/2, p-STAT1 | [19] |
| Survival in the circulation | |||||||
| HIF-1α | Hypoxia inducible factor-1 alpha | Transcription factor in response to hypoxia | GC tissue | IHC | - | - | [25] |
| EGFL7 | Epidermal growth factor-like domain-containing protein 7 | Epidermal growth factor for vasculogenesis | GC tissue, GC cell line | qPCR, WB, IHC | Yes | AKT, SNAI1 | [30] |
| Premetastatic niche | |||||||
| CXCL1 | C-X-C motif chemokine ligand 1 | Inflammatory chemokine binding CXCR2 | CRC cell line, liver (M)1, lung (M)1, cecum (M)1 | ELISA, FCM | Yes | CXCR2, VEGF-A, MDSCs | [36] |
| TIMP1 | Tissue inhibitor of metallopeptidase 1 | Inhibitor of MMPs | Plasma, CRC tissue, CRC cell line, liver (M)1 | qPCR, ELISA | Yes | SDF-1, Neutrophil | [43] |
| Plasma, PDAC tissue, PDAC cell line (M), liver (M) | qPCR, ELISA, IHC | Yes | PI3K, CD63, SDF-1, HSC, Neutrophil | [44] | |||
| Exosome | - | Cell-derived membrane vesicle | CRC tissue, serum, CRC cell line | qPCR, WB | Yes | miR-203 | [51] |
| PDAC cell line, liver (M)1, lung (M)1, spleen (M)1, kidney (M)1, brain (M)1, bone marrow (M)1 | WB, IHC, IF, FCM, Proteomics | Yes | Proteins2 | [52] | |||
| Migration, invasion and proliferation at the target organs | |||||||
| NFKB1/p105 | Nuclear factor kappa B subunit 1 | Transcription factor | GC tissue | FCM | - | - | [55] |
| GC tissue | FCM | - | Ki-67 | [57] | |||
| MAP1LC3 | Microtubule associated protein 1 light chain 3 | Subunit of MAP1 and associated with autophagy | GC tissue | IHC | - | - | [63] |
| BECN1 | Beclin1 | Autophagy regulator and component of PI3K complex | GC tissue | IHC | - | - | [63] |
| SQSTM1/p62 | sequestosome 1 | Activator of NF-kB signaling | GC tissue | IHC | - | - | [63] |
| MFSD4 | Major facilitator superfamily domain containing 4 | Multi-pass transmembrane protein | GC tissue, GC cell line | qPCR | - | BMP2, NUDT13, OCLN | [64] |
| PAK1 | p21 (RAC1) activated kinase 1 | serine/threonine p21-activating kinase | GC tissue, GC cell line | qPCR, WB, IHC, IF | Yes | ATF2, miR-132, CD44, FN1 | [66] |
| Angiogenesis | |||||||
| VEGF-D | Vascular endothelial growth factor-D | Growth factor for angiogenesis | GC tissue | IHC | - | - | [70] |
| TYMP | Thymidine phosphorylase | Angiogenic factor | GC tissue | IHC | - | - | [72] |
| GC tissue | IHC | - | - | [73] | |||
| Biomarkers predicting hematogenous metastasis from GC | |||||||
| IL-6 | Interleukin-6 | Inflammatory cytokines | Serum | ELISA, CLEIA | - | HGF | [78] |
| Glut1 | Glucose transporter-1 | Glucose transporter | GC tissue | IHC | - | - | [79] |
| HER2 | Human epidermal growth factor receptor 2 | Epidermal growth factor receptor | GC tissue | IHC, FISH | - | - | [80] |
| GC tissue | IHC, FISH | - | - | [81] | |||
| NCPAP3 | NTase domain containing non-canonical poly(A) polymerase 3 | mRNA stabilizing factor | GC tissue, GC cell line | qPCR | - | - | [82] |
| NPM1 | Nucleophosmin 1 | Nucleolar protein | GC tissue | IHC | - | - | [83] |
| CXCR4 | C-X-C motif chemokine receptor 4 | Inflammatory chemokine receptor binding CXCL12 | GC tissue | IHC | - | CXCL12 | [84] |
| CXCL12 | C-X-C motif chemokine ligand 12 | Inflammatory chemokine binding CXCR4 | GC tissue | IHC | - | CXCR4 | [84] |
| D-Dimer | - | Fibrin degradation product | Plasma | LEIA | - | - | [85] |
| Fibrinogen | - | Coagulation factor | Plasma | Clauss clotting method | - | - | [86] |
| CD44v6 | CD44 variant 6 | Adhesion molecule | GC tissue | qPCR, IHC | - | - | [87] |
GC is the third leading cause of cancer-related death in both sexes worldwide[8]. The prognosis of patients with GC is dismal: The 5-year survival for all patients is approximately 50% and is only 25%-30% for patients with advanced GC due to a lack of curative therapeutic agents and sensitive biomarkers predicting recurrence[9]. Concerning peritoneal dissemination that is the most frequent metastasis from GC, development of recent therapeutic strategies might improve the prognosis of GC patients[10,11]. Surgical resection of hepatic metastasis can improve the outcome of GC patients, though the adaptation of surgical treatment for hematogenous metastasis is limited[12]. The development of remedies against hematogenous metastasis has stalled. Elucidating the molecular biological mechanisms specific for hematogenous metastasis from GC will be a significant and effectual step for the development of novel biomarkers and therapeutic target molecules, which will lead to the improvement of patients’ prognoses.
Epithelial mesenchymal transition and invasion into the circulation are the first steps for distant metastasis from the primary lesion. To spread to other organs through the blood stream, tumor cells must invade the basal lamina, reach and invade vessels, and detach from the primary tumor nodule. Then, single tumor cells or tumorspheres must acquire a mesenchymal phenotype and resist anoikis to arrive at a target organ. We have listed the genes that reportedly contribute to these steps and summarized the studies below.
Vimentin (VIM) is a type III intermediate filament protein that is mainly expressed in mesenchymal cells and an important marker of epithelial mesenchymal transition (EMT)[13]. Epithelial cancer cells acquire motility and metastatic potential by cellular re-programming to a mesenchymal phenotype. Increased vimentin expression has been reported in various cancers including gastrointestinal cancers[14,15]. Zhao et al[16] explored the clinical significance of VIM expression and human epidermal growth factor receptor 2 (HER2) status in GC tissues by immunohistochemistry (IHC). They found that VIM expression was significantly correlated with older age, advanced stage, poorly differentiated type, venous invasion, hepatic metastasis and recurrence and that HER2 status was correlated with advanced cancer, poor differentiation, venous invasion, hepatic metastasis and recurrence. There was a significant correlation between VIM expression and HER2-positivity. VIM expression was detected in 9.8% in GC patients and was not detected in early GC patients. The 3-year survival of the patients with vimentin-positive GC was significantly poorer than that of patients with vimentin-negative GC. VIM positivity was an independent prognostic factor in multivariate analysis with respect to overall survival. VIM plays an important role in metastasis and may have a more requisite role in the establishment of hematogenous metastasis in GC. EMT inhibitors including TGF-β signaling pathway inhibitor might be a therapeutic agent for hematogenous metastasis from GC[17].
G protein-coupled receptors (GPCRs) are seven-pass transmembrane receptors that participate in diverse physiological processes including visual sensing, immune responce, cell viability, and tumor metastasis[18]. Ligand binding to GPCRs activates the G protein and intracellular signaling. Because there are numerous GPCRs and they are the origin of many intracellular signals, GPCRs represent 30%-50% of the targets of currently marketed therapeutic drugs[19]. GPR155 is a member of the GPCR family and little is known about its function. Our recent global expression analysis of primary GC tissues obtained from patients with synchronous hepatic metastasis and without metastasis to the peritoneal cavity or distant lymph nodes uncovered that GPR155 was a molecule specific for hematogenous metastasis[20]. GPR155 was the most downregulated gene in GC tissues with synchronous hepatic metastasis compared with GC tissues without hepatic metastasis. In stage IV GC, the expression level of GPR155 was significantly lower in patients with synchronous hematogenous metastasis compared with patients without hematogenous metastasis. In stage II/III GC, the patients in the GPR155 low expression group had significantly higher cumulative incidence of hematogenous recurrence. Multivariate analysis showed that downregulated expression of GPR155 mRNA was an independent predictor of hematogenous metastasis. Furthermore, we revealed that the expression level of GPR155 was inversely correlated with the expression of TWIST1 and WNT5B, which have been well known to play pivotal roles in EMT. Inhibition of GPR155 expression using siRNA specific for GPR155 increased the level of p-ERK1/2 and p-STAT1 and cell proliferation and invasion capacity in vitro. We found that GPR155 may represent a molecule specific for hematogenous metastasis from GC via EMT and cell viability promotion and may be a putative biomarker for diagnosing and predicting hematogenous metastasis from GC. GPR155 is a transmembrane receptor, is expected to be a druggable target.
When tumor cells detach from the primary nodule and enter the circulation, they are exposed to stress from hypoxia in the portal vein and a non-adherent state. Activation of an alternative metabolic pathway under hypoxia and acquisition of anoikis resistance are necessary to endure these environmental selective pressures[21,22]. A subclone that evolves to adapt itself to this severe environment for epithelial cells can reach the new soil alive. Here, we review the molecules that contribute to environmental adaptation that are reportedly related to hematogenous metastasis from GC.
The hypoxic environment is known to be related to angiogenesis, a malignant tumor phenotype and resistance to therapies[23]. The adaptation to a hypoxic environment is an important advantage for the development of distant metastases[24]. Hypoxia inducible factor-1 alpha (HIF-1α) expression is suppressed under normal oxygen partial pressure by the ubiquitin-proteasome pathway. When oxygen supply becomes deficient, the concentration of HIF-1α is elevated, promoting transcription of vascular endothelial growth factor (VEGF), glucose transporter 1, platelet derived growth factor subunit B, carbonic anhydrase 9, etc., by forming a heterodimer with HIF-1β[25]. Some studies have suggested the utility of HIF-1α inhibitor to suppress cancer cell activity[26,27]. GC cells that have detached from a primary lesion can survive and engraft in the portal vein, which is hypoxic, to form metastatic loci. Chen et al[28] showed that HIF-1α overexpression in GC tissue was more frequent in patients with hepatic metastases than without hepatic metastasis. They also reported that HIF-1α was higher in patients with peritoneal metastasis than in patients without peritoneal metastasis, but the population of high HIF-1α still tended to be large in patients with hepatic metastasis. HIF-1α must play an important role in distant metastases including hematogenous metastasis from GC.
In a physiological state, epithelial cells, including neoplastic cells, suppress anoikis by adhering to the extracellular matrix (ECM) and adjacent cells via integrin or cadherin, and a loss of adhesion induces apoptosis[29]. Anoikis resistance is an important factor for metastasizing to distant organs in various cancers[30]. In GC, anoikis resistance has been relatively well investigated in peritoneal metastasis, which is the most frequent metastasis from GC[31,32]. Luo et al[33] demonstrated that epidermal growth factor-like domain-containing protein 7 (EGFL7) promoted metastasis by activating EMT through an EGFR-AKT-Snail signaling pathway and by protecting GC cells from anoikis. Overexpression of EGFL7 significantly decreased apoptotic GC cells in suspension culture, and GC cells treated with EGFL7-specific shRNA had a significantly higher percentage of apoptotic cells. Moreover, they showed that EGFL7-overexpressing cells grew into larger tumors and were more likely to metastasize to the liver compared to EGFL7-underexpressing CG cells in vivo. Although the mouse xenografts in their study were ectopic subcutaneous tumors, the results suggested that EGFL7 should play a pivotal role in the establishment of hematogenous metastasis via anoikis resistance.
In 1978, Schofield[34] postulated that the microenvironment could maintain hematopoietic stem cells and advocated the concept of the niche for stemness in the spleen. Recently, the concept has been extended to a metastatic niche as the microenvironment that is conducive to the survival and proliferation of metastatic cancer cells[35]. A premetastatic niche is the soil in secondary organs that is formed before the arrival of circulating tumor cells by factors from primary tumor cells that adjust the premetastatic niche. Studies on blocking premetastatic niche formation may provide novel treatment strategies to prevent distant metastasis and cure cancers, as cancers cannot be cured when they metastasize to distant organ. To the best of our knowledge, there have been no reports regarding a hematogenous premetastatic niche. Hence, elements reported to be involved in premetastatic niche formation in gastrointestinal cancers are introduced below.
C-X-C motif chemokine ligand 1 (CXCL1) encodes an 11 kDa chemokine and is a member of the CXC family. CXCL1 is secreted by macrophages and epithelial cells and acts as a chemoattractant for neutrophils[36]. CXCL1 participates in angiogenesis, inflammation, wound healing and development of the spinal cord, and its aberrant expression facilitates tumorigenesis, cell proliferation and metastasis in certain cancers[37,38]. In colorectal cancer (CRC), CXCL1 contributes to premetastatic niche formation by recruiting C-X-C motif chemokine receptor (CXCR2)-positive myeloid-derived suppressor cells (MDSCs)[39]. VEGF-A secreted by primary CRC cells stimulates tumor associated macrophages (TAMs) in the primary focus to produce CXCL1. The secreted CXCL1 drives circulating MDSCs to the premetastatic liver. MDSCs isolated from premetastatic livers of xenograft mice bearing human CRC cells in the cecal wall promote CRC cell survival. The cancer cells in the primary focus drive MDSCs to the liver via CXCL1 from TAMs and might form a premetastatic niche to evade innate and adaptive immune responses.
The balance of proteases and their inhibitors is essential to maintain homeostasis in the ECM. Matrix metalloproteinases (MMPs) are proteinases that decompose the ECM, and their overexpression is reportedly associated with tumor spread and metastasis[40]. Several studies have reported the correlation between MMP overexpression and poor prognosis in several malignant tumors[41]. Thus, it was hypothesized that inhibition of MMPs would result in a therapeutic anticancer effect[42]. Tissue inhibitor of metallopeptidase (TIMP) inhibits MMP activity and prevents tissue destruction by forming a complex with MMPs[43]. However, increased expression of TIMP1 is negatively correlated with survival in patients with several cancer types[44,45]. Additionally, in GC patients, the association between TIMP1 overexpression and poor outcome has been reported[46]. Seubert et al[47] described that TIPM1 created a premetastatic niche for hepatic metastasis from CRC, which explains the paradoxical phenomenon where TIMP expression correlated with poor prognosis in cancer patients even though TIMP inhibits MMPs. In their study, high TIMP1 levels in plasma and CRC tissue were associated with hepatic metastasis in CRC patients, and TIMP1-overexpressing tumors transplanted subcutaneously diverted intravenously injected cancer cells to the liver in a mouse model. Additionally, they demonstrated that TIMP1 established a premetastatic niche by recruiting stromal cell-derived factor 1 (SDF-1)-dependent neutrophils to the liver. Grünwald et al[48] demonstrated that TIMP1 secreted by pancreatic cancer activated hepatic stellate cells (HSCs) via CD63 and phosphatidylinositol 3-kinase signaling and increased susceptibility of the liver to pancreatic cancer cells. Activated HSCs expressed SDF-1, attracting neutrophils to the liver, which formed a premetastatic niche. In vivo, systemic increases in TIMP1 lead to more hepatic metastases after injections of pancreatic cancer cells, which did not occur in TIMP1 or CD63 knockout mice. HSCs were reported to participate in the formation of a premetastatic niche in other studies[49]. TIMP1 overexpression was observed in GC tissue, and therefore TIMP1 might contribute to the formation a hepatic premetastatic niche in GC[46].
Exosomes are small membrane vesicles derived from most eukaryotic cells in vivo and in vitro[50]. Derived exosomes exist not only in the ECM but also in bodily fluids, including blood, urine and cerebrospinal fluid, circulating in the body. Past studies have indicated that exosomes are associated with various biological processes, participating in apoptosis, angiogenesis, inflammation, coagulation and antigen presentation[51]. Moreover, exosomes function as a cargo transporting proteins and nucleic acids to target cells and act as a communication tool between distant cells[52]. Recently, exosomes from cancer cells were reported to facilitate cancer progression and metastasis and to suppress anti-tumor immunity[53,54]. Takano et al[55] described that circulating exosomal microRNA (miR)-203 was associated with distant metastasis in CRC patients. Exosomal miR-203 that originated from primary CRC was reportedly incorporated into monocytes and promoted the differentiation of monocytes to M2-tumor-associated macrophages (TAMs). In a xenograft mouse model, miR-203-transfected CRC cells developed more liver metastases than control CRC cells. Their result suggested that exosomes bearing miR-203 contribute to the establishment of a premetastatic niche via TAM promotion in the liver of CRC patients. Yu et al[56] demonstrated that exosomes derived from pancreatic cancer induced a liver premetastatic niche. They performed proteomic analysis on exosomal proteins and revealed that these proteins were involved in pancreatic cancer growth, invasion and metastasis. Interestingly, another study showed that exosomes had respective organotropism, which was prescribed by integrin on their membranes[57]. The organotropism of exosomes depended on the combination of the alpha chain and β chain of integrin and distinct cells in the target organ took up the circulating exosomes. Exosomes from cancer cells were delivered by integrin to a particular organ and formed a premetastatic niche via contained proteins or nucleic acids, leading to metastatic organotropism. Further exploration of exosomes should uncover more insights on organotropism and the mechanisms of metastasis.
Cancer cells that arrive at a metastatic organ are trapped in a capillary plexus. Subsequently, the adhesion of cancer cells to epithelial cells is driven by selectin and integrin families[58]. Then, cancer cells migrate to the interval of epithelial cells and invade target organ tissue via adhesion to and decomposition of the basal lamina. Among the cancer cells that arrive at a metastatic target organ, only the cells that have acquired the capacities of adhesion, migration, invasion and proliferation can form a new tumor focus. We summarized the reported molecules associated with these steps.
Ohyama et al[59] examined nuclear factor kappa B subunit 1 (NFKB1/p105) immunofluorescence intensity in GC cells isolated from 43 clinical specimens using flow cytometry. The intensity was higher in patients with hepatic metastasis than in patients without hepatic metastasis and was positively correlated with venous invasion. In contrast, the intensity was lower in patients with peritoneal metastasis than in patients without peritoneal metastasis. NFKB1/p105 intensity was not associated with nodal metastasis, lymphatic invasion, serosal invasion or histological type. Kimura et al[60] also reported a correlation between the p105-labeling rate detected by flow cytometry and hepatic metastasis from GC. In addition, they reported that NFKB1/p105 positivity by flow cytometry correlated positively with Ki-67 positivity, an index widely used for cell proliferation[61]. NFKB1/p105 in GC cells is a putative biomarker specific for hematogenous metastasis. It was also reported that 5-FU resistance might be overcome via suppression of phosphorylated NFKB in Epstein-Barr virus-positive gastric cancer[62]. The development of therapeutic agent targeting NFKB might lead to improvement of GC patients’ prognosis.
Autophagy is an intracellular degradation system that delivers cytoplasmic proteins and organelles to the lysosome, and it is an important system for maintaining intracellular homeostasis against pathogens and nutrient stress[63]. Additionally, there are contradictory aspects in autophagy regarding neoplasia. In non-cancerous cells, autophagy protects cells from adverse effects leading to malignant transformation, such as reactive oxygen species, aberration of organelles and DNA damage[64]. In contrast, autophagy acts as an important anti-apoptotic mechanism in established cancer cells resisting hypoxia, malnutrition and therapeutic agents[65]. Therefore, inhibiting autophagy should be a viable therapeutic strategy for cancers, and effective treatments with an anti-autophagy agent have been reported[66]. Sharifi et al[67] showed that autophagy was necessary for metastatic cells to migrate and invade by focal adhesion disassembly via proteolysis of paxillin. Their work marked the first anti-metastatic effect via autophagy inhibition and was a notable achievement. Masuda et al[68] indicated the correlation between autophagy-related proteins, microtubule associated protein 1 light chain 3, beclin1 and sequestosome 1/p62, and clinicopathological features. They investigated the expression of these proteins by IHC in 510 GC tissues. Autophagy, as determined by the expression of these proteins, was significantly correlated with poor survival rates and incidence of hepatic metastasis, but not with incidence of peritoneal metastasis. Understanding the role of autophagy in tumor survival and metastasis would help develop specific autophagy inhibitors and might improve the outcome of patients with GC.
Major facilitator superfamily domain containing 4 (MFSD4) is located on chromosome 1q32.1 and encodes a multi-pass transmembrane protein, and its biological function has not yet been determined. We recently detected MFSD4 as a biomarker specific for hepatic metastasis of GC by sequencing RNA from the GC tissue of patients with or without hepatic metastasis[69]. Patients with low MFSD4 expression in primary GC tissues had significantly higher cumulative incidence of hepatic recurrence, and reduced MFSD4 expression was an independent risk factor of metachronous and synchronous hepatic metastasis. We indicated that DNA methylation in CpG islands of MFSD4 was one of the suppressive mechanisms of transcription. Furthermore, GC cell migration and invasion abilities were significantly increased by inhibition of MFSD4 expression using siRNA. MFSD4 should be a promising biomarker predicting hepatic metastasis in GC patients.
p21 (RAC1) activated kinase 1 (PAK1) is a serine/threonine-protein kinase that plays a critical role in cytoskeleton dynamics, cell adhesion, migration, proliferation, apoptosis and mitosis[70]. Liu et al[71] delineated the downstream pathway of PAK1 in which PAK1 acted as an oncogenic factor. PAK1 suppressed the expression of miR-132 via phosphorylating activating transcription factor 2 (ATF2), which bound to the promoter of miR-132. Phosphorylation of ATF2 inhibited its nuclear translocation and resulted in the diminution of miR-132. Additionally, their bioinformatics analysis revealed direct targets of miR-132, including CD44 and fibronectin 1, whose inhibition induced tumor apoptosis. Furthermore, miR-132 overexpression inhibited cell adhesion and migration in vitro and hematogenous metastasis in vivo. The patients with lower miR-132 expression in GC tissue had significantly poorer prognoses, and hepatic metastatic tissues expressed significantly lower miR-132 compared with primary GC tissues while there were no differences between primary GC tissues and lymph node metastases or peritoneal metastases. PAK1 and its downstream pathway should be a useful biomarker and therapeutic target for hematogenous metastasis from GC.
As in the primary lesion, growth factors and angiogenic factors are required for metastatic focus growth. Tumor angiogenesis is necessary to supply nutrients and oxygen, and to carry out metabolites for tumor growth[72]. Additionally, an increase in blood vessels leads to further metastatic opportunities. In this section, we introduce studies that investigated the relationship between angiogenic factors and hematogenous metastasis.
Effectiveness of anti-VEGF and anti-VEGFR monoclonal antibodies were proved in clinical management[73-75]. Several studies have shown the association between vascular endothelial growth factor-D (VEGF-D) and lymph node metastasis in GC[76,77]. Deng et al[78] indicated that VEGF-D is associated with hepatic metastasis from GC. They investigated the correlation between hepatic metastasis and the expression levels of VEGF-A, VEGF-C, VEGF-D, VEGFR-3, and CD34 by IHC. VEGF-D, VEGFR-3, CD34, Lauren classification and lymph node metastasis were associated with hepatic metastasis after radical surgery in univariate analysis, and VEGF-D was the only independent indicator of hepatic metastasis in multivariate analysis. They concluded that VEGF-D is an important factor for predicting hepatic metastasis of GC. The VEGF family plays a key role in angiogenesis and lymphangiogenesis. Their study lacks evidence of the molecular mechanism of hepatic metastasis establishment, though VEGF-D might contribute to hepatic metastasis via angiogenesis, which increases intratumor blood flow and nourishes the metastatic tumor.
Thymidine phosphorylase (TYMP) is an enzyme involved in pyrimidine nucleotide metabolism and was recently reported to be identical to platelet-derived endothelial cell growth factor (PD-ECGF). PD-ECGF has angiogenic activity in vitro and in vivo[79]. Kimura et al[80] investigated the association of clinicopathological features with the expression of VEGF and TYMP in IHC analysis. In their study, there was a significant correlation between the positive expression of VEGF and lymphatic invasion. Additionally, the positive expression of TYMP and VEGF was significantly correlated with the frequency of hepatic recurrence. Moreover, patients with positivity of both TYMP and VEGF had significantly unfavorable prognoses. Their results indicated that combination analyses of TYMP and VEGF expression in GC appear to be well-characterized indicators of prognosis and suggested that co-expression of TYMP and VEGF, molecules contributing to angiogenesis, supported hepatic metastasis formation. Maeda et al[81] also reported that TYMP was associated with angiogenesis and hepatic metastasis from GC. They showed a correlation between TYMP expression and microvessel density in GC tissue by IHC. TYMP-positive patients had higher microvessel density and a significantly higher frequency of hepatic metastasis. Their result suggested that microangiogenesis promotes the establishment of hepatic metastasis.
Many cancer-related genes that should be putative biomarkers and therapeutic targets have been reported in the past[82,83]. In recent decades, the progress and generalization of sequencing technologies have enriched our molecular knowledge regarding cancers and revealed the molecular mechanisms specific for distinct metastatic organs and hematogenous metastasis[84,85]. While some studies have described mechanisms contributing to the establishment of hematogenous metastasis or downstream pathways, other studies have described biomarkers for predicting hematogenous metastasis. These biomarkers are useful for patient stratification, selection of therapeutic strategy and postoperative surveillance according to individual risk of metastasis and recurrence. Additionally, further investigation of molecular mechanisms might lead to the development of novel therapeutic target molecules. We listed the molecules reported as biomarkers of hematogenous metastasis in Table 1 and outlined some of them below[86-95].
Interleukin-6 (IL-6) is a representative inflammatory cytokine that participates in B cell maturation, T cell differentiation, activation of natural killer cells, suppression of regulatory T cells and cancer cachexia[96]. Additionally, IL-6 involvement in the biological activity of cancer cells has been previously reported[97,98]. High IL-6 expressing tumor cells formed more distant metastases in breast cancer, lung cancer and hepatocellular carcinoma[99,100]. Furthermore, adhesion of tumor cells to targeted organs, which leads to metastatic focus formation, is facilitated in high IL-6 expressing organs such as brain, lung, liver and bone marrow[101]. In GC, the association between IL-6 and clinicopathological factors has been reported[102,103]. Ashizawa et al[86] assessed the correlation of preoperative serum levels of IL-6 with GC patients’ characteristics. They found that serum IL-6 level was significantly related to advanced stage, tumor depth, lymphatic invasion, venous invasion and hepatic metastasis. IL-6 expression in GC tissue or serum might be related to distant metastasis, and in particular, hepatic metastasis.
Glucose intake is increased in malignant tumor cells, which is facilitated by glucose transporters. Glucose transporter-1 (Glut1), a member of the glucose transporter family, is overexpressed in several cancers and is correlated with malignant phenotypes[104]. The association between Glut1 and GC was first reported in 2000. Kim et al[105] showed that high Glut1 protein expression was associated with an intestinal type of GC. In 2001, Kawamura et al[87] demonstrated that Glut1-positive GC by IHC had a significantly higher incidence of hepatic metastasis whereas there was no statistical significance regarding the correlation between Glut1 and peritoneal metastasis. Additionally, they showed that Glut1-positive GC cells were localized mainly in the central part of the tumor. The result was consistent with an adaptation to a hypoxic environment at the center of the tumor. It is expected that the transcription of Glut1 is activated via increased HIF1-α. Glut1 could be a putative biomarker for hepatic metastasis from GC.
HER is a member of the epidermal growth factor receptor family. HER2 is involved in the pathogenesis and poor prognosis of breast cancer and GC, and monoclonal antibodies to HER2, trastuzumab and pertuzumab have been applied clinically worldwide[106,107]. The major role of HER2 is to promote cell proliferation, suppress apoptosis, and facilitate tumorigenesis[108]. A few studies have reported the association between HER2 expression and GC patients’ prognoses[109,110]. Lee et al[88] analyzed the relationship between HER2 expression and computed tomography (CT) imaging in GC patients. In their cohort of 276 patients, hepatic metastases were more frequently found in HER2-positive GC while peritoneal metastasis was more often found in HER2-negative GC. Hepatic metastases were significant independent factors that predict HER2-positive cancers. Similarly, Matsusaka et al[89] reported the correlation between HER2 positivity and hepatic metastasis in 1466 GC patients. In their data, the incidence of hepatic metastasis was significantly higher in HER2-positive patients, and moreover, HER2-positive patients had a significantly lower incidence of peritoneal metastasis. These two studies suggested that HER2 positivity was associated with hepatic metastasis from GC specifically and was negatively associated with peritoneal metastasis. As supportive data, a meta-analysis also demonstrated that HER2 positivity was associated with differentiated type and intestinal type, and differentiated type and Borrmann type1/2 are reportedly risk factors for hepatic metastasis from GC[111]. The mechanism is unknown; however, HER2 positivity may be a predictive biomarker for hepatic metastasis from GC.
We recently focused on genes reflecting the metastatic potential of GC cells and identified a NTase domain containing non-canonical poly(A) polymerase 3 (NCPAP3) as a predictor for hepatic metastasis[90]. NCPAP3 has been shown to regulate translation by acting as an mRNA stability factor and the gene has a mutation predicting worse prognosis in multiple myeloma[112]. NCPAP3 expression was decreased in GC tissue compared with adjacent noncancerous mucosae in most patients. Patients with lower NCPAP3 expression have a shorter overall survival and disease-free survival. Furthermore, lower NCPAP3 expression was significantly correlated with the cumulative incidence of hepatic metastasis while there was no significant difference in the cumulative incidence of peritoneal metastasis by NCPAP3 expression. Additionally, we revealed the mechanisms for suppression of NCPAP3 expression. Copy number alterations at the NCPAP3 locus were observed in the GC tissues of 35% of patients and in 50% of GC cell lines. Additionally, 42% of GC cell lines harbored single nucleotide variants, and all of these cell lines expressed lower NCPAP3 mRNA. Aberrant DNA methylation was not observed in GC cell lines. NCPAP3 not only associates with the malignant phenotype of GC but may also be a predictive biomarker specific for hepatic metastasis.
Nucleophosmin 1 (NPM1) is a nucleolar phosphoprotein involved in numerous cellular processes, including centrosome duplication, histone assembly, protein chaperoning and cell proliferation[113,114]. NPM1 downregulates the tumor suppressor cyclin dependent kinase inhibitor 2A in the nucleolus and has inhibitory effects by activating transcription factor 5 (ATF5) and abrogating ATF5-induced G(2)/M cell cycle blockade[115,116]. Some studies revealed that positive expression of NPM1 in GC tissue was associated with poor prognosis in postoperative GC patients. Zhou et al[117] and Li et al[118] found that NPM1 level was linked to more advanced tumor stages and was an independent indicator for prognosis and recurrence. Ding et al[91] indicated a correlation between NPM1 expression and clinicopathological features including metastatic site. Patients with NPM1-positive GC had significantly higher rates of hepatic metastasis and recurrence. While the molecular basis remains to be elucidated, NPM1 expression might predict hepatic metastasis from GC.
The development of molecular techniques and bioinformatics has led to accumulated knowledge and an understanding of the mechanisms of distant metastasis from cancer. Cancers generate manifold subclones as seeds based on their genomic instability and heterogeneity. Subsequently, subclones that pass through the selection of each step for metastasis and adapt to the secondary organ, the so-called soil, have the opportunity to metastasize. Moreover, cancer cells create a premetastatic niche via secretion of exosomes. However, knowledge of the mechanism specific for hematogenous metastasis is scarce, and the full picture of organotropism has not yet been elucidated. Hematogenous metastasis is a factor that strongly contributes to poor prognosis in GC. Therefore, understanding and controlling its mechanism are significant issues. Further studies on this theme should improve GC patients’ prognoses.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Ding SZ, Liu SH, Yip D S- Editor: Cui LJ L- Editor: A E- Editor: Huang Y
| 1. | Ewing J. Neoplastic diseases; a treatise on tumors. 2ed. Philadelphia London: W.B. Saunders company 1922; . [Cited in This Article: ] |
| 2. | Weiss L. Metastasis of cancer: a conceptual history from antiquity to the 1990s. Cancer Metastasis Rev. 2000;19:I-XI, 193-383. [PubMed] [Cited in This Article: ] |
| 3. | Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98-101. [PubMed] [Cited in This Article: ] |
| 4. | De Mattos-Arruda L, Bidard FC, Won HH, Cortes J, Ng CK, Peg V, Nuciforo P, Jungbluth AA, Weigelt B, Berger MF. Establishing the origin of metastatic deposits in the setting of multiple primary malignancies: the role of massively parallel sequencing. Mol Oncol. 2014;8:150-158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Shimizu D, Kanda M, Kodera Y. Review of recent molecular landscape knowledge of gastric cancer. Histol Histopathol. 2018;33:11-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 23] [Reference Citation Analysis (0)] |
| 6. | Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, Cai T, Clevers H, Swanton C, Nowak MA. Origins of lymphatic and distant metastases in human colorectal cancer. Science. 2017;357:55-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 306] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 7. | Makohon-Moore AP, Zhang M, Reiter JG, Bozic I, Allen B, Kundu D, Chatterjee K, Wong F, Jiao Y, Kohutek ZA. Limited heterogeneity of known driver gene mutations among the metastases of individual patients with pancreatic cancer. Nat Genet. 2017;49:358-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 263] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 8. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20108] [Cited by in F6Publishing: 19780] [Article Influence: 2197.8] [Reference Citation Analysis (17)] |
| 9. | Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385:977-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1579] [Cited by in F6Publishing: 1613] [Article Influence: 179.2] [Reference Citation Analysis (0)] |
| 10. | Kanda M, Kodera Y, Sakamoto J. Updated evidence on adjuvant treatments for gastric cancer. Expert Rev Gastroenterol Hepatol. 2015;9:1549-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Ishigami H, Kitayama J, Kaisaki S, Hidemura A, Kato M, Otani K, Kamei T, Soma D, Miyato H, Yamashita H. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol. 2010;21:67-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 12. | Kodera Y, Fujitani K, Fukushima N, Ito S, Muro K, Ohashi N, Yoshikawa T, Kobayashi D, Tanaka C, Fujiwara M. Surgical resection of hepatic metastasis from gastric cancer: a review and new recommendation in the Japanese gastric cancer treatment guidelines. Gastric Cancer. 2013;17:206-212. [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4877] [Cited by in F6Publishing: 4961] [Article Influence: 225.5] [Reference Citation Analysis (0)] |
| 14. | Singh S, Sadacharan S, Su S, Belldegrun A, Persad S, Singh G. Overexpression of vimentin: role in the invasive phenotype in an androgen-independent model of prostate cancer. Cancer Res. 2003;63:2306-2311. [PubMed] [Cited in This Article: ] |
| 15. | Shirahata A, Sakata M, Sakuraba K, Goto T, Mizukami H, Saito M, Ishibashi K, Kigawa G, Nemoto H, Sanada Y. Vimentin methylation as a marker for advanced colorectal carcinoma. Anticancer Res. 2009;29:279-281. [PubMed] [Cited in This Article: ] |
| 16. | Zhao W, Yue L, Zhou F, Xu C, Liang W, Sui A, Ding A, Qiu W. Clinical significance of vimentin expression and Her-2 status in patients with gastric carcinoma. Clin Transl Sci. 2013;6:184-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Bartscht T, Rosien B, Rades D, Kaufmann R, Biersack H, Lehnerta H, Ungefroren H. Inhibition of TGF-β Signaling in Tumor Cells by Small Molecule Src Family Kinase Inhibitors. Anticancer Agents Med Chem. 2017;17:1351-1356. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 980] [Cited by in F6Publishing: 964] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 19. | Jo M, Jung ST. Engineering therapeutic antibodies targeting G-protein-coupled receptors. Exp Mol Med. 2016;48:e207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Shimizu D, Kanda M, Tanaka H, Kobayashi D, Tanaka C, Hayashi M, Iwata N, Niwa Y, Takami H, Yamada S. GPR155 Serves as a Predictive Biomarker for Hematogenous Metastasis in Patients with Gastric Cancer. Sci Rep. 2017;7:42089. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Gilkes DM, Semenza GL, Wirtz D. Hypoxia and the extracellular matrix: drivers of tumour metastasis. Nat Rev Cancer. 2014;14:430-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1028] [Cited by in F6Publishing: 941] [Article Influence: 94.1] [Reference Citation Analysis (0)] |
| 22. | Douma S, Van Laar T, Zevenhoven J, Meuwissen R, Van Garderen E, Peeper DS. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature. 2004;430:1034-1039. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 445] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 23. | Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckl). 2015;3:83-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 842] [Cited by in F6Publishing: 1140] [Article Influence: 126.7] [Reference Citation Analysis (0)] |
| 24. | Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res. 2010;16:5928-5935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 466] [Cited by in F6Publishing: 502] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 25. | Wan J, Chai H, Yu Z, Ge W, Kang N, Xia W, Che Y. HIF-1α effects on angiogenic potential in human small cell lung carcinoma. J Exp Clin Cancer Res. 2011;30:77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Riby JE, Firestone GL, Bjeldanes LF. 3,3’-diindolylmethane reduces levels of HIF-1alpha and HIF-1 activity in hypoxic cultured human cancer cells. Biochem Pharmacol. 2008;75:1858-1867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Sutendra G, Dromparis P, Kinnaird A, Stenson TH, Haromy A, Parker JM, McMurtry MS, Michelakis ED. Mitochondrial activation by inhibition of PDKII suppresses HIF1a signaling and angiogenesis in cancer. Oncogene. 2013;32:1638-1650. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 28. | Chen L, Shi Y, Yuan J, Han Y, Qin R, Wu Q, Jia B, Wei B, Wei L, Dai G. HIF-1 alpha overexpression correlates with poor overall survival and disease-free survival in gastric cancer patients post-gastrectomy. PLoS One. 2014;9:e90678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Buchheit CL, Weigel KJ, Schafer ZT. Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat Rev Cancer. 2014;14:632-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 259] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 30. | Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Focal adhesion kinase gene silencing promotes anoikis and suppresses metastasis of human pancreatic adenocarcinoma cells. Surgery. 2004;135:555-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Nishimura S, Adachi M, Ishida T, Matsunaga T, Uchida H, Hamada H, Imai K. Adenovirus-mediated transfection of caspase-8 augments anoikis and inhibits peritoneal dissemination of human gastric carcinoma cells. Cancer Res. 2001;61:7009-7014. [PubMed] [Cited in This Article: ] |
| 32. | Sakai H, Ohuchida K, Mizumoto K, Cui L, Nakata K, Toma H, Nagai E, Tanaka M. Inhibition of p600 expression suppresses both invasiveness and anoikis resistance of gastric cancer. Ann Surg Oncol. 2011;18:2057-2065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Luo BH, Xiong F, Wang JP, Li JH, Zhong M, Liu QL, Luo GQ, Yang XJ, Xiao N, Xie B. Epidermal growth factor-like domain-containing protein 7 (EGFL7) enhances EGF receptor-AKT signaling, epithelial-mesenchymal transition, and metastasis of gastric cancer cells. PLoS One. 2014;9:e99922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7-25. [PubMed] [Cited in This Article: ] |
| 35. | Peinado H, Zhang H, Matei IR, Costa-Silva B, Hoshino A, Rodrigues G, Psaila B, Kaplan RN, Bromberg JF, Kang Y. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1003] [Cited by in F6Publishing: 1108] [Article Influence: 158.3] [Reference Citation Analysis (0)] |
| 36. | Amiri KI, Richmond A. Fine tuning the transcriptional regulation of the CXCL1 chemokine. Prog Nucleic Acid Res Mol Biol. 2003;74:1-36. [PubMed] [Cited in This Article: ] |
| 37. | Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol. 2002;72:9-18. [PubMed] [Cited in This Article: ] |
| 38. | Verbeke H, Geboes K, Van Damme J, Struyf S. The role of CXC chemokines in the transition of chronic inflammation to esophageal and gastric cancer. Biochim Biophys Acta. 2012;1825:117-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 39. | Wang D, Sun H, Wei J, Cen B, DuBois RN. CXCL1 Is Critical for Premetastatic Niche Formation and Metastasis in Colorectal Cancer. Cancer Res. 2017;77:3655-3665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 220] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 40. | Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1413] [Cited by in F6Publishing: 1456] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 41. | Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161-174. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4447] [Cited by in F6Publishing: 4393] [Article Influence: 199.7] [Reference Citation Analysis (0)] |
| 42. | Brown PD. Ongoing trials with matrix metalloproteinase inhibitors. Expert Opin Investig Drugs. 2000;9:2167-2177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 133] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267-283. [PubMed] [Cited in This Article: ] |
| 44. | Krüger A, Fata JE, Khokha R. Altered tumor growth and metastasis of a T-cell lymphoma in Timp-1 transgenic mice. Blood. 1997;90:1993-2000. [PubMed] [Cited in This Article: ] |
| 45. | Holten-Andersen MN, Stephens RW, Nielsen HJ, Murphy G, Christensen IJ, Stetler-Stevenson W, Brünner N. High preoperative plasma tissue inhibitor of metalloproteinase-1 levels are associated with short survival of patients with colorectal cancer. Clin Cancer Res. 2000;6:4292-4299. [PubMed] [Cited in This Article: ] |
| 46. | Grunnet M, Mau-Sørensen M, Brünner N. Tissue inhibitor of metalloproteinase 1 (TIMP-1) as a biomarker in gastric cancer: a review. Scand J Gastroenterol. 2013;48:899-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Seubert B, Grünwald B, Kobuch J, Cui H, Schelter F, Schaten S, Siveke JT, Lim NH, Nagase H, Simonavicius N. Tissue inhibitor of metalloproteinases (TIMP)-1 creates a premetastatic niche in the liver through SDF-1/CXCR4-dependent neutrophil recruitment in mice. Hepatology. 2015;61:238-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 48. | Grünwald B, Harant V, Schaten S, Frühschütz M, Spallek R, Höchst B, Stutzer K, Berchtold S, Erkan M, Prokopchuk O. Pancreatic Premalignant Lesions Secrete Tissue Inhibitor of Metalloproteinases-1, Which Activates Hepatic Stellate Cells Via CD63 Signaling to Create a Premetastatic Niche in the Liver. Gastroenterology. 2016;151:1011-1024.e7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 49. | Eveno C, Hainaud P, Rampanou A, Bonnin P, Bakhouche S, Dupuy E, Contreres JO, Pocard M. Proof of prometastatic niche induction by hepatic stellate cells. J Surg Res. 2015;194:496-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1110] [Cited by in F6Publishing: 1240] [Article Influence: 103.3] [Reference Citation Analysis (0)] |
| 51. | Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3512] [Cited by in F6Publishing: 3750] [Article Influence: 170.5] [Reference Citation Analysis (0)] |
| 52. | Belting M, Wittrup A. Nanotubes, exosomes, and nucleic acid-binding peptides provide novel mechanisms of intercellular communication in eukaryotic cells: implications in health and disease. J Cell Biol. 2008;183:1187-1191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 138] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 53. | Ruivo CF, Adem B, Silva M, Melo SA. The Biology of Cancer Exosomes: Insights and New Perspectives. Cancer Res. 2017;77:6480-6488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 375] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 54. | Nakajima K, Nangia-Makker P, Hogan V, Raz A. Cancer Self-Defense: An Immune Stealth. Cancer Res. 2017;77:5441-5444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 55. | Takano Y, Masuda T, Iinuma H, Yamaguchi R, Sato K, Tobo T, Hirata H, Kuroda Y, Nambara S, Hayashi N. Circulating exosomal microRNA-203 is associated with metastasis possibly via inducing tumor-associated macrophages in colorectal cancer. Oncotarget. 2017;8:78598-78613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 56. | Yu Z, Zhao S, Ren L, Wang L, Chen Z, Hoffman RM, Zhou J. Pancreatic cancer-derived exosomes promote tumor metastasis and liver pre-metastatic niche formation. Oncotarget. 2017;8:63461-63483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 57. | Hoshino A, Costa-Silva B, Shen TL, Rodrigues G, Hashimoto A, Tesic Mark M, Molina H, Kohsaka S, Di Giannatale A, Ceder S. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2697] [Cited by in F6Publishing: 3218] [Article Influence: 357.6] [Reference Citation Analysis (0)] |
| 58. | Kannagi R. Carbohydrate-mediated cell adhesion involved in hematogenous metastasis of cancer. Glycoconj J. 1997;14:577-584. [PubMed] [Cited in This Article: ] |
| 59. | Ohyama S, Yonemura Y, Kimura H, Kosaka T, Miyazaki I, Sasaki T. Proliferation-associated nuclear antigen p105 as a marker for metastasis of human gastric cancer. J Cancer Res Clin Oncol. 1991;117:583-586. [PubMed] [Cited in This Article: ] |
| 60. | Kimura H, Yonemura Y, Epstein AL. Flow cytometric quantitation of the proliferation-associated nuclear antigen p105 and DNA content in advanced gastric cancers. Cancer. 1991;68:2175-2180. [PubMed] [Cited in This Article: ] |
| 61. | Kimura H, Yonemura Y, Miyazaki I. Proliferative activity in gastric cancer determined with cell cycle-related monoclonal antibodies Ki-67 and p105: analysis by flow cytometry. J Surg Oncol. 1992;51:174-178. [PubMed] [Cited in This Article: ] |
| 62. | Shin JY, Kim JO, Lee SK, Chae HS, Kang JH. LY294002 may overcome 5-FU resistance via down-regulation of activated p-AKT in Epstein-Barr virus-positive gastric cancer cells. BMC Cancer. 2010;10:425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:1845-1846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 444] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 64. | Cuervo AM. Autophagy: in sickness and in health. Trends Cell Biol. 2004;14:70-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 590] [Cited by in F6Publishing: 595] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 65. | Kimmelman AC. The dynamic nature of autophagy in cancer. Genes Dev. 2011;25:1999-2010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 426] [Cited by in F6Publishing: 454] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 66. | Shao Y, Gao Z, Marks PA, Jiang X. Apoptotic and autophagic cell death induced by histone deacetylase inhibitors. Proc Natl Acad Sci USA. 2004;101:18030-18035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 445] [Cited by in F6Publishing: 466] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 67. | Sharifi MN, Mowers EE, Drake LE, Collier C, Chen H, Zamora M, Mui S, Macleod KF. Autophagy Promotes Focal Adhesion Disassembly and Cell Motility of Metastatic Tumor Cells through the Direct Interaction of Paxillin with LC3. Cell Rep. 2016;15:1660-1672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 68. | Masuda GO, Yashiro M, Kitayama K, Miki Y, Kasashima H, Kinoshita H, Morisaki T, Fukuoka T, Hasegawa T, Sakurai K. Clinicopathological Correlations of Autophagy-related Proteins LC3, Beclin 1 and p62 in Gastric Cancer. Anticancer Res. 2016;36:129-136. [PubMed] [Cited in This Article: ] |
| 69. | Kanda M, Shimizu D, Tanaka H, Shibata M, Iwata N, Hayashi M, Kobayashi D, Tanaka C, Yamada S, Fujii T. Metastatic pathway-specific transcriptome analysis identifies MFSD4 as a putative tumor suppressor and biomarker for hepatic metastasis in patients with gastric cancer. Oncotarget. 2016;7:13667-13679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Kelly ML, Chernoff J. Getting smart about p21-activated kinases. Mol Cell Biol. 2011;31:386-387. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 71. | Liu F, Cheng Z, Li X, Li Y, Zhang H, Li J, Liu F, Xu H, Li F. A novel Pak1/ATF2/miR-132 Signaling Axis is involved in the hematogenous metastasis of gastric cancer cells. Mol Ther Nucleic Acids. 2017;8:370-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Deryugina EI, Quigley JP. Tumor angiogenesis: MMP-mediated induction of intravasation- and metastasis-sustaining neovasculature. Matrix Biol. 2015;44-46:94-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 291] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 73. | Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, Lim HY, Yamada Y, Wu J, Langer B. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011;29:3968-3976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 795] [Cited by in F6Publishing: 887] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 74. | Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, Sugimoto N, Lipatov O, Kim TY. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224-1235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1613] [Cited by in F6Publishing: 1583] [Article Influence: 158.3] [Reference Citation Analysis (0)] |
| 75. | Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, Safran H, Dos Santos LV, Aprile G, Ferry DR. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1541] [Cited by in F6Publishing: 1463] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 76. | Shida A, Fujioka S, Kobayashi K, Ishibashi Y, Nimura H, Mitsumori N, Yanaga K. Expression of vascular endothelial growth factor (VEGF)-C and -D in gastric carcinoma. Int J Clin Oncol. 2006;11:38-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 77. | Shida A, Fujioka S, Ishibashi Y, Kobayashi K, Nimura H, Mitsumori N, Suzuki Y, Kawakami M, Urashima M, Yanaga K. Prognostic significance of vascular endothelial growth factor D in gastric carcinoma. World J Surg. 2005;29:1600-1607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 78. | Deng J, Liang H, Sun D, Pan Y, Wang B, Guo Y. Vascular endothelial growth factor-D is correlated with hepatic metastasis from gastric cancer after radical gastrectomy. Surgery. 2009;146:896-905. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 79. | Ishikawa F, Miyazono K, Hellman U, Drexler H, Wernstedt C, Hagiwara K, Usuki K, Takaku F, Risau W, Heldin CH. Identification of angiogenic activity and the cloning and expression of platelet-derived endothelial cell growth factor. Nature. 1989;338:557-562. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 514] [Cited by in F6Publishing: 510] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 80. | Kimura H, Konishi K, Nukui T, Kaji M, Maeda K, Yabushita K, Tsuji M, Miwa A. Prognostic significance of expression of thymidine phosphorylase and vascular endothelial growth factor in human gastric carcinoma. J Surg Oncol. 2001;76:31-36. [PubMed] [Cited in This Article: ] |
| 81. | Maeda K, Chung YS, Ogawa Y, Takatsuka S, Kang SM, Ogawa M, Sawada T, Onoda N, Kato Y, Sowa M. Thymidine phosphorylase/platelet-derived endothelial cell growth factor expression associated with hepatic metastasis in gastric carcinoma. Br J Cancer. 1996;73:884-888. [PubMed] [Cited in This Article: ] |
| 82. | Shimizu D, Kanda M, Nomoto S, Oya H, Takami H, Hibino S, Suenaga M, Inokawa Y, Hishida M, Takano N. Identification of intragenic methylation in the TUSC1 gene as a novel prognostic marker of hepatocellular carcinoma. Oncol Rep. 2014;31:1305-1313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 83. | Tanaka H, Kanda M, Koike M, Iwata N, Shimizu D, Ezaka K, Sueoka S, Tanaka Y, Takami H, Hashimoto R. Adherens junctions associated protein 1 serves as a predictor of recurrence of squamous cell carcinoma of the esophagus. Int J Oncol. 2015;47:1811-1818. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Kanda M, Shimizu D, Tanaka H, Tanaka C, Kobayashi D, Hayashi M, Iwata N, Niwa Y, Yamada S, Fujii T. Significance of SYT8 For the Detection, Prediction, and Treatment of Peritoneal Metastasis From Gastric Cancer. Ann Surg. 2018;267:495-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 85. | Kanda M, Kodera Y. Molecular mechanisms of peritoneal dissemination in gastric cancer. World J Gastroenterol. 2016;22:6829-6840. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 99] [Cited by in F6Publishing: 101] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 86. | Ashizawa T, Okada R, Suzuki Y, Takagi M, Yamazaki T, Sumi T, Aoki T, Ohnuma S, Aoki T. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric Cancer. 2005;8:124-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 87. | Kawamura T, Kusakabe T, Sugino T, Watanabe K, Fukuda T, Nashimoto A, Honma K, Suzuki T. Expression of glucose transporter-1 in human gastric carcinoma: association with tumor aggressiveness, metastasis, and patient survival. Cancer. 2001;92:634-641. [PubMed] [Cited in This Article: ] |
| 88. | Lee JS, Kim SH, Im SA, Kim MA, Han JK. Human Epidermal Growth Factor Receptor 2 Expression in Unresectable Gastric Cancers: Relationship with CT Characteristics. Korean J Radiol. 2017;18:809-820. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Matsusaka S, Nashimoto A, Nishikawa K, Miki A, Miwa H, Yamaguchi K, Yoshikawa T, Ochiai A, Morita S, Sano T. Clinicopathological factors associated with HER2 status in gastric cancer: results from a prospective multicenter observational cohort study in a Japanese population (JFMC44-1101). Gastric Cancer. 2016;19:839-851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 90. | Tanaka H, Kanda M, Shimizu D, Tanaka C, Kobayashi D, Hayashi M, Iwata N, Yamada S, Fujii T, Nakayama G. FAM46C Serves as a Predictor of Hepatic Recurrence in Patients with Resectable Gastric Cancer. Ann Surg Oncol. 2017;24:3438-3445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 91. | Ding A, Zhao W, Shi X, Yao R, Zhou F, Yue L, Liu S, Qiu W. Impact of NPM, TFF3 and TACC1 on the prognosis of patients with primary gastric cancer. PLoS One. 2013;8:e82136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 92. | Iwasa S, Yanagawa T, Fan J, Katoh R. Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric cancer is associated with lymph node and liver metastasis. Anticancer Res. 2009;29:4751-4758. [PubMed] [Cited in This Article: ] |
| 93. | Diao D, Wang Z, Cheng Y, Zhang H, Guo Q, Song Y, Zhu K, Li K, Liu D, Dang C. D-dimer: not just an indicator of venous thrombosis but a predictor of asymptomatic hematogenous metastasis in gastric cancer patients. PLoS One. 2014;9:e101125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 94. | Yamashita H, Kitayama J, Kanno N, Yatomi Y, Nagawa H. Hyperfibrinogenemia is associated with lymphatic as well as hematogenous metastasis and worse clinical outcome in T2 gastric cancer. BMC Cancer. 2006;6:147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 95. | Yamaguchi A, Goi T, Yu J, Hirono Y, Ishida M, Iida A, Kimura T, Takeuchi K, Katayama K, Hirose K. Expression of CD44v6 in advanced gastric cancer and its relationship to hematogenous metastasis and long-term prognosis. J Surg Oncol. 2002;79:230-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Dong C. TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8:337-348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 787] [Cited by in F6Publishing: 810] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 97. | Oka M, Yamamoto K, Takahashi M, Hakozaki M, Abe T, Iizuka N, Hazama S, Hirazawa K, Hayashi H, Tangoku A. Relationship between serum levels of interleukin 6, various disease parameters and malnutrition in patients with esophageal squamous cell carcinoma. Cancer Res. 1996;56:2776-2780. [PubMed] [Cited in This Article: ] |
| 98. | Yoshida N, Ikemoto S, Narita K, Sugimura K, Wada S, Yasumoto R, Kishimoto T, Nakatani T. Interleukin-6, tumour necrosis factor alpha and interleukin-1beta in patients with renal cell carcinoma. Br J Cancer. 2002;86:1396-1400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 125] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 99. | Takeda K, Fujii N, Nitta Y, Sakihara H, Nakayama K, Rikiishi H, Kumagai K. Murine tumor cells metastasizing selectively in the liver: ability to produce hepatocyte-activating cytokines interleukin-1 and/or -6. Jpn J Cancer Res. 1991;82:1299-1308. [PubMed] [Cited in This Article: ] |
| 100. | Reichner JS, Mulligan JA, Palla ME, Hixson DC, Albina JE, Bland KI. Interleukin-6 production by rat hepatocellular carcinoma cells is associated with metastatic potential but not with tumorigenicity. Arch Surg. 1996;131:360-365. [PubMed] [Cited in This Article: ] |
| 101. | Ara T, Declerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer. 2010;46:1223-1231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 269] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 102. | Fu XL, Duan W, Su CY, Mao FY, Lv YP, Teng YS, Yu PW, Zhuang Y, Zhao YL. Interleukin 6 induces M2 macrophage differentiation by STAT3 activation that correlates with gastric cancer progression. Cancer Immunol Immunother. 2017;66:1597-1608. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 103. | Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X, Li J, Li C, Yan M, Zhu Z. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget. 2017;8:20741-20750. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 210] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 104. | Yamamoto T, Seino Y, Fukumoto H, Koh G, Yano H, Inagaki N, Yamada Y, Inoue K, Manabe T, Imura H. Over-expression of facilitative glucose transporter genes in human cancer. Biochem Biophys Res Commun. 1990;170:223-230. [PubMed] [Cited in This Article: ] |
| 105. | Kim WS, Kim YY, Jang SJ, Kimm K, Jung MH. Glucose transporter 1 (GLUT1) expression is associated with intestinal type of gastric carcinoma. J Korean Med Sci. 2000;15:420-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 22] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 106. | Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8204] [Cited by in F6Publishing: 7836] [Article Influence: 340.7] [Reference Citation Analysis (0)] |
| 107. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4615] [Cited by in F6Publishing: 4839] [Article Influence: 345.6] [Reference Citation Analysis (1)] |
| 108. | Ménard S, Pupa SM, Campiglio M, Tagliabue E. Biologic and therapeutic role of HER2 in cancer. Oncogene. 2003;22:6570-6578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 276] [Cited by in F6Publishing: 280] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 109. | Dang HZ, Yu Y, Jiao SC. Prognosis of HER2 over-expressing gastric cancer patients with liver metastasis. World J Gastroenterol. 2012;18:2402-2407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 37] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 110. | Terashima M, Kitada K, Ochiai A, Ichikawa W, Kurahashi I, Sakuramoto S, Katai H, Sano T, Imamura H, Sasako M; ACTS-GC Group. Impact of expression of human epidermal growth factor receptors EGFR and ERBB2 on survival in stage II/III gastric cancer. Clin Cancer Res. 2012;18:5992-6000. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 155] [Cited by in F6Publishing: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 111. | Wang HB, Liao XF, Zhang J. Clinicopathological factors associated with HER2-positive gastric cancer: A meta-analysis. Medicine (Baltimore). 2017;96:e8437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 112. | Chapman MA, Lawrence MS, Keats JJ, Cibulskis K, Sougnez C, Schinzel AC, Harview CL, Brunet JP, Ahmann GJ, Adli M. Initial genome sequencing and analysis of multiple myeloma. Nature. 2011;471:467-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1116] [Cited by in F6Publishing: 1117] [Article Influence: 85.9] [Reference Citation Analysis (0)] |
| 113. | Grisendi S, Mecucci C, Falini B, Pandolfi PP. Nucleophosmin and cancer. Nat Rev Cancer. 2006;6:493-505. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 609] [Cited by in F6Publishing: 636] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 114. | Lin CY, Liang YC, Yung BY. Nucleophosmin/B23 regulates transcriptional activation of E2F1 via modulating the promoter binding of NF-kappaB, E2F1 and pRB. Cell Signal. 2006;18:2041-2048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 115. | Luchinat E, Chiarella S, Franceschini M, Di Matteo A, Brunori M, Banci L, Federici L. Identification of a novel nucleophosmin-interaction motif in the tumor suppressor p14arf. FEBS J. 2018;285:832-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 116. | Liu X, Liu D, Qian D, Dai J, An Y, Jiang S, Stanley B, Yang J, Wang B, Liu X. Nucleophosmin (NPM1/B23) interacts with activating transcription factor 5 (ATF5) protein and promotes proteasome- and caspase-dependent ATF5 degradation in hepatocellular carcinoma cells. J Biol Chem. 2012;287:19599-19609. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 117. | Zhou F, Qiu W, Sun L, Xiang J, Sun X, Sui A, Ding A, Yue L. Clinical significance of nucleophosmin/B23 and human epidermal growth factor receptor 2/neu expressions in gastric cancers. APMIS. 2013;121:582-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 118. | Li Y, Sun Z, Liu K, Qiu W, Yao R, Feng T, Xin C, Yue L. Prognostic significance of the co-expression of nucleophosmin and trefoil factor 3 in postoperative gastric cancer patients. Mol Clin Oncol. 2014;2:1055-1061. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |