Published online Aug 16, 2017. doi: 10.4253/wjge.v9.i8.396
Peer-review started: February 8, 2017
First decision: May 10, 2017
Revised: May 19, 2017
Accepted: May 30, 2017
Article in press: May 31, 2017
Published online: August 16, 2017
To assess the impact of laparoscopic liver resection (LLR) on surgical blood loss (SBL), especially in patients with antithrombotics for thromboembolic risks.
Consecutive 258 patients receiving liver resection at our institution between 2010 and 2016 were retrospectively reviewed. Preoperative antithrombotic therapy (ATT; antiplatelets and/or anticoagulation) was regularly used in 100 patients (ATT group, 38.8%) whereas not used in 158 (non-ATT group, 61.2%). Our perioperative management of high thromboembolic risk patients included maintenance of preoperative aspirin monotherapy for patients with antiplatelet therapy and bridging heparin for patients with anticoagulation. In both ATT and non-ATT groups, outcome variables of patients undergoing LLR were compared with those of patients receiving open liver resection (OLR), and the independent risk factors for increased SBL were determined by multivariate analysis.
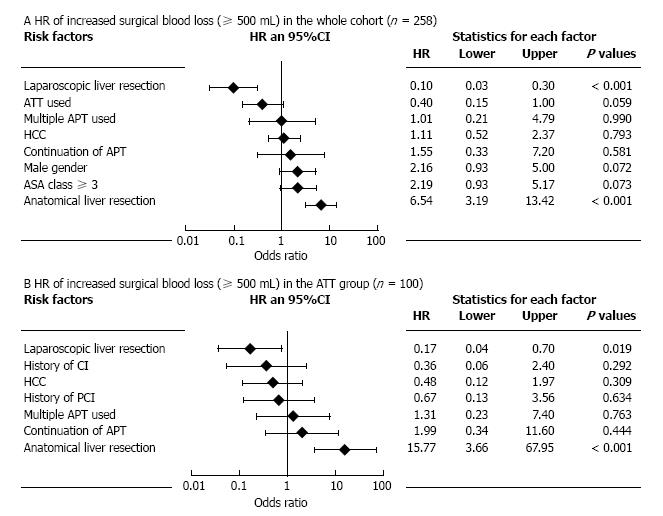
This series included 77 LLR and 181 OLR. There were 3 thromboembolic events (1.2%) in a whole cohort, whereas increased SBL (≥ 500 mL) and postoperative bleeding complications (BCs) occurred in 66 patients (25.6%) and 8 (3.1%), respectively. Both in the ATT and non-ATT groups, LLR was significantly related to reduced SBL and low incidence of BCs, although LLR was less performed as anatomical resection. Multivariate analysis showed that anatomical liver resection was the most significant risk factor for increased SBL [risk ratio (RR) = 6.54, P < 0.001] in the whole cohort, and LLR also had the significant negative impact (RR = 1/10.0, P < 0.001). The same effects of anatomical resection (RR = 15.77, P < 0.001) and LLR (RR = 1/5.88, P = 0.019) were observed when analyzing the patients in the ATT group.
LLR using the two-surgeon technique is feasible and safely performed even in the ATT-burdened patients with thromboembolic risks. Independent from the extent of liver resection, LLR is significantly associated with reduced SBL, both in the ATT and non-ATT groups.
Core tip: Analyzing consecutive 258 patients undergoing liver resection using the two-surgeon technique, we showed that laparoscopic liver resection is significantly associated with reduced surgical blood loss and low postoperative bleeding complications even in antithrombotic-burdened patients with thromboembolic risks.
- Citation: Fujikawa T, Kawamoto H, Kawamura Y, Emoto N, Sakamoto Y, Tanaka A. Impact of laparoscopic liver resection on bleeding complications in patients receiving antithrombotics. World J Gastrointest Endosc 2017; 9(8): 396-404
- URL: https://www.wjgnet.com/1948-5190/full/v9/i8/396.htm
- DOI: https://dx.doi.org/10.4253/wjge.v9.i8.396
In recent years, with the arrival of an aging society, surgical cases with heart disease and cerebrovascular disease have become more common, and most of them are undergoing antithrombotic therapy [ATT; antiplatelet therapy (APT) and/or anticoagulation therapy (ACT)] to prevent thromboembolism. Although the indication for ATT is expanding, perioperative management of antithrombotic drugs during gastroenterological surgery is often at high risk of hemorrhagic and thromboembolic complications and can become difficult[1-4].
In our institution, a protocol of risk stratification and perioperative antithrombotic management has been established for patients receiving ATT (“Kokura Protocol”)[5,6]. So far, the feasibility and safety of the Kokura Protocol during laparoscopic and/or open abdominal surgery have been reported[5,6]. Moreover, our recent paper demonstrated that laparoscopic liver resection (LLR) using the “two-surgeon technique” is safely performed without critical intraoperative or postoperative bleeding even in patients receiving APT[7]. But the effect of LLR on increased surgical blood loss (SBL) and postoperative bleeding complications (BCs), especially in patients undergoing ATT, still remains unclear.
The aim of the current research is to investigate the impact of LLR on increased SBL and BCs with special reference to the presence or absence of ATT.
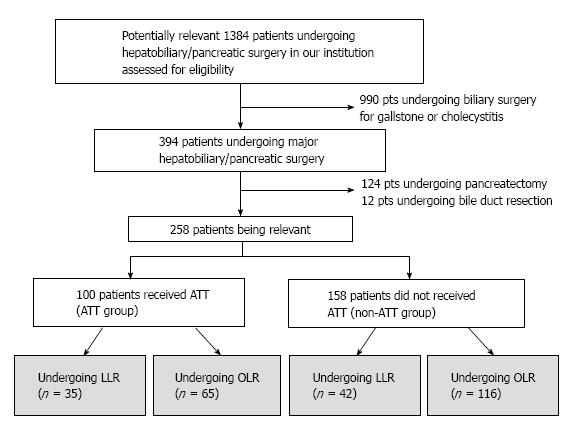
Following institutional review board approval, we searched potentially relevant cases from the single institution prospectively collected surgery database. After excluding cases with emergency surgery or other types of surgery, we included 258 consecutive liver resections performed from January 2010 to October 2016 in the current study (Figure 1). ATT was regularly used in 100 patients (ATT group, 38.8%) whereas not used in 158 patients (non-ATT group, 61.2%). Background, perioperative and outcome variables of the patients were collected through the surgery database as well as hospital and clinic charts.
The status of patients’ symptoms and functions regarding ambulatory status was described according to the ECOG scale of performance status (PS)[8]. Postoperative complications were assessed and categorized by Clavien-Dindo classification (CDC)[9] and CDC class II or higher was considered significant. Postoperative bleeding and thromboembolic complications were defined as previously described[5,6]. BCs included luminal bleeding, abdominal bleeding, and abdominal wall hematoma; thromboembolic complications included myocardial infarction, cerebral infarction, mesenteric infarction, and pulmonary thromboembolism. Operative mortality included death within 30 d after surgery.
Surgical procedures in this cohort included 163 partial liver resection and 95 anatomical liver resection. All procedures were performed by or under the guidance of one of the board-certified attending surgeons at our institution. We have adopted the “two-surgeon technique” during open liver resection (OLR)[10], and also introduced and maintained this procedure even in LLR, in order to perform safe liver parenchymal transection without critical intraoperative bleeding[7]. The indications for LLR at our institution were initially limited to the lesions in S2, S3, S5, S6 and the ventral side of S4, but were later expanded to almost all areas including S1. Patients having a large tumor more than 10 cm in diameter, those requiring bile duct resection or lymph node dissection, those with tumors involving major hepatic veins or inferior vena cava were excluded. We currently perform both pure and hybrid LLR and select the procedure depending on the tumor location and patient condition. Especially, if the ATT-burdened patients with high thromboembolic risks require major anatomical resection, we definitely choose hybrid LLR or open hepatectomy to avoid elevation of thromboembolic risks due to reduced central venous pressure.
The primary outcome included increased SBL (500 mL or more) and BCs. Both in the ATT and non-ATT groups, background characteristics, perioperative factors, and outcome variables of patients undergoing LLR were compared with those of patients receiving OLR, and the independent risk factors for increased SBL were determined by multivariate analysis.
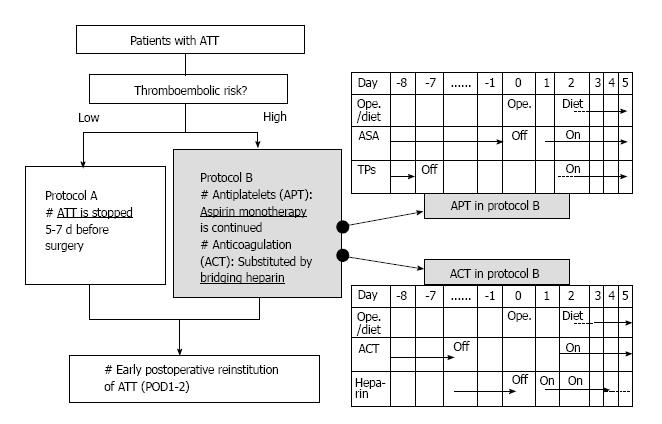
We have established our perioperative antithrombotic management system including thromboembolic risk stratification and perioperative antithrombotic management protocol (“Kokura Protocol”), and have shown that both open and laparoscopic abdominal surgeries in patients with antithrombotic therapy can be performed safely under Kokura Protocol[5,6]. Figure 2 demonstrated perioperative flowchart of patients with ATT in the Kokura Protocol. The management generally consisted of interrupting ATT 5 to 7 d before surgery and early postoperative reinstitution in low thromboembolic risk patients. However, in case of high thromboembolic risks, single aspirin therapy is continued for APT patients, and ACT was substituted by bridging heparin; early reinstitution of the antithrombotic drugs is executed. In patients using both APT and ACT, perioperative management of APT was also combined with those of ACT.
The collected data were checked and statistically analyzed by using the package of SPSS software. The categorized variables between the groups were compared by Fisher’s exact probability test. The continuous data, expressed as a median with range, and non-parametric variables were compared by Kruskal-Wallis test or Student’s t test. The analytic method of multivariable logistic regression model was performed to assess significant risk factors affecting increased SBL and BC. Statistical significance was determined at the level of P < 0.05.
The current cohort included 77 LLR and 181 OLR. Table 1 demonstrates various characteristics of patient background in the both groups. The type of patient race in the present cohort was exclusively Asian. Both in the ATT and non-ATT groups, age, gender, the rate of high body mass index, and PS class were identical between LLR and OLR. Also, there were no differences between LLR and OLR groups in the occurrence of underlying diseases including history of coronary artery disease, congestive heart failure, cerebral infarction, or diabetes mellitus. Among the ATT group, the rates of APT and ACT were 32.6% (84/258) and 11.6% (30/258), respectively. Totally, 57 (22.1%) of patients, including 35 (13.6%) of APT patients and 26 (10.1%) of ACT patients, were regarded as high thromboembolic risk and required to continue preoperative aspirin monotherapy and/or bridging heparin.
| Variables | Total (n = 258) | ATT (n = 100) | Non-ATT (n = 158) | ||||
| LLR (n = 35) | OLR (n = 65) | P value | LLR (n = 42) | OLR (n = 116) | P value | ||
| Age, yr, median (range) | 69 (36-89) | 78 (59-90) | 76 (52-92) | 0.067 | 71 (45-89) | 69 (36-86) | 0.106 |
| Gender | 0.312 | 1 | |||||
| Female | 80 (31.0) | 10 (28.6) | 12 (18.5) | 15 (35.7) | 43 (37.1) | ||
| Male | 178 (69.0) | 25 (71.4) | 53 (81.6) | 27 (64.3) | 73 (62.9) | ||
| BMI | 0.662 | 1 | |||||
| < 30 kg/m2 | 247 (95.7) | 34 (97.1) | 60 (92.3) | 41 (97.6) | 112 (96.6) | ||
| ≥ 30 kg/m2 | 11 (4.3) | 1 (2.9) | 5 (7.7) | 1 (2.4) | 4 (3.4) | ||
| Performance status | 0.124 | 1 | |||||
| 0, 1 | 242 (93.8) | 30 (85.7) | 62 (95.4) | 40 (95.2) | 110 (94.8) | ||
| 2, 3 | 16 (6.2) | 5 (14.3) | 3 (4.6) | 2 (4.8) | 6 (5.2) | ||
| Concurrent diseases | |||||||
| Diabetes mellitus | 58 (22.5) | 10 (28.6) | 17 (26.2) | 0.817 | 7 (16.7) | 24 (20.7) | 0.656 |
| Hx of congestive heart failure | 21 (8.1) | 8 (22.9) | 11 (16.9) | 0.594 | 1 (2.4) | 1 (0.9) | 0.462 |
| Coronary artery disease | |||||||
| Hx of PCI | 49 (19.0) | 17 (48.6) | 31 (47.7) | 1 | 1 (2.4) | 0 (0.0) | 0.266 |
| Hx of CABG | 7 (2.7) | 4 (11.4) | 3 (4.6) | 0.236 | 0 (0.0) | 0 (0.0) | - |
| Hx of cerebral infarction | 26 (10.1) | 5 (14.3) | 17 (26.2) | 0.212 | 0 (0.0) | 4 (3.4) | 0.574 |
| Current hemo-/peritoneal dialysis | 11 (4.3) | 2 (5.7) | 5 (7.7) | 1 | 1 (2.4) | 3 (2.6) | 1 |
| Anticoagulation therapy | 30 (11.6) | 8 (22.9) | 22 (33.8) | 0.360 | - | - | - |
| Periop. heparin bridging | 26 (10.1) | 7 (20.0) | 19 (29.2) | 0.350 | - | - | - |
| Preop. aspirin continuation | 35 (13.6) | 14 (40.0) | 21 (32.3) | 0.382 | - | - | - |
Table 2 shows factors concerning operative procedures and postoperative morbidity in the both groups. Totally, the diagnoses of the diseases were hepatocellular carcinoma (HCC) in 97 (37.6%) and other diseases in 161 (62.4%), including liver metastases from gastrointestinal malignancy and benign diseases. Type of operation consisted of partial resection in 163 (63.2%), sub-sectionectomy (S5, S6 or S8) in 9 (3.5%), left lateral sectionectomy in 19 (7.4%), and other anatomical hepatectomy (mono-/bi-/tri-sectionectomy) in 67 (26.0%). Both in the ATT and non-ATT groups, there was no difference in the type of liver diseases, although LLR comprised less anatomical resections (ATT, P < 0.001; non-ATT, P = 0.004), shorter duration of operations (ATT, P = 0.011; non-ATT, P = 0.049), and less SBL (ATT, P < 0.001; non-ATT, P = 0.007). Increased SBL (≥ 500 mL) was more frequently observed in OLR compared to LLR in the whole cohort [34.3% (62/181) vs 5.2% (4/77), P < 0.001]. One patient (0.4%) undergoing LLR in the non-ATT group was converted to open surgery due to massive bleeding but none was converted in the ATT group.
| Variables | Total (n = 258) | ATT (n = 100) | Non-ATT (n = 158) | ||||
| LLR (n = 35) | OLR (n = 65) | P value | LLR (n = 42) | OLR (n = 116) | P value | ||
| Liver diseases | 0.393 | 0.271 | |||||
| HCC | 97 (37.6) | 15 (42.9) | 22 (33.8) | 19 (45.2) | 41 (35.3) | ||
| Non HCC | 161 (62.4) | 20 (57.1) | 43 (66.2) | 23 (54.8) | 75 (64.7) | ||
| Type of operation | < 0.001 | 0.004 | |||||
| Partial resection | 163 (63.2) | 24 (68.6) | 38 (58.5) | 31 (73.8) | 70 (60.3) | ||
| Sub-sectionectomy (S5, 6, 8) | 9 (3.5) | 0 (0.0) | 2 (3.1) | 4 (9.5) | 3 (2.6) | ||
| Lateral sectionectomy | 19 (7.4) | 9 (25.7) | 1 (1.5) | 4 (9.5) | 5 (4.3) | ||
| Other anatomical hepatectomy | 67 (26.0) | 2 (5.7) | 24 (36.9) | 3 (7.1) | 38 (32.8) | ||
| Duration of ope., min, median (range) | 230 (74-705) | 198 (98-418) | 257 (86-587) | 0.011 | 204 (104-420) | 242 (74-705) | 0.049 |
| Surgical blood loss, mL, median (range) | 200 (1-11070) | 80 (1-850) | 310 (5-2100) | < 0.001 | 50 (1-530) | 265 (2-11070) | 0.007 |
| Intraoperative RBC transfusion | 45 (17.4) | 4 (11.4) | 12 (18.5) | 0.408 | 3 (7.1) | 26 (22.4) | 0.035 |
| Postop. complication | |||||||
| None | 217 (84.1) | 34 (97.1) | 50 (76.9) | 0.009 | 41 (97.6) | 92 (79.3) | 0.005 |
| Superficial SSI | 8 (3.1) | 0 (0.0) | 2 (3.1) | 1 (2.4) | 7 (6.0) | ||
| Deep SSI | 5 (1.9) | 0 (0.0) | 3 (4.6) | 0 (0.0) | 2 (1.7) | ||
| Bile leakage | 11 (4.3) | 0 (0.0) | 4 (6.2) | 0 (0.0) | 7 (6.0) | ||
| Bleeding complication | 8 (3.1) | 0 (0.0) | 3 (4.6) | 0 (0.0) | 5 (4.3) | ||
| Major bleeding | 6 (2.3) | 0 (0.0) | 3 (4.6) | 0 (0.0) | 3 (2.6) | ||
| Minor bleeding | 2 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.7) | ||
| Thromboembolic complication | 3 (1.2) | 0 (0.0) | 1 (1.5) | 0 (0.0) | 2 (1.7) | ||
| Cerebral infarction | 2 (0.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (1.7) | ||
| Coronary stent thrombosis | 1 (0.4) | 0 (0.0) | 1 (1.5) | 0 (0.0) | 0 (0.0) | ||
| Cardiopulmonary arrest | 1 (0.4) | 1 (2.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Operative mortality | 1 (0.4) | 1 (2.9) | 0 (0.0) | 0.350 | 0 (0.0) | 0 (0.0) | - |
| Length of postop. stay, d, median (range) | 14 (4-103) | 12 (7-23) | 15 (8-103) | 0.174 | 11 (6-19) | 15 (4-92) | 0.321 |
An overall rate of postoperative complication was 15.9% (41/258), and LLR included less complications both in the ATT group (2.9% vs 23.1%, P = 0.009) and non-ATT group (2.4% vs 20.7%, P = 0.005). The most common complication was bile leakage (8/258, 4.3%), all of which were experienced after OLR. Only 3 thromboembolic complications (1.2%) occurred after OLR (cerebral infarction in 2 and coronary stent thrombosis in 1), but LLR was free from these events. Eight BCs were experienced only after OLR (3.1%), including 6 major and 2 minor bleedings, although there was no postoperative BC after LLR. One case of operative mortality was experienced in the ATT group. This patient had high thromboembolic risks, including long-term treatment of hemodialysis and history of multiple DES implantation, underwent partial LLR for HCC under continuation of aspirin monotherapy, and had a good postoperative course, but just the day before discharge (10 d after surgery), suddenly developed cardiopulmonary arrest (pulmonary embolism or coronary thrombosis were denied by urgent cardiopulmonary catheterization) and expired. The cause of arrest was unknown, but may not be related to surgical procedures.
Table 3 shows potential factors affecting increased SBL in the whole cohort (n = 258) and in the ATT group (n = 100). In the whole cohort, male gender (P = 0.009), HCC (P = 0.008), OLR (P < 0.001), and anatomical liver resection (P < 0.001) were the factors affecting increased SBL. When the analysis target was narrowed down to the ATT group, however, not only OLR (P = 0.013) and anatomical liver resection (P < 0.001) but also use of multiple APT (P = 0.035) and preoperative aspirin continuation (P = 0.046) were significantly associated with increased SBL. To control potential confounding and interaction, multivariate analyses for increased SBL in the whole cohort and in the ATT group were performed and shown in Figure 3 as forest plots. In the whole cohort, anatomical liver resection was the most significant risk factor for increased SBL [risk ratio (RR) = 6.54, P < 0.001] and LLR also had the significant negative impact (RR = 1/10.0, P < 0.001). The same effects of anatomical resection (RR = 15.77, P < 0.001) and LLR (RR = 1/5.88, P = 0.019) were observed when analyzing the patients in the ATT group.
| Variables | Increased surgical blood loss (≥ 500 mL) | |||
| The whole cohort (n = 258) | ATT group (n = 100) | |||
| Present/total | P value | Present/total | P value | |
| Total | 66/258 (25.6) | 23/100 (23.0) | ||
| Age | 0.664 | 0.811 | ||
| ≥ 75 yr | 25/105 (23.8) | 14/57 (24.6) | ||
| < 75 yr | 41/153 (26.8) | 9/43 (20.9) | ||
| Gender | 0.009 | 0.389 | ||
| Female | 12/80 (15.0) | 3/22 (13.6) | ||
| Male | 54/178 (30.3) | 20/78 (25.6) | ||
| BMI | 0.734 | 0.332 | ||
| < 30 kg/m2 | 64/247 (25.9) | 23/94 (24.5) | ||
| ≥ 30 kg/m2 | 2/11 (18.2) | 0/6 (0.0) | ||
| Performance status | 1 | 0.192 | ||
| 0, 1 | 62/242 (25.6) | 23/92 (25.0) | ||
| 2-4 | 4/16 (25.0) | 0/8 (0.0) | ||
| ASA class | 0.148 | 0.789 | ||
| I, II | 34/153 (22.2) | 5/25 (20.0) | ||
| III, IV | 32/105 (30.5) | 18/75 (24.0) | ||
| Diabetes mellitus | 0.733 | 1 | ||
| Yes | 16/58 (27.6) | 6/27 (22.2) | ||
| No | 50/200 (25.0) | 17/73 (23.3) | ||
| Hx of PCI | 0.589 | 0.234 | ||
| Yes | 14/49 (28.6) | 14/48 (29.2) | ||
| No | 52/209 (24.9) | 9/52 (17.3) | ||
| ATT used | 0.468 | - | ||
| Yes | 23/100 (23.0) | - | ||
| No | 43/158 (27.2) | - | ||
| Multiple APT used | 0.117 | 0.035 | ||
| Yes | 11/29 (37.9) | 11/29 (37.9) | ||
| No | 55/229 (24.0) | 12/71 (16.9) | ||
| Preop. aspirin continuation | 0.215 | 0.046 | ||
| Yes | 12/35 (34.3) | 12/34 (35.3) | ||
| No | 54/223 (24.2) | 11/66 (16.7) | ||
| Liver diseases | 0.008 | 0.138 | ||
| HCC | 34/97 (35.1) | 12/37 (32.4) | ||
| Non HCC | 32/161 (19.9) | 11/63 (17.5) | ||
| Laparoscopic liver resection | < 0.001 | 0.013 | ||
| Yes | 4/77 (5.2) | 3/35 (8.6) | ||
| No | 62/181 (34.3) | 20/65 (30.8) | ||
| Anatomical liver resection | < 0.001 | < 0.001 | ||
| Yes | 46/95 (48.4) | 19/38 (50.0) | ||
| No | 20/163 (12.3) | 4/62 (6.5) | ||
Various types of abdominal surgery are currently being performed laparoscopically thanks to development of many energy devices and techniques. Compared to OLR, many reports have demonstrated advantages of LLR, such as minimal degree of body wall damage, fewer intra- and post-operative complications, and decreased SBL[11-14]. However, the impact of LLR on SBL and BC in patients receiving ATT has not been investigated and is still largely unknown. Our study demonstrates that the cohort comprised 258 liver resection, including 77 LLR and 181 OLR, among which 38% of patients received ATT regularly. LLR was significantly related to reduced SBL and low incidence of BC. Multivariate analyses also showed that both in the whole cohort and in the ATT group, not only anatomical liver resection was significantly associated with increased SBL, but also LLR independently had the impact on reduction of SBL. This is the first study to elucidate the effect of LLR on reduced SBL in patients receiving ATT. Using the two-surgeon technique, LLR is feasible and safely performed without increase of SBL or thromboembolic events even in the ATT-burdened patients with thromboembolic risks.
Minimizing intraoperative SBL during liver resection is one of the most important tasks, and improvement of several technical aspects has been reported, such as the liver hanging manoeuvre, Pringle manoeuvre, and the two-surgeon technique[10,15,16]. The two-surgeon technique during liver surgery, which was first recommended by Aloia, is a novel technique for decreasing SBL and postoperative bile leakage as well as shortening operative time by allowing two surgeons to simultaneously participate in the parenchymal transection[10]. The primary surgeon dissects the liver parenchyma by ultrasonic dissection device; the assistant surgeon performs meticulous hemostasis using the saline-linked electrocautery. We also applied this manoeuvre during both conventional OLR and LLR.
In our hospital, the occurrence of ATT-received patients who need to undergo major hepatobiliary/pancreatic surgery is as many as 40%, and the number is increasing further in the future. In ATT-burdened patients undergoing major hepatobiliary/pancreatic surgery, both excessive surgical stress and inappropriate antithrombotic management are considered to affect bad postoperative outcome. The surgical stress has been demonstrated to make an inflammatory response which generates plaque fissure and subsequently causes acute thrombosis[17,18]. Therefore, we should consider an application of LLR to even more troublesome ATT-burdened patients. If the patient has high thromboembolic risks and preoperative ATT cannot be stopped, the intraoperative and postoperative bleeding risks will increase. To minimize SBL especially in this critical patient population, we thought that the appropriate devices and techniques for rigid hemostasis must be applied during LLR. As shown in our previous report, LLR using the two-surgeon technique is safe and feasible, and can be applied to even ATT-burdened patients[7].
Minimizing SBL to maintain a dry operative field is extremely crucial especially during pure LLR. To control hepatic inflow, Pringle maneuver (intermittent hepatic vascular inflow occlusion) is usually employed during liver parenchymal transection. To control backflow bleeding from the hepatic vein, the maintenance of low central venous pressure (CVP) is commonly used, and decreasing CVP combined with the maintenance of low airway pressure and high pneumoperitoneum pressure (PPP) is also reported to be useful[19-22]. However, maintenance of low CVP and high PPP during liver parenchymal transection in pure LLR may expose the ATT-burdened patients to the elevated risks of thromboembolism. Therefore, if the patients with high thromboembolic risks require major anatomical resection, we definitely choose and perform “hybrid LLR” (in which the parenchymal transection is performed through mini-laparotomy) or OLR under the maintenance of normal CVP levels to avoid low CVP-induced thromboembolic events. Our data demonstrated that even though the procedures were associated with increased bleeding tendency due to normal CVP levels, hybrid LLR using the two-surgeon technique was performed safely without increase of SBL or thromboembolic complications.
Concerning perioperative thromboembolic complications including cerebrovascular stroke, pulmonary embolism, or major adverse cardiovascular event (MACE), the rates of perioperative thromboembolisms vary depending on differences in target patient population, study design, and changing of clinical practices. The reported incidence of stroke following noncardiac, nonneurosurgical surgery ranges between 0.1%-0.4% overall, and 2.9%-3.5% in patients at risk of perioperative stroke[23-26]. In consideration of thromboembolic events after liver resection, the prevalence of thromboembolism seems to be higher. Schroeder et al[27] reported that analyzing 587 patients undergoing liver resection from ACS-National Surgical Quality Improvement Program (NSQIP) database, rates of MACE and overall thromboembolic complications after liver resection were at 4.4% and 3.6%, respectively. Another research of 5227 liver resections from ACS-NSQIP database showed that the rate of critical cardiac complications including myocardial infarction and cardiac arrest after liver resection was at 4.8% in patients with underlying cardiac disease and at 1.6% in those without[28]. The present study demonstrated that the incidence of perioperative thromboembolic complication was maintained at 1.2%, a relatively low rate compared to the previous report. Hence, it is suggested that liver resections including both OLR and LLR can be performed safely under the Kokura Protocol, the rigorous perioperative antithrombotic management protocol, with successful inhibition of thromboembolic events even in high thromboembolic risk patients.
There are limitations to the present study. This single-center retrospective observational design of the current study has inherent potential for bias, which lessens the effect of the statistical analysis. This restriction will be alleviated by follow-up investigation, or by multi-institutional prospective studies. Since we continuously manage ATT-received cases that are required to undergo liver resection using the Kokura Protocol and the same surgical policies, we are going to analyze more cases to investigate the safety and feasibility of LLR on this high-risk patient population.
LLR using the two-surgeon technique is feasible and safely performed without increase of SBL or thromboembolic events even in the ATT-burdened patients with thromboembolic risks. Independent from the extent of liver resection, LLR is significantly associated with reduced SBL, both in the ATT and non-ATT groups.
Nowadays, patients who have histories of cardiovascular or cerebrovascular diseases have been seen more often with aging of patients, and those patients frequently receive antithrombotic therapy (ATT) for the purpose of primary and secondary prevention of thromboembolic diseases. While indications for ATT use have expanded, antithrombotic management during gastrointestinal and/or hepatobiliary-pancreatic surgery is difficult and always bothersome because of high risks of perioperative bleeding or thromboembolic events. Recently, laparoscopic liver resection (LLR) using the “two-surgeon technique” is safely performed without critical intraoperative or postoperative bleeding even in patients receiving ATT, but the effect of LLR on increased surgical blood loss (SBL) and postoperative bleeding complications (BCs), especially in patients undergoing ATT, still remains unclear.
In the authors’ institution, a protocol of risk stratification and perioperative antithrombotic management has been established for patients receiving ATT (“Kokura Protocol”). So far, the feasibility and safety of both open and laparoscopic abdominal surgeries under the Kokura Protocol have been reported. Moreover, the authors’ recent paper demonstrated that LLR using the “two-surgeon technique” is safely performed without critical intraoperative or postoperative bleeding even in patients receiving ATT.
The impact of LLR on BCs in patients receiving ATT has not been investigated and is still largely unknown. The authors’ study demonstrates that the cohort comprised 258 liver resection, including 77 LLR and 181 OLR, among which 38% of patients received ATT regularly. LLR was significantly related to reduced SBL and low incidence of postoperative BCs. Multivariate analyses also showed that both in the whole cohort and in the ATT group, LLR independently had the impact on reduction of SBL. This is the first study to elucidate the effect of LLR on reduced SBL in patients receiving ATT.
Using the two-surgeon technique, LLR is feasible and safely performed without increase of SBL or thromboembolic events even in the ATT-burdened patients with thromboembolic risks.
ATT includes antiplatelet therapy (APT) and/or anticoagulation therapy (ACT) for the purpose of primary and secondary prevention of thromboembolic diseases. LLR has been innovated and currently accepted as minimally-invasive procedures for both hepatocellular carcinoma and metastatic liver diseases in selected patients. LLR is reportedly related to reduced degree of body wall damage, fewer intraoperative and postoperative complications, and decreased SBL.
It is an interesting work.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Anadol Z, Santambrogio R S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Fujikawa T, Maekawa H, Shiraishi K, Tanaka A. Successful resection of complicated bleeding arteriovenous malformation of the jejunum in patients starting dual anti-platelet therapy just after implanting drug-eluting coronary stent. BMJ Case Rep. 2012;2012. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Fujikawa T, Noda T, Tada S, Tanaka A. Intractable intraoperative bleeding requiring platelet transfusion during emergent cholecystectomy in a patient with dual antiplatelet therapy after drug-eluting coronary stent implantation (with video). BMJ Case Rep. 2013;2013. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Mita K, Ito H, Murabayashi R, Sueyoshi K, Asakawa H, Nabetani M, Kamasako A, Koizumi K, Hayashi T. Postoperative bleeding complications after gastric cancer surgery in patients receiving anticoagulation and/or antiplatelet agents. Ann Surg Oncol. 2012;19:3745-3752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Thachil J, Gatt A, Martlew V. Management of surgical patients receiving anticoagulation and antiplatelet agents. Br J Surg. 2008;95:1437-1448. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Fujikawa T, Tanaka A, Abe T, Yoshimoto Y, Tada S, Maekawa H. Effect of antiplatelet therapy on patients undergoing gastroenterological surgery: thromboembolic risks versus bleeding risks during its perioperative withdrawal. World J Surg. 2015;39:139-149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Fujikawa T, Tanaka A, Abe T, Yoshimoto Y, Tada S, Maekawa H, Shimoike N. Does antiplatelet therapy affect outcomes of patients receiving abdominal laparoscopic surgery? Lessons from more than 1,000 laparoscopic operations in a single tertiary referral hospital. J Am Coll Surg. 2013;217:1044-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Fujikawa T, Yoshimoto Y, Kawamura Y, Kawamoto H, Yamamoto T, Tanaka A. Safety and feasibility of laparoscopic liver resection in antiplatelet-burdened patients with arterial thromboembolic risks. J Gastroenterol Hepatol Res. 2016;5:2165-2172. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Sørensen JB, Klee M, Palshof T, Hansen HH. Performance status assessment in cancer patients. An inter-observer variability study. Br J Cancer. 1993;67:773-775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 313] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 9. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18532] [Cited by in F6Publishing: 21934] [Article Influence: 1096.7] [Reference Citation Analysis (0)] |
| 10. | Aloia TA, Zorzi D, Abdalla EK, Vauthey JN. Two-surgeon technique for hepatic parenchymal transection of the noncirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg. 2005;242:172-177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 11. | Belli G, Fantini C, D’Agostino A, Cioffi L, Langella S, Russolillo N, Belli A. Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: short- and middle-term results. Surg Endosc. 2007;21:2004-2011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 187] [Cited by in F6Publishing: 206] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Kaneko H, Takagi S, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, Maeda T, Shiba T. Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg. 2005;189:190-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 248] [Cited by in F6Publishing: 266] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 13. | Laurent A, Cherqui D, Lesurtel M, Brunetti F, Tayar C, Fagniez PL. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. Arch Surg. 2003;138:763-769; discussion 769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 234] [Cited by in F6Publishing: 252] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 14. | Morino M, Morra I, Rosso E, Miglietta C, Garrone C. Laparoscopic vs open hepatic resection: a comparative study. Surg Endosc. 2003;17:1914-1918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 190] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 15. | Imamura H, Takayama T, Sugawara Y, Kokudo N, Aoki T, Kaneko J, Matsuyama Y, Sano K, Maema A, Makuuchi M. Pringle’s manoeuvre in living donors. Lancet. 2002;360:2049-2050. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 90] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Nitta H, Sasaki A, Fujita T, Itabashi H, Hoshikawa K, Takahara T, Takahashi M, Nishizuka S, Wakabayashi G. Laparoscopy-assisted major liver resections employing a hanging technique: the original procedure. Ann Surg. 2010;251:450-453. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Breslow MJ, Parker SD, Frank SM, Norris EJ, Yates H, Raff H, Rock P, Christopherson R, Rosenfeld BA, Beattie C. Determinants of catecholamine and cortisol responses to lower extremity revascularization. The PIRAT Study Group. Anesthesiology. 1993;79:1202-1209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 120] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Landesberg G, Beattie WS, Mosseri M, Jaffe AS, Alpert JS. Perioperative myocardial infarction. Circulation. 2009;119:2936-2944. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 284] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 19. | Chouillard EK, Gumbs AA, Cherqui D. Vascular clamping in liver surgery: physiology, indications and techniques. Ann Surg Innov Res. 2010;4:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Kobayashi S, Honda G, Kurata M, Tadano S, Sakamoto K, Okuda Y, Abe K. An Experimental Study on the Relationship Among Airway Pressure, Pneumoperitoneum Pressure, and Central Venous Pressure in Pure Laparoscopic Hepatectomy. Ann Surg. 2016;263:1159-1163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 21. | Okuda Y, Honda G, Kurata M, Kobayashi S. Useful and convenient procedure for intermittent vascular occlusion in laparoscopic hepatectomy. Asian J Endosc Surg. 2013;6:100-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Yamamoto K, Fukumori D, Yamamoto F, Yamamoto M, Igimi H, Yamashita Y. First report of hepatectomy without endotracheal general anesthesia. J Am Coll Surg. 2013;216:908-914. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Landercasper J, Merz BJ, Cogbill TH, Strutt PJ, Cochrane RH, Olson RA, Hutter RD. Perioperative stroke risk in 173 consecutive patients with a past history of stroke. Arch Surg. 1990;125:986-989. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 50] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Larsen SF, Zaric D, Boysen G. Postoperative cerebrovascular accidents in general surgery. Acta Anaesthesiol Scand. 1988;32:698-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 77] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Limburg M, Wijdicks EF, Li H. Ischemic stroke after surgical procedures: clinical features, neuroimaging, and risk factors. Neurology. 1998;50:895-901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 92] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Parikh S, Cohen JR. Perioperative stroke after general surgical procedures. N Y State J Med. 1993;93:162-165. [PubMed] [Cited in This Article: ] |
| 27. | Schroeder RA, Marroquin CE, Bute BP, Khuri S, Henderson WG, Kuo PC. Predictive indices of morbidity and mortality after liver resection. Ann Surg. 2006;243:373-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 28. | Tran TB, Worhunsky DJ, Spain DA, Dua MM, Visser BC, Norton JA, Poultsides GA. The significance of underlying cardiac comorbidity on major adverse cardiac events after major liver resection. HPB (Oxford). 2016;18:742-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |