Published online May 15, 2018. doi: 10.4251/wjgo.v10.i5.108
Peer-review started: February 7, 2018
First decision: March 8, 2018
Revised: March 14, 2018
Accepted: April 10, 2018
Article in press: April 11, 2018
Published online: May 15, 2018
Hepatocellular carcinoma (HCC) is the fifth leading cause of cancer mortality in the United States and the second leading cause of cancer mortality worldwide. Sorafenib is the only food and drug administration (FDA) approved as first line systemic treatment in HCC. Regorafenib and nivolumab are the only FDA approved second line treatment after progression on sorafenib. We will discuss all potential first and second line options in HCC. In addition, we also will explore sequencing treatment options in HCC, and examine biomarkers that can potentially predict benefits from treatments such as immune checkpoint inhibitor. This minireview summarizes potential treatments in HCC based on clinical trials that have been published in manuscript or abstract format from 1994-2018.
Core tip: Hepatocellular carcinoma (HCC) is the fifth leading cause of cancer mortality in the United States and the second leading cause of cancer mortality worldwide. There are some potential treatment options for first and second line HCC, there are also new biomarkers that can predict benefits from treatments such as immune checkpoint inhibitors.
- Citation: Contratto M, Wu J. Targeted therapy or immunotherapy? Optimal treatment in hepatocellular carcinoma. World J Gastrointest Oncol 2018; 10(5): 108-114
- URL: https://www.wjgnet.com/1948-5204/full/v10/i5/108.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v10.i5.108
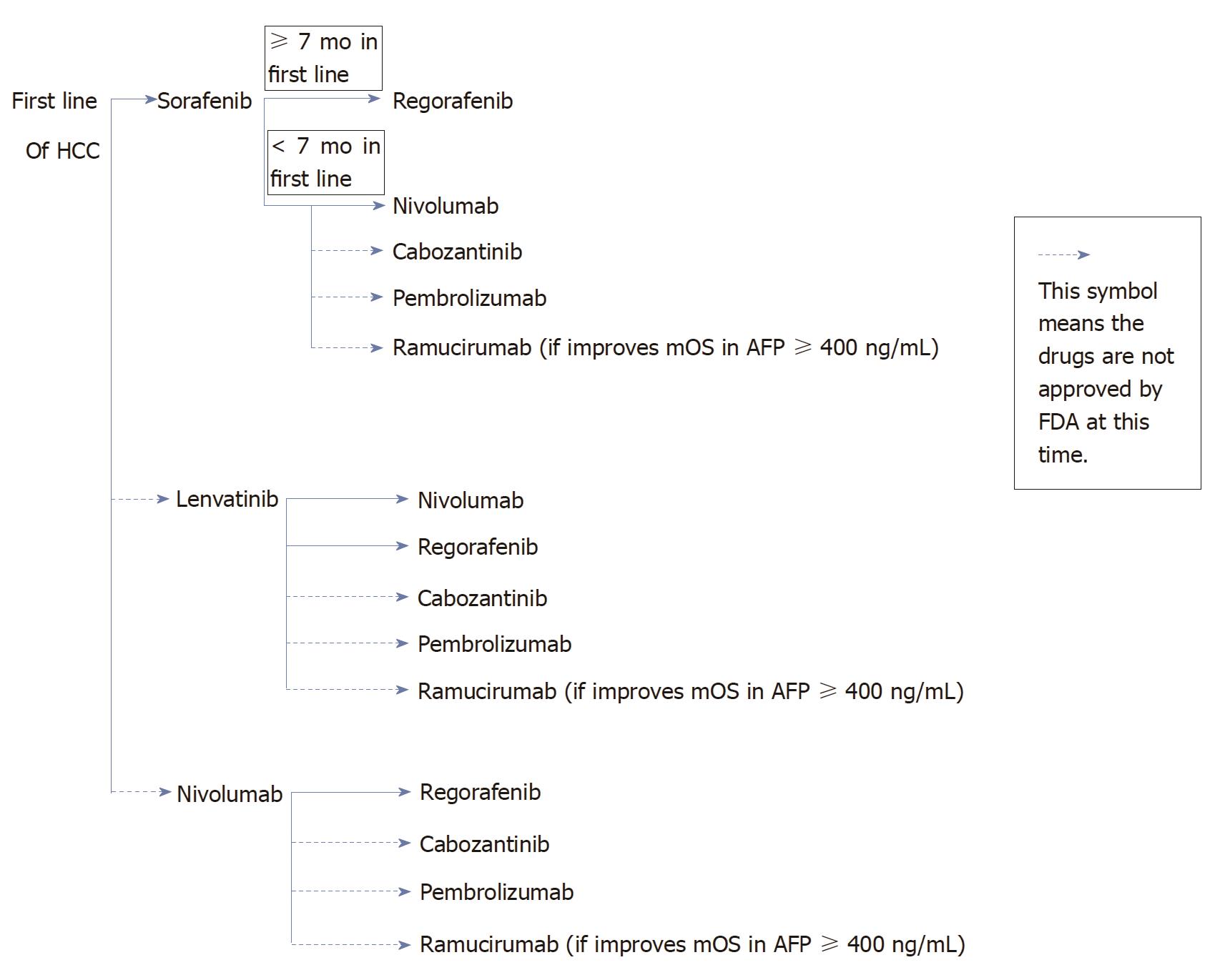
Hepatocellular carcinoma (HCC) is the fifth leading cause of cancer mortality in the United States and the second leading cause of cancer mortality worldwide[1]. Sorafenib has been the only food and drug administration (FDA) approved first line treatment in HCC since 2007. Lenvatinib is another promising treatment in first line HCC, demonstrated non-inferiority in median overall survival (mOS) compared to sorafenib[2]. Nivolumab also might have activity in first line HCC. In the second line treatment of HCC, there are 2 FDA approved medications regorafenib and nivolumab. In addition, other targeted therapies such as cabozantinib or pembrolizumab might be beneficial in second line treatment of HCC.
We will discuss the options of systemic treatment in HCC both for first and second line, the optimal sequencing of treatments, their side effects, and potential biomarkers that may predict benefits of therapy.
Sorafenib is a tyrosine kinase inhibitor that inhibits vascular endothelial growth factor receptor 1 (VEGFR1), VEGFR2, VEGFR3, platelet-derived growth factor receptor-beta, KIT and RAF/ mitogen-activated protein/MEK. In the phase III (SHARP trial) of 602 HCC patients with Child Pugh Class A (preserved liver function), mOS in sorafenib was 10.7 mo[3]. Although it is the first line and only therapy that improves mOS in first line patients, most of patients could not tolerate at the full dose of sorafenib due to the side effects. In the oncology community, most patients are started on lower dose, for example 200 mg PO BID with potential up titration. The most common adverse events (AEs) were diarrhea (39%), fatigue (22%), hand-foot skin reaction (21%), rash (16%), and alopecia (14%)[3]. The common grade 3/4 AEs were hypophosphatemia (11%), diarrhea (8%), hand-foot skin reaction (8%), thrombocytopenia (4%), and hypertension (2%)[3]. Even though there was no difference in survival benefits whether or not patients are started at a full dose (400 mg BID) or reduced dose (200 mg BID), it improved cost-effective in sorafenib treatment[4,5]. Therefore sorafenib is most beneficial for patients with Child Pugh Class A with preserved liver function. In a retrospective subanalyses of phase III SHARP study, sorafenib has shown mOS of 14 mo in HCV patients[6]. In the SHARP study, the top 3 risk factors for HCC in the sorafenib group were Hepatitis C (29%), alcohol (26%), and hepatitis B (19%). In the phase III of Asia Pacific study in 226 HCC patients with Child Pugh Class A, up to 73% patients were HBV positive. This study reported the mOS was 6.5 mo in sorafenib vs 4.2 mo in placebo group[7]. In a retrospective study of 59 unresectable HCC patients who received sorafenib that included Child Pugh Class A (26), B (23), and C (10)[8]. The mOS were 8.3, 4.3, and 1.5 mo, respectively[8]. In this study, the top 3 risk factors for HCC were alcohol (38%) and viral hepatitis B/C (26%). This retrospective study suggested that sorafenib may exert the maximum benefit in Child Pugh Class A patient, regardless of etiology for HCC.
Some of the side effects emerged from sorafenib suggested that hypertension (HTN) and diarrhea may be correlated with efficacy. In a retrospective study in 41 HCC patients (Child Pugh Class A/B, 25/16 patients), showed development of HTN led to better response to sorafenib treatment, with mOS of 18.2 mo vs 4.5 mo in patients without HTN[9]. Another retrospective study in 112 patients with advanced HCC showed that diarrhea can also predict the response to sorafenib treatment as well. Patients with diarrhea demonstrated longer mOS of 14.1 mo vs 7.1 mo when compared to patients without diarrhea[10].
Lenvatinib is a multiple kinase inhibitor that inhibits VEGFR 1-3, fibroblast growth factor receptor 1-4, platelet derived growth factor receptor (PDGFR) alpha, c-Kit and RET proto-oncogene. In the randomized phase III (REFLECT) study of lenvatinib vs sorafenib in first line treatment of unresectable HCC in 954 patients (1:1) with Child Pugh Class A, it showed mOS in lenvatinib vs sorafenib was 13.6 mo and 12.3 mo, respectively. It met its primary endpoint of non-inferiority and it achieved the secondary endpoints with the median progression free survival (PFS) of 7.4 mo vs 3.7 mo and the time to progression (TTP) was 8.9 mo vs 3.7 mo[2]. The most common AEs were hypertension (42%), diarrhea (39%), decreased appetite (34%), decreased weight (31%), and fatigue (30%)[2]. The common grade 3/4 AEs were hypertension (23%), decreased weight (8%), decreased platelet count (6%), elevated aspartate aminotransferase (5%), and decreased appetite (5%)[2]. The usage dose is oral 8 mg (weight < 60 kg) or 12 mg (weight ≥ 60 kg) once daily. In the phase 2 study of lenvatinib in 46 HCC patients with Child Pugh Class A, the objective response rate (ORR) was 37%[11]. The most common causes of HCC in phase 2 study were Hepatitis C (58.7%), Hepatitis B (32.6%), and Alcohol (4.3%).
Nivolumab is an immune checkpoint inhibitor that inhibits PD-1. In a phase I/II study (CHECKMATE 040) of nivolumab in advanced HCC patients in the dose-expansion phase, there were 56 sorafenib naïve patients. All patients were uninfected with viral hepatitis (55 with Child Pugh Class A and only 1 Child Pugh Class B)[12]. This study showed ORR of 23% and OS rate of 82% at 9 mo[12]. Nivolumab showed 23% of partial response (PR) in HCC sorafenib naïve patients, it could be considered as a potential first line treatment[12]. It demonstrated that nivolumab might be beneficial for first line treatment in HCC patients. A phase III study of nivolumab compared to sorafenib as a first line treatment is ongoing.
Regorafenib, is an oral multikinase inhibitor specifically inhibits VEGFR-1, 2, 3. It was approved by FDA on April 27, 2017 as a second line treatment in HCC patients who have been previously progressed with sorafenib. In this study, the median treatment time on first line sorafenib was 7.8 mo for both patient groups[13]. This study showed mOS of 10.6 mo in regorafenib groups (379) vs 7.8 mo in placebo groups (194)[13]. The median PFS was 3.1 mo in regorafenib vs 1.5 mo in placebo group[13]. The ORR in regorafenib group was 11%[13]. In the phase III (RESORCE) study of regorafenib in 573 HCC patients with Child Pugh Class A, the most common AEs were hand-foot skin reaction (52%), diarrhea (33%), fatigue (29%), anorexia (24%), and hypertension (23%)[13]. The common grade 3/4 AEs were hypertension (13%), hand-foot skin reaction (13%), fatigue (6%), increased blood bilirubin (6%), and increased AST (4%)[13]. The etiologies of HCC in this study were hepatitis B (38%), alcohol use (24%), and hepatitis C (21%)[13]. In this study (RESORCE) showed that 199 patients out of 374 patients who received regorafenib had experience of hand-foot skin reaction during cycle 1, these patients had better mOS of 14.1 mo vs 6.6 mo in patients who did not experience hand-foot skin reaction. It also showed HR of 0.52[14]. It suggests that hand-foot skin reaction should be managed properly to get a better response of regorafenib and mOS benefit.
Nivolumab, is an immunotherapy that inhibits PD-1. It was granted approval by FDA on September 22, 2017 as a second line systemic treatment in HCC patients who have been treated with or intolerant to sorafenib. The phase I/II study of nivolumab with dose escalation that included 48 patients with Child Pugh Class A and B7, in addition to dose expansion in 214 patients (Child Pugh Class A)[12]. In the dose-escalation phase, ORR was 15%, 6 mo and 9 mo OS rates were both 66%, and mOS was 15 mo[12]. In the dose expansion phase, ORR was 20%, 6 mo and 9 mo OS rates were 83% and 74%, only the group in sorafenib progressor without viral hepatitis reached mOS of 13.2 mo and the rest of the groups did not reach mOS[12]. In the dose expansion phase, the patients were divided into 113 patients without HBV or HCV (56 untreated/intolerant of sorafenib and 57 progressed post sorafenib)[12]. In addition, this phase also included 51 patients with HBV and 50 patients with HCV[12]. The study demonstrated transient decreased HCV RNA in some HCV infected patients and no reactivation in HBV infected patients. The most common AEs were fatigue (25%), pruritus (20%), diarrhea (18%), rash (11%), and increased AST level (11%)[12]. The grade 3/4 AEs were increased AST (4%), rash (2%), diarrhea (2%), and fatigue (2%)[12]. The dose is 3 mg/kg (240 mg) every 2 wk.
In a retrospective analysis of this study, PD-L1 was showed as biomarker that predicted response to nivolumab in 174 out of 214 patients. The ORR was 26% vs 19% in patients with PD-L1 ≥ 1% compared with PD-L1 < 1%, it suggested that PD-L1 could be a potential biomarker associated with nivolumab treatment[12].
Cabozantinib is an oral tyrosine kinase inhibitor including VEGFR, MET, RET, KIT, and FLT3. In the phase III (CELESTIAL) study of cabozantinib vs placebo in 707 HCC patients with Child Pugh Class A who previously received sorafenib[15]. The characteristics of the patients were the median age of patients was 64 years, 82% male patients, 38% HBV infected, 25% HCV infected, 78% had extrahepatic spread, 30% had macrovascular invasion, and 27% had received two prior systemic therapy[15]. This study has achieved mOS of 10.2 mo in cabozantinib vs 8 mo in placebo group[15]. It also achieved median PFS of 5.2 mo in cabozantinib vs 1.9 mo in placebo group, and ORR of 4% in cabozantinib group vs 0.4% in placebo group[15]. The most common grade 3/4 AEs were hand-foot syndrome (17%), HTN (16%), increased AST (12%), fatigue (10%), and diarrhea (10%)[15]. It suggested that cabozantinib has the potential to be an effective treatment for second line HCC.
Pembrolizumab is an immunotherapy that inhibits PD-1. In the Phase 2 study (KEYNOTE-224) of Pembrolizumab in 104 HCC patients with Child Pugh Class A who progressed on sorafenib treatment. The primary endpoint of this study was achieved with ORR of 16.3% with 1 CR[16]. The median PFS was 4.8 mo and the 6 mo PFS and OS rates were 43.1% and 77.9%, respectively[16]. About 94% of patients who responded, continue to respond at 6 mo[16]. The most common AEs were fatigue (21.2%) and increased AST (12.5%)[16]. The etiologies of HCC were HBV (21.2%) and HCV (26%)[16]. The grade 3-5 AE was reported in 25% of patient with 1 death due to ulcerative esophagitis[16]. This study showed that pembrolizumab might have a good response in advanced HCC patients who progressed on sorafenib.
Ramucirumab is a fully monoclonal antibody (IgG1) that inhibits VEGFR2. In the phase III study of ramucirumab vs placebo as a second line treatment in 565 HCC patients with Child Pugh Class A (REACH)[17]. Eventhough there was no significantly improvement in mOS between patients who received ramucirumab vs placebo (9.2 mo vs 7.6 mo), ORR in ramucirumab group was higher than the placebo group (7% vs < 1%)[17]. The most common AEs were peripheral edema (36%), liver injury (30%), bleeding or haemorrhage (26%), ascites (22%), and fatigue (21%)[17]. The grade 3/4 AEs were liver injury (14%), hypertension (13%), ascites (5%), bleeding or haemorrhage (5%), and asthenia (5%)[17]. The etiologies of HCC in this study were Hepatitis B (35%) and Hepatitis C (27%)[17]. In the prespecified subgroup retrospective analysis of 250 patients with α-fetoprotein (AFP) ≥ 400 ng/mL, the mOS was 7.8 mo (ramucirumab group) vs 4.2 mo (placebo group)[17]. It suggested that ramucirumab could be beneficial in HCC patients with AFP ≥ 400 ng/mL. AFP can potentially be used as a biomarker to predict the response of ramucirumab treatment in HCC patients. A phase III study looking for HCC patients with AFP ≥ 400 ng/mL not prespecified is ongoing.
Sorafenib is the only FDA approved first line treatment in HCC. It is beneficial in HCC patients with Child Pugh Class A and especially in patients with HCV. As demonstrated in a retrospective analysis of HCV patients which comprised 29% of the total patient populations in SHARP study, the mOS was 14 mo, while mOS of the overall population was only 10.9 mo. When patients experience side effects such as HTN or diarrhea, these side effects should be managed aggressively to minimize premature discontinuation of sorafenib. In a two retrospective studies in patients who had HTN or diarrhea were linked to a better mOS compared to patients who did not experience HTN or diarrhea. For instance, the mOS in HTN group was 18.2 mo vs 4.5 mo in group without HTN, the mOS in patients with diarrhea was 14.1 mo vs 7.1 mo in patients without diarrhea (Figure 1).
If patients with Child Pugh Class A tolerate sorafenib well in the first line setting, regorafenib would be a good choice as a second line treatment due to similar toxicities profiles of the two medications. Regorafenib was only studied in patients with Child Pugh Class A. For patients who have difficulty tolerating toxicities of sorafenib, nivolumab could be a good option as a second line treatment, it achieved ORR of 15%-20%. Nivolumab will be beneficial in patients with Child Pugh Class A/B7. Nivolumab achieved higher RR in PD-L1 ≥ 1% (positive) compared to tumors with PD-L1 < 1% (negative), 26% and 19% respectively. However nivolumab does not seem to offer differential outcomes regardless of the length of treatment on first line therapy. Even though cabozantinib or pembrolizumab or ramucirumab have not been FDA approved at this time. Once become FDA approved, then cabozantinib or pembrolizumab could be other second line options. If the phase III study in HCC patients with AFP ≥ 400 ng/mL shows improvement mOS with ramucirumab, then the strategy for second line treatment may include testing of AFP. For patients with AFP ≥ 400 ng/mL, ramucirumab could be a second line option.
Lenvatinib has shown non-inferiority to sorafenib in a phase III study, therefore it would be a first line treatment in HCC if granted FDA approval. It could be a good alternate to sorafenib for patients who prefer to have less hand-foot syndrome and/or diarrhea. Once patients progress, the second line treatment options are nivolumab (in patients with Child Pugh Class A or B7 only and PD-L1 +) and regorafenib (in Child Pugh Class A). Other potential second line options are cabozantinib, pembrolizumab, or ramucirumab.
Nivolumab as first line treatment if granted FDA approval, it will be beneficial for patients who have no contraindication to immunotherapy or who have severe HTN at baseline. If patients could not tolerate or progressed while on nivolumab, the second line options could be regorafenib. Other potential second line options are cabozantinib, pembrolizumab, or ramucirumab.
AFP stands for alpha-feto protein, it is used as a diagnostic and prognosis marker in HCC patients. In a single-institution prospective study, preoperative value of AFP > 400 ng/mL in 108 resectable HCC patients, correlated with higher recurrence rates and lower survival rates at 2 years[18]. In a prespecified group of 250 HCC patients in a phase III ramucirumab trial (REACH) with a baseline AFP ≥ 400 ng/mL, mOS of ramucirumab and placebo was 7.8 mo and 4.2 mo, respectively[17]. In the group (310 patients) where baseline AFP < 400 ng/mL, there was no difference in mOS between ramucirumab and placebo. Therefore, AFP could be used as a marker to predict response with ramucirumab treatment. Phase III of ramucirumab study is ongoing in HCC patients with AFP ≥ 400 ng/mL and the mOS benefit needs to be validated in patients with AFP ≥ 400 ng/mL, once the preliminary data is available.
A programmed death ligand-1 could be a potential biomarker to predict the efficacy of immune checkpoint inhibitors. PD-L1 can be detected using several assays, and the definition of PD-L1 positivity and the methodology of measuring PD-L1 are required to understand about the role of PD-L1 in HCC[19]. In a phase II dose expansion cohort study of nivolumab in HCC patients either progressed or intolerant of sorafenib, RR was 26% vs 19% in patients with PD ≥ 1% and PD-L1 < 1%, respectively[12]. PD-L1 ≥ 1% therefore appears to indicate higher RR in HCC and it also predicts response of nivolumab treatment with mOS benefit.
A tumor-specific mutated peptides on the surface of cancer cells initiate neoantigen production. Each tumor cell causes genetic mutations due to alteration of peptides (amino acid sequencing), it produces neoantigen signature that contains four amino acid strings of peptides[20]. Neoantigen signature is seen in patients with long term clinical benefit of therapy (no evidence of disease for > 6 mo)[20]. Neoantigen was investigated using whole exome sequencing in DNA of tumor cell. Neoantigen can be used as a biomarker to predict the response to immune checkpoint inhibitor treatment. The higher number of neoantigen in a tumor that binds to major histocompatibility complex (MHC) class I, it would be recognized easier by T cells to activate T cells. A prospective study of 18 non-small cell lung cancer (NSCLC) samples from patients who received pembrolizumab (anti-PD-1, an immunotherapy), high mutational burden related to high neoantigen (median of 112 candidate neoantigen per tumor) and associated with improvement of PFS for 14.5 mo[21]. This study showed high mutational burden at least 200 nonsynonymous mutations (mutations that altered protein in cancer cells) per sample, it related to durable clinical benefit (partial or stable response > 6 mo). High mutational burden by itself was not enough to predict durable clinical benefit, because in a few patients without durable clinical benefit also had high mutational burden. In addition to high mutational burden, high number of neoantigen was a better prediction of treatment response. It showed better PFS in patients with high neoantigen compared to low neoantigen group, with PFS of 14.5 mo vs 3.5 mo, respectively[21]. Another prospective study in 64 stage IV melanoma patients who received ipilimumab or tremelimumab (anti-CTLA-4) demonstrated long term clinical benefit in 11 out of 25 patients with high number of mutational load, in addition 14 patients with high number of mutational load without long term clinical benefit[20]. In the second set of 39 melanoma patients who received anti-CTLA-4, 25 patients with high neoantigen had long term clinical benefit to anti-CTLA-4[20].
Tumor mutational burden (TMB) refers to DNA sample that can be detected in blood, and it is considered one example liquid biopsy. This non-invasive test is helpful and convenience especially if tumor tissue is inadequate. This biomarker might help to predict the response of immune checkpoint inhibitor. In a retrospective analysis of atezolizumab (anti-PD-L1) in NSCLC patients, blood was used to extract TMB to predict benefit in patients who received atezolizumab. It included 211 NSCLC patients in POPLAR and 583 NSCLC patients in OAK trial[22]. The TMB was minimum 10 single nucleotide variants (SNV) from cell free-DNA in plasma. In the POPLAR study, patients with TMB ≥ 10, the atezolizumab group showed better PFS hazard ratio (HR) of 0.68 and OS HR of 0.59 compared to docetaxel group[22]. In the OAK study, PFS and OS were also better in the atezolizumab group compared to docetaxel group with HR of 0.73 and 0.69, respectively[22]. From this data, tumor mutational burden could be beneficial as a biomarker for the efficacy of immune checkpoint inhibitor. Prospective studies using TMB in NSCLC patients are ongoing. It needs further investigation for HCC patients in the future.
A cytokine that is produced by several cells including CD4+ T helper cell type 1 (Th1 cells), CD8+ cytotoxic T cell, macrophage, mucosal epithelial cell, natural killer cell (NK), and NK T cell[23-25]. It inhibits cellular proliferation and causes apoptosis[26]. A study in 48 HCC patients who received curative treatment (surgery/RFA), a higher risk of tumor recurrence was observed in patients with lower levels of interferon gamma (IFN-γ)[27]. IFN-γ can therefore be a potential marker to predict HCC recurrence. In two prospective studies from 17 NSCLC and 21 melanoma patients who received pembrolizumab (anti-PD-1), these studies analyzed IFN-γ mRNA to predict response treatment of pembrolizumab. It showed longer PFS and OS in NSCLC patients with high level vs low level of IFN-γ (5.12 vs 2 mo; 10.15 vs 4.86 mo). It also showed longer PFS in melanoma patients with high level vs low level of IFN-γ (4.99 mo vs 1.86 mo)[28].
HCC is the second leading cause of cancer mortality worldwide. Sorafenib is the only FDA approved first line treatment in unresectable HCC. Sorafenib has shown median OS response in HCC patients with HCV infection. There are others potential first line treatments in HCC such as lenvatinib and nivolumab, although not FDA approved, hold great promise based on phase III studies. The second line treatments of HCC patients who progressed or intolerant to sorafenib, include regorafenib and nivolumab. Regorafenib demonstrated higher median OS in HCC patients who tolerated sorafenib for at least 7 mo. Nivolumab has been reported to be more beneficial in HCC patients with Child Pugh Class A/B7, and achieved higher RR in patients with PD-L1 ≥ 1%. Other potential options for second line treatments are cabozantinib (phase III) or pembrolizumab (phase II).
There are two current biomarkers that used to predict response of treatment such as PD-L1 and AFP. For instance, PD-L1 indicates higher RR in nivolumab study, and AFP ≥ 400 ng/mL shows a trend for higher median OS in ramucirumab subgroup analysis phase III study. In addition, other future biomarkers that might be used to predict response of treatment are neoantigens, tumor mutational burden and IFN-γ. These biomarkers need further validation in large randomized clinical trials.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Jin B, Ma J, Neninger E, Tabll AA, Wakao H S- Editor: Cui LJ L- Editor: A E- Editor: Tan WW
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11573] [Cited by in F6Publishing: 12481] [Article Influence: 2080.2] [Reference Citation Analysis (3)] |
| 2. | Cheng AL, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia . Phase III trial of lenvatinib (LEN) vs sorafenib (SOR) in first-line treatment of patients with unresectable hepatocellular carcinoma (uHCC). J Clin Oncol. 2017;35. [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9016] [Cited by in F6Publishing: 9515] [Article Influence: 594.7] [Reference Citation Analysis (1)] |
| 4. | Kaplan DE, Yu S, Taddei TH, Reiss KA, Mehta R, D’Addeo K, Aytaman A, Hunt K, Fox RK, Baytarian M. Up-titration of sorafenib for hepatocellular carcinoma: impact on duration of exposure and cost. J Clin Oncol. 2017;35. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Alghamdi MA, Lee-Ying R, Swiha M, Chan KK, Cheung WY, Ho M, and Tam VC. The effect of sorafenib (S) starting dose and dose intensity on survival in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol. 2017;35. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M, Sangiovanni A. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821-829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 565] [Cited by in F6Publishing: 615] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 7. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3854] [Cited by in F6Publishing: 4336] [Article Influence: 271.0] [Reference Citation Analysis (0)] |
| 8. | Pinter M, Sieghart W, Graziadei I, Vogel W, Maieron A, Königsberg R, Weissmann A, Kornek G, Plank C, Peck-Radosavljevic M. Sorafenib in unresectable hepatocellular carcinoma from mild to advanced stage liver cirrhosis. Oncologist. 2009;14:70-76. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 128] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Estfan B, Byrne M, Kim R. Sorafenib in advanced hepatocellular carcinoma: hypertension as a potential surrogate marker for efficacy. Am J Clin Oncol. 2013;36:319-324. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Bettinger D, Schultheiss M, Knüppel E, Thimme R, Blum HE, Spangenberg HC. Diarrhea predicts a positive response to sorafenib in patients with advanced hepatocellular carcinoma. Hepatology. 2012;56:789-790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda M, Okusaka T, Tamai T, Suzuki T, Hisai T, Hayato S. Phase 2 study of lenvatinib in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2017;52:512-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 235] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 12. | El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492-2502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2536] [Cited by in F6Publishing: 2871] [Article Influence: 410.1] [Reference Citation Analysis (1)] |
| 13. | Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2160] [Cited by in F6Publishing: 2376] [Article Influence: 339.4] [Reference Citation Analysis (0)] |
| 14. | Bruix J, Merle P, Granitor A, Huang YH, Bodoky G, Yokosuka O, Rosmorduc O, Breder VV, Gerolami R, Masi G. Hand-foot skin reaction (HFSR) and overall survival (OS) in the phase 3 RESORCE trial of regorafenib for treatment of hepatocellular carcinoma (HCC) progressing on sorafenib. J Clin Oncol. 2018;36. [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 15. | Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Park JW, Blanc JF. Cabozantinib (C) versus placebo (P) in patients with advanced hepatocellular carcinoma (HCC) who have received prior sorafenib: results from randomized phase III CELESTIAL trial. J Clin Oncol. 2018;36. [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Zhu AX, Finn RS, Cattan S, Edeline J, Ogasawara S, Palmer DH, Verslype C, Zagonel V, Rosmorduc O, Vogel A. KEYNOTE-224: Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib. J Clin Oncol. 2018;36. [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Zhu AX, Park JO, Ryoo BY, Yen CJ, Poon R, Pastorelli D, Blanc JF, Chung HC, Baron AD, Pfiffer TE. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16:859-870. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 598] [Cited by in F6Publishing: 591] [Article Influence: 65.7] [Reference Citation Analysis (0)] |
| 18. | Ma WJ, Wang HY, Teng LS. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol. 2013;11:212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15:971-979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 563] [Cited by in F6Publishing: 625] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 20. | Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189-2199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3101] [Cited by in F6Publishing: 3164] [Article Influence: 316.4] [Reference Citation Analysis (0)] |
| 21. | Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5919] [Cited by in F6Publishing: 5862] [Article Influence: 651.3] [Reference Citation Analysis (0)] |
| 22. | Gandara DR, Kowanetz M, Mok TSK, Rittmeyer A, Fehrenbacher L, Fabrizio D, Otto G, Malboeuf C, Lieber D, Paul SM, Amler L, Riehl T, Schleifman E, Cummings CA, Hegde PS, Zou W, Sandler A, Ballinger M, Shames DS. 12950-Blood-based biomarkers for cancer immunotherapy: tumor mutational burden in blood (bTMB) is associated with improved atezolizumab (atezo) efficacy. 2017 ESMO Congress; 2017 Sep 9-12; Madrid, Spain; Abstract 12950. . [Cited in This Article: ] |
| 23. | Shin T, Nakayama T, Akutsu Y, Motohashi S, Shibata Y, Harada M, Kamada N, Shimizu C, Shimizu E, Saito T. Inhibition of tumor metastasis by adoptive transfer of IL-12-activated Valpha14 NKT cells. Int J Cancer. 2001;91:523-528. [PubMed] [Cited in This Article: ] |
| 24. | Gately MK, Warrier RR, Honasoge S, Carvajal DM, Faherty DA, Connaughton SE, Anderson TD, Sarmiento U, Hubbard BR, Murphy M. Administration of recombinant IL-12 to normal mice enhances cytolytic lymphocyte activity and induces production of IFN-gamma in vivo. Int Immunol. 1994;6:157-167. [PubMed] [Cited in This Article: ] |
| 25. | Frucht DM, Fukao T, Bogdan C, Schindler H, O'Shea JJ, Koyasu S. IFN-gamma production by antigen-presenting cells: mechanisms emerge. Trends Immunol. 2001;22:556-560. [PubMed] [Cited in This Article: ] |
| 26. | Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163-189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2690] [Cited by in F6Publishing: 2850] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 27. | Lee IC, Huang YH, Chau GY, Huo TI, Su CW, Wu JC, Lin HC. Serum interferon gamma level predicts recurrence in hepatocellular carcinoma patients after curative treatments. Int J Cancer. 2013;133:2895-2902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Karachaliou N, Crespo G, Aldeguer E, Drozdowskyj A, Capitan AG, Teixido C. Interferon-gamma (INFG), an important marker of response to immune checkpoint blockade (ICB) in non-small cell lung cancer (NSCLC) and melanoma patients. J Clin Oncology. 2017;35. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |