Published online Oct 16, 2020. doi: 10.4253/wjge.v12.i10.388
Peer-review started: May 13, 2020
First decision: June 15, 2020
Revised: June 29, 2020
Accepted: September 8, 2020
Article in press: September 8, 2020
Published online: October 16, 2020
Conventional endoscopy is based on full spectrum white light. However, different studies have investigated the use of fluorescence based endoscopy systems where the white light has been supplemented by infrared light and the use of relevant fluorophores. Fluorescence endoscopy utilizes the fluorescence emitted from a fluorophore, visualizing what is not visible to the naked eye.
To explore the feasibility of fluorescence endoscopy and evaluate its use in diagnosing and evaluating gastrointestinal disease.
We followed the PRISMA guidelines for this systematic review. The research covered five databases; PubMed, Scopus, Web of Science, Embase, and the Cochrane Collection, including only studies in English and Scandinavian languages. Authors screened title and abstract for inclusion, subsequently full-text for inclusion according to eligibility criteria listed in the protocol. The risk of bias was assessed for all studies according to the Newcastle-Ottawa Scale. The authors extracted the data and reported the results in both text and tables.
We included seven studies in the systematic review after screening a total of 2769 papers. The most prominent fluorophore was indocyanine green (n = 6), and whereas one study (n = 1) used Bevacizumab 800-CW. Three studies investigated fluorescence endoscopy in detecting varices, adenomas in patients with familial adenomatous polyposis and neoplasms in the gastrointestinal tract. Four studies evaluated the usefulness of fluorescence endoscopy in assessing tumor invasion. Three of the four studies reported an exceptional diagnostic accuracy (93%, 89% and 88%) in assessing tumor invasion, thus representing better visualization and more correct diagnosis by fluorescence endoscopy compared with the conventional endoscopy. The relationship between the endoscopic findings, tumor invasion, and tumor vascularity was evaluated in two studies showing a significant correlation (dP < 0.05 and bP < 0.01).
The use of fluorescence endoscopy is a promising method adding diagnostic value in the detection of neoplasia, adenomas, and assessment of tumor invasion within the gastrointestinal tract. More studies are needed to utilize the feasibility of fluorescence endoscopy compared with other endoscopic methods.
Core Tip: In the evaluation of tumor invasion, detection of neoplasia and adenomas, studies on fluorescence endoscopy reports promising results.
- Citation: Mortensen OE, Nerup N, Thorsteinsson M, Svendsen MBS, Shiwaku H, Achiam MP. Fluorescence guided intraluminal endoscopy in the gastrointestinal tract: A systematic review. World J Gastrointest Endosc 2020; 12(10): 388-400
- URL: https://www.wjgnet.com/1948-5190/full/v12/i10/388.htm
- DOI: https://dx.doi.org/10.4253/wjge.v12.i10.388
Gastrointestinal diseases are the third most common cause of death with gastrointestinal cancer as the leading cause; in 2018 gastric cancer was estimated to cause 738000 deaths worldwide[1]. The high prevalence is, among others, correlated to multifactorial reasons like lifestyle, physical inactivity, stress, and genetics[2,3]. Conventional endoscopy is widely used for gastrointestinal diseases because it is a minimally invasive and potentially curative procedure, facilitating diagnosis, staging, and treatment. The method of flexible conventional endoscopy is based on the visualization by white light. Thus, allowing the surgeon to visualize the gastrointestinal tract from the inside[4,5]. Recently, studies have examined flexible endoscopy in combination with infrared light, and administration of a fluorophore[6].
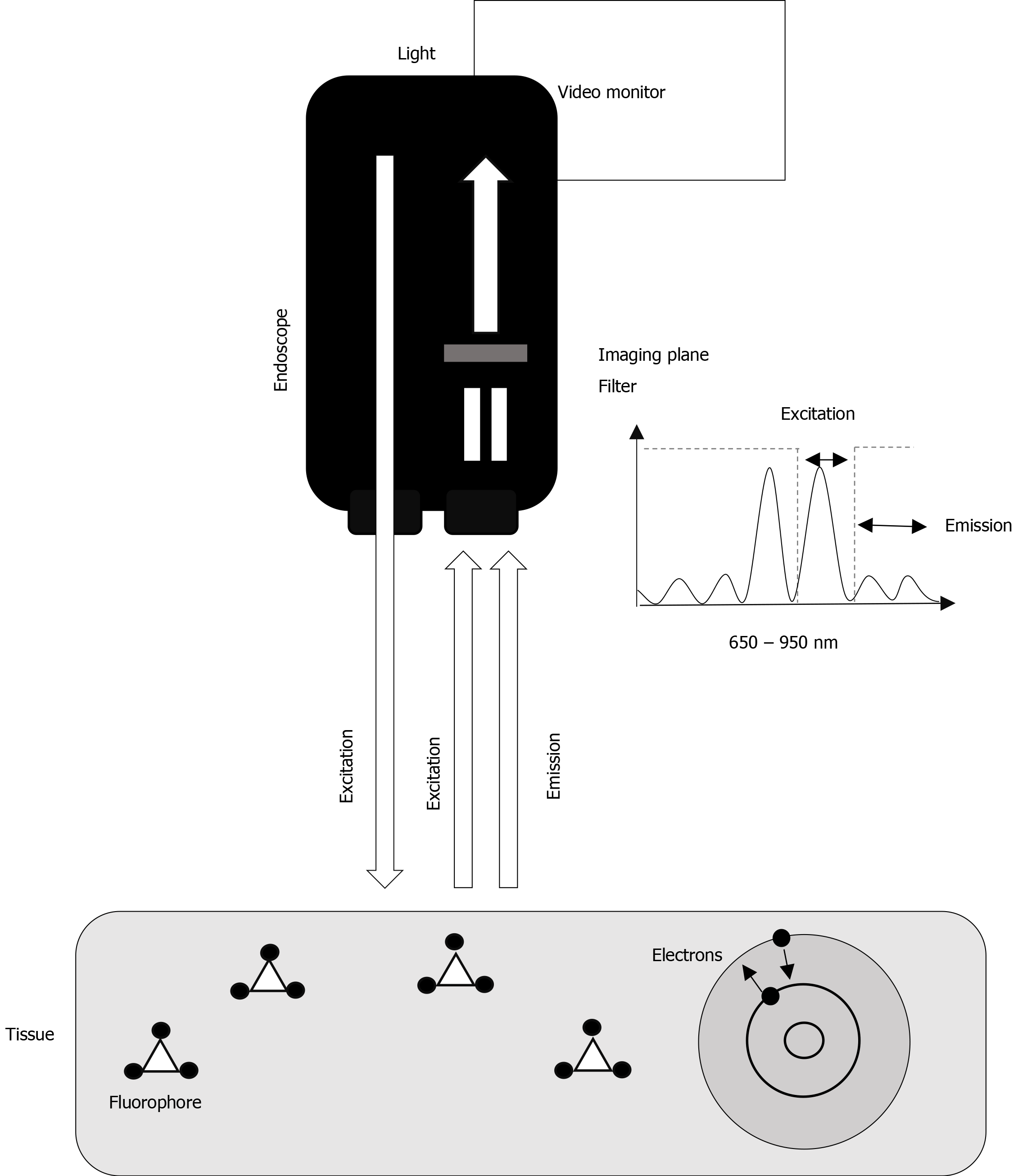
Fluorescence arises when a fluorophore is in circulation, and the tissue of interest is exposed to light in a wavelength, that the fluorophore absorbs. When the fluorophore absorbs the photons from the light, an excitation happens where the electrons are shifted to a higher state of energy. Spontaneously, the electrons will shift back to their state of energy releasing the extra energy (emission) as light at another wavelength seen as fluorescence[7,8] (Figure 1). Fluorescence guided flexible intraluminal endoscopy is based on the principle of fluorescence and the spectrum of infrared (IR) light, including near-infrared light. IR light has a wavelength of about 780 nm to 1000 nm. IR light has a limited scattering when it reaches the tissue and a low absorption by water and hemoglobin, thus facilitating a less obstructed penetration through tissue compared with standard white light[9]. The mucosal and submucosal vessels are not visible to the naked eye (in white light), but after intravenous injection of a fluorophore and illumination by IR light, profound structures can be visualized. As angiogenesis and neovascularization are essential factors in carcinogenesis and tumor invasion, visualization of mucosal and submucosal vessels may increase the diagnostic value of the endoscopy[10,11].
Conceptually, the endoscope consists of a light source and an imaging plane–light fibers within the endoscope, with an external camera chip on the tip of the distal end of the camera. The light entering the endoscope for illumination can be white light for standard visualization, whereas when in fluorescence mode, the light primarily consists of the excitatory wavelengths of the fluorophore used. Still in fluorescence mode, after reaching the tissue, the total amount of light reenters the endoscope at the tip. Before reaching the camera chip, the excitatory light needs to be filtered by an optical filter (Figure 1)[12,13]. A frequently used fluorophore is Indocyanine green (ICG), which is excited at the wavelength at 805 nm. Intravenously administered ICG binds to the lipoproteins in the circulation[7]; however, several kinds of other fluorophores exist. The IRDye-800CW is another cyanine fluorophore used for specific protein labeling e.g., Bevacizumab-800CW[13-15]. The aim of this systematic review was to evaluate the diagnostic and therapeutic value of fluorescence-guided flexible intraluminal endoscopy.
The protocol, flow diagram, and the present manuscript adhered to the PRISMA guidelines for systematic reviews[16]. The protocol was submitted for PROSPERO with the registration number CRD42020147516[17].
The eligibility criteria for this systematic review was made according to the principals of participants, interventions, comparison, and outcome. Only human studies examining gastrointestinal diseases and surgical advantages, in general, were included. The studies should use fluorescence endoscopy and compare this method with the use of standard endoscopy or endoscopic expert knowledge, or histopathological examinations. Outcomes of interest were a result representing an increase or decrease in the diagnostic or therapeutic value of fluorescence endoscopy. According to the study design, animal studies and other reviews were excluded. We included randomized controlled trials, case-series with more than five subjects, and prospective/retrospective cohort studies independent of the year of publication and the publication status. Only studies written in English or Scandinavian languages were included.
The search string was built in PubMed (Table 1) and adapted to Scopus, Web of Science, Embase, and the Cochrane Collection to identify all the relevant articles for this systematic review. The search string covers all organs from the mouth to the anus, but it does not include the accessory glandular organs. The key words used in the search strategy is shown in Table 1. The database search was performed on June 9th, 2019. Titles and abstracts were screened using an online tool Rayyan[18,19] by four authors (Mortensen OE, Achiam MP, Nerup N, and Thorsteinsson M) to meet the inclusion and exclusion criteria. Consecutively, with two of the authors performing a full-text screening. Subsequently, the reference lists of the included studies were screened to find additional studies. If any discrepancies about inclusion or exclusion, the full-text studies were brought to a meeting and re-examined until consensus. The authors used the web application Rayyan to manage all the data in the screening process. Two authors (Mortensen OE and Thorsteinsson M) performed a data extraction. The handling of data and data from the studies have been extracted from the studies without any modifications and statistical measurements. We extracted data about patients, patient characteristics, diagnosis, fluorophores and dosage used, adverse events, endoscopic findings, diagnostic accuracy, vessel count, and conclusions. No additional analyses were performed.
| Classification |
| (Endoscop OR Esophagoscop OR Gastroscop OR Gastroscopic Surgical Procedure OR Gastroscopic Surgical Procedures OR Colonoscop OR Colonoscopic Surgical Procedure OR Colonoscopic Surgical Procedures OR Surgery Gastroscopic OR Surgery Colonoscopic) AND (Indocyanine green fluorescence OR Indocyanine Green OR ICG OR fluorescent OR fluorescent dye OR fluorescence OR fluorescein OR near-infrared OR near infrared) AND (Upper Gastrointestinal Tract OR Lower Gastrointestinal Tract OR Upper gastrointestinal disease OR Lower gastrointestinal disease OR Upper gastrointestinal diseases OR Lower gastrointestinal diseases OR gastrointestinal tract OR gastrointestinal diseases OR GI diseases OR GI-diseases OR Upper GI-Diseases OR Lower GI-diseases) |
The Newcastle-Ottawa scale for cohort studies was used to assess the risk of bias of the included studies[20]. The risk of bias assessment focused on the three main subjects; selection, comparability, and outcome (Table 2).
| Ref. | Selection | Comparability | Outcome | Total score | ||||||||
| 1 | 2 | 3 | 4 | Score | 1 | Score | 1 | 2 | 3 | Score | ||
| Iseki et al[25], 2000 | a | b | a | a | ●○●● | - | ○○ | a | a | a | ●●● | Poor quality |
| Mataki et al[21], 2003 | a | b | a | b | ●○●○ | - | ○○ | a | a | a | ●●● | Poor quality |
| Okamoto et al[22], 2005 | a | b | a | b | ●○●○ | - | ○○ | c | a | a | ○●● | Poor quality |
| Ishihara et al[12], 2006 | a | b | a | a | ●○●● | - | ○○ | a | a | a | ●●● | Poor quality |
| Kimura et al[23], 2007 | a | b | a | b | ●○●○ | - | ○○ | a | a | a | ●●● | Poor quality |
| Ortiz- Fernandez-Sordo et al[24], 2018 | a | b | a | a | ●○●● | - | ○○ | a | a | a | ●●● | Poor quality |
| Hartmans et al[15], 2018 | a | b | a | a | ●○●● | - | ○○ | c | a | a | ○●● | Poor quality |
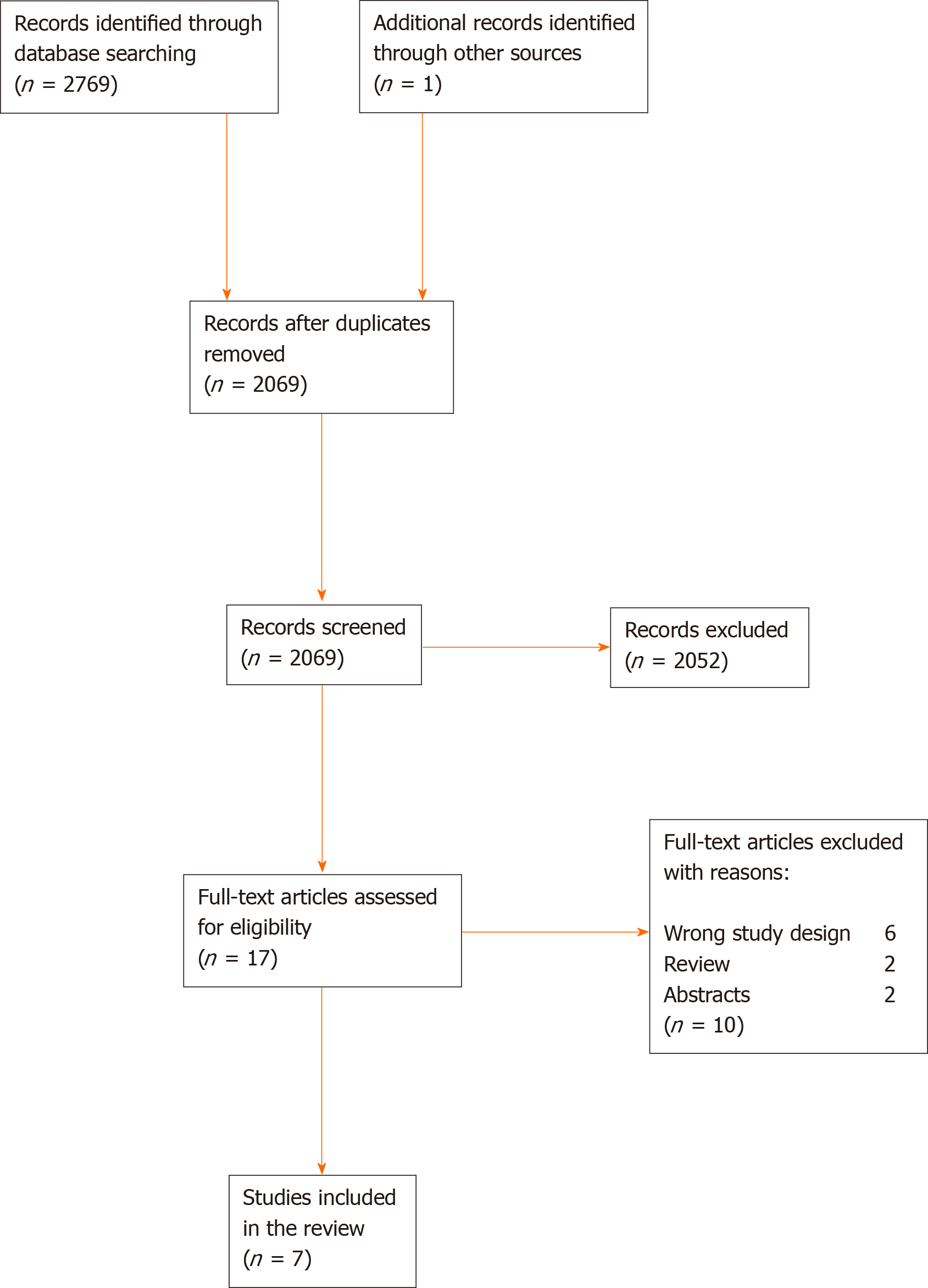
The authors screened 2769 articles in Rayyan and added one study from the reference lists of other studies. The authors screened 2069 articles after the removal of duplicates, of those 2052 articles were excluded after the screening of title and abstract. Seventeen articles were assessed for full-text screening, where additional ten studies were excluded due to wrong study design or if full-text versions were not available. Finally, seven studies were included comprising a total of 190 patients (Table 3), selected according to the criteria listed. The full screening process is shown in the PRISMA flow diagram (Figure 2).
| Ref. | Study design | Patients (n) | Age (yr) | Gender (M/W) | Diagnosis | Contrast | Dosage (mg/kg) | Adverse events | Endoscopic findings | Diagnostic accuracy (%) | Vessel count | Applicability |
| Iseki et al[25], 2000 | Retrospective | 37 | 59 (me) | 25/12 | Gastric cancer | ICG | 2-5 | N/A | 16/18 M tumors: No stain or homogeneous stain. 17/19 SM or more invasive tumors: Inhomogeneous stain or pooling of the dye | 89 | Yes | Tumor invasion |
| Mataki et al[21], 2003 | Retrospective | 33 | N/A | N/A | Early stage gastric cancer and gastric adenoma | ICG | 1 | None | 0/8 adenomas: + fluorescence. 9/14 M tumors: + fluorescence. 11/11 SM tumors: + fluorescence | N/A | N/A | Tumor invasion |
| Okamoto et al[22], 2005 | Retrospective | 20 | 65 (me) | 12/8 | Varices | ICG | 2, 0.1, 0.01, 0.005 or 0.001 | None | Clear fluorescence with doses of ICG in 0.005 to 0.01 mg/kg | N/A | N/A | Detection of varices |
| Ishihara et al[12], 2006 | Retrospective | 30 | N/A | N/A | Gastriccancer | ICG | 2 | N/A | 21/23 M or SM tumors < 1 mm: No stain or homogeneous stain. 7/7 SM tumors > 1 mm: Inhomogeneous stain or pooling of the dye | 93 | N/A | Tumor invasion |
| Kimura et al[23], 2007 | Retrospective | 30 | 71.5 (me) | 20/10 | Early stage gastric cancer and gastric adenoma | ICG | 0.01 | None | 1/20 M tumors: + fluorescence. 8/10 SM tumors: + fluorescence | N/A | Yes | Tumor invasion |
| Ortiz- Fernandez-Sordo et al[24], 2018 | Pilot study | 23 | 69 (49-85) (med) | 20/3 | Early neoplastic lesions within Barrett’s esophagus | ICG | 2 | None | 7/23 tumors: No stain (5/7 were less than HGD) 18/23 tumors: Stain (17/18 were at least HGD, MC or SMC) | 88 | N/A | Detection of neoplasms |
| Hartmans et al[15], 2018 | Retrospective | 17 | 42 (20-65) (med) | 5/12 | FAP | Bevacizumab800CW | 4.5, 10 or 25 mg | None | Colorectal adenomas detected at all doses by fluorescence | N/A | N/A | Detection of colorectal adenomas |
The studies were rated for bias according to the Newcastle-Ottawa scale and reported according to their quality (Table 2). All studies were assessed as poor quality due to the lack of comparability and missing control groups. No risk of bias was made across the studies because of the limited number of studies included.
All the included studies used a system from Olympus (Tokyo, Japan). Intravenous injection of the fluorophore was done in all included studies visualizing the vascularity of the tissue of interest. Six of the seven studies investigated the diagnostic value of fluorescence endoscopy in patients with previously diagnosed adenomas, neoplasms, or cancer (n = 170)[12,15,21], and one study investigated the use in detecting esophageal varices (n = 20)[22-25].
All studies categorized and evaluated the endoscopic findings differently according to the observed fluorescence appearance. Two studies classified the fluorescence staining as no tumor stain, homogeneous tumor stain, inhomogeneous tumor stain, or pooling of the dye[12,25], while another study categorized the staining as no stain, faint stain, dense stain, homogeneous stain, and pooling of the dye. The definitions were as follows; no stain: A decreased dye accumulation in the tumor compared to surrounding mucosa, homogeneous stain: A diffusely increased dye accumulation in the tumor compared with the surrounding mucosa, inhomogeneous stain: A scattered dye accumulation in the tumor, and pooling of the dye: A substantial dye accumulation in the tumor[24]. In another two studies, they categorized the pooling of the dye/fluorescence categorized as positive or negative[21,23]. The staining definitions and diagnostic values accordingly are shown in Table 3.
Six of seven studies used ICG as a fluorophore[12,21-25]. The dose of ICG ranged from 0.001 to 5 mg/kg bodyweight varying between a fixed dose or different doses of ICG. Four studies reported no adverse events according to ICG[21-24], and the remaining two did not report the frequency or absence of adverse events[12,25]. One study made a dose-response test for Bevacizumab-800CW, which was used as a fluorophore labeling Vascular Endothelial Growth Factor A present in colorectal adenomas and reported no adverse events according to the injections and doses (Table 3)[15].
Three studies assessed inter- or intraobserver agreement in the infrared fluorescence endoscopic examination. One study reported 90% in interobserver agreement[23], while another study reported a 97% interobserver agreement[21]. The third study reported 97% (kappa 0.97) in intraobserver agreement and a 85% (kappa 0.85) in interobserver agreement[25].
Five studies reported infrared fluorescence endoscopy as useful to assess tumor invasion or detect neoplasia[12,21,23-25]. In a retrospective study of 30 patients with depressed gastric cancers, the authors reported that 21 of 23 intramucosal and submucosal tumors smaller than 1 mm were observed with no stain or faint stain. Seven of seven submucosal tumors larger than 1 mm and more invasive tumors were observed with dense staining or pooling of the dye. Consequently, 28 of 30 both mucosal and submucosal tumors were correctly diagnosed (diagnostic accuracy 93%, Table 3). Additionally, 18 of 19 (accuracy 95%) of tumors with ulcerative changes were correctly diagnosed. Diagnostic accuracy was described as the level of compliance for endoscopic findings by using IR-light and a fluorophore compared with the histopathological examinations[12].
Iseki et al[25] (n = 37) reported that 16 of 18 mucosal tumors were observed with no stain or homogeneous tumor stain. Seventeen of 19 submucosal or deeper tumors were observed with inhomogeneous tumor stain or pooling of the dye. Consequently, 33 of 37 mucosal and submucosal tumors correctly diagnosed (diagnostic accuracy 89%, Table 3). Additionally, 33 of 37 (accuracy 89%) tumors correctly diagnosed as depressed or ulcerative. The study compared the diagnostic accuracy of fluorescence endoscopy and chromoendoscopy in assessing tumor invasion. Chromoendoscopy had a diagnostic accuracy at 68%, compared with fluorescence endoscopy (89%, aP < 0.02). Furthermore, the authors reported that tumor invasion assessed by fluorescence endoscopy was strongly correlated to the degree of tumor vascularity (bP < 0.01).
The study of Mataki et al[21] (n = 33) reported all eight gastric adenomas (accuracy 100%) negative for pooling of dye as in contrast to 20 of 25 (80%) for both mucosal and submucosal tumors which were positive for pooling of dye (Table 3) (cP < 0.03 for mucosal and submucosal). The authors suggested the fluorescence endoscopy as a diagnostic staging tool to determine if a tumor was eligible to make an endoscopic mucosal resection.
Kimura et al[23] (n = 30) reported one of 20 gastric adenomas or intramucosal tumors as being positive in fluorescence, and eight of ten submucosal tumors as being positive in fluorescence. The study did not state diagnostic accuracy, but the numbers correspond to a sensitivity of 80% and specificity of 95%. Also, a significant correlation between the invasiveness of the tumor, fluorescence, and vessel count was found (dP < 0.05).
One study examined early neoplastic lesions within Barrett’s esophagus in 23 cases[24]. Seven cases showed no stain, and histology showed less than high-grade dysplasia in five of those seven cases. Eighteen of 23 showed staining, and histology showed at least high-grade dysplasia, intramucosal carcinoma or submucosal carcinoma. Diagnostic accuracy was 88% (Table 3), sensitivity 90%, specificity 83%, and negative predictive value 71% in identifying the high-grade dysplasia or more advanced histopathology.
Two studies made a dose-response examination[15,22]. Okamoto et al[22] investigated esophageal varices (n = 20) with two studies-a clinical study, and an experimental study to evaluate tissue permeability. The clinical study suggested the optimal dose range of ICG between 0.005-0.01 mg/kg bodyweight based on their evaluation of the fluorescent signal to differentiate between normal mucosa and varices.
One study made a dose-response study with another fluorophore, Bevacizumab-800CW, investigating patients with Familial Adenomatous Polyposis (n = 17). Colorectal adenomas were detected with all doses of the fluorophore; 4.5 mg, 10 mg, and 25 mg, whereas normal mucosa showed no fluorescence[15].
In this systematic review, we identified seven studies using fluorescence endoscopy to assess and evaluate tumor invasion, detect neoplasms, adenomas and esophageal varices. Although fluorescence endoscopy was first described many years ago, this method with interesting results has become even more promising for therapeutic and diagnostic purposes with the recent advances within the field of fluorescence-guided surgery and cancer-specific imaging[26].
Six studies evaluated fluorescence endoscopy according to tumor development and invasion. In one study, fluorescence endoscopy was compared with chromoendoscopy, which is another method used to visualize and detect neoplasia in the gastrointestinal tract. The authors found a significantly higher diagnostic accuracy using fluorescence endoscopy (68% vs 89%, aP < 0.02)[25]. Furthermore, the authors reported a significant correlation between tumor invasion and tumor vascularity when using fluorescence endoscopy (bP < 0.01) as tumors with a tumor stain had significantly more vessels than did tumors without a tumor stain[25-27]. Additionally, the vessels were more varied in size in tumors showing inhomogeneous stain than tumors with a homogeneous stain. The authors suggested that tumor invasion to the submucosa will induce new, permeable vessels, which will result in extravasation of blood observed as pooling of the dye. The association between tumor invasion, fluorescence and vessel count was reproduced in another study with a significant correlation (dP < 0.05)[23]. The association of vascularity and tumor invasion was also demonstrated in a study of 44 patients which reported a color change in the endoscopic findings based on the tumor vascularity. The study assessed the tumor vascularity with an endoscopic quantitative analysis of the hemoglobin index[28]. Additionally, another study of 25 specimens from resections of early gastric cancer investigated color changes appearing during endoscopy. They suggested that blood flow, angiogenesis, and the microvasculature in tumors as factors responsible for the endoscopic findings[29]. Nevertheless, these mechanisms are not fully understood and need further assessment.
For evaluating vascularity, ICG has been used for many years, first for photography, later for angiography in 1969[30]. The contrast has been commercially available for many years, as it has a high level of safety and a very low incidence of adverse events has been reported[31,32]. In this systematic review, four of six included studies (n = 106) using ICG specifically reported that no adverse effects occurred[21-24], while the remaining two studies reporting nothing on adverse events. Usually, the recommended dosage of ICG is 0.2-0.25 mg/kg, which must not exceed 2 mg/kg in total[33]. One study included in this review reported an optimal dose of ICG at 0.005 to 0.01 mg/kg body weight[22], while another study reported a very high dosage of ICG at 2-5 mg/kg body weight[25]. However, no consensus about the ICG dosage exists in the studies.
Another subject of emerging clinical interest is the potentially cancer-specific fluorescent probes. Studies investigating the cancer-specific probes reflect the need for developing cancer-specific, optically detectable imaging agents to detect cancers and to add diagnostic and therapeutic value to fluorescence endoscopy. Both cancer-specific probes and the fluorescence endoscopy has been validated by several studies[15,34-37].
Recently, several studies have investigated the urokinase-type plasminogen activator receptor (uPAR) as a cancer-specific probe[38-41]. Using uPAR as a probe, one study subsequently demonstrated the feasibility of uPAR-coupled fluorescent probes. The promising results pointed towards a future using ICG-coupled uPAR probes for imaging and image-guided surgery as the tumor-targeted fluorophores may improve the discrimination between normal and neoplastic tissue. Cancer-specific fluorescent probes may also enable fluorescence-guided endoscopic resection with real-time assessment of the tumor margins, as well as prove to be a novel tool in response evaluation of tumors after chemoradiotherapy. The latter being possible by evaluation of fluctuations in fluorescence intensity caused by changes in tumor vascularity[42].
Fluorescence endoscopy is still lacking a method to quantify the fluorescent signal to decrease the subjectivity and increase objectivity, sensitivity, and specificity of the method. Some studies have investigated methods to quantitate the fluorescent signal. In the studies included in this review, the fluorescent signal was judged qualitative, meaning visually subjectively, except for one study which quantified the fluorescent signal ex vivo[15]. In a series of animal studies[43-45], a new method named quantitative-ICG for quantification of perfusion using ICG fluorescence was presented and validated. The quantification of the fluorescent signal will add an important factor to all technologies using fluorescence as a diagnostic marker.
This systematic review with a focus on human studies using fluorescence endoscopy led to 2769 articles screened, but only seven studies included in the final review, which reflects the limited research within the field. Notwithstanding the limited number of studies, seven of seven studies were rated as poor quality in the Newcastle Ottawa Scale for bias assessment. The low score reflects potential unreliability within the studies, as they all lacked control groups and non-exposed cohorts, thus indicating that this method needs further investigation. However, less strict criteria may have led to more heterogeneous studies included and a more challenging comparison of the endoscopic findings. The exclusion criteria were to keep a homogeneity in the studies and to reflect high clinical applicability of this systematic review.
In conclusion, this systematic review found that fluorescence endoscopy may add both diagnostic and therapeutic value within the field of gastrointestinal diseases. The majority of the studies included investigated the value within tumor staging, and the detection of adenomas, and neoplasms, thus indicating this method as an opportunity for a more precise diagnosis in the early development of neoplasms and tumors. More studies are needed to examine the usefulness of fluorescence endoscopy compared with other endoscopic methods. Furthermore, the combination of fluorescence endoscopy, quantification of the fluorescent signal, and cancer-specific fluorescent probes has the potential to improve the endoscopic diagnosis, monitoring and therapy of gastrointestinal diseases.
Different studies have investigated the use of fluorescence based endoscopy systems where the white light has been supplemented by infrared light and the use of relevant fluorophores. Fluorescence endoscopy is among the recent advances within the field of fluorescence-guided surgery and cancer-specific imaging.
The aim of this systematic review was to evaluate both the diagnostic and therapeutic value of fluorescence-guided flexible intraluminal endoscopy. Angiogenesis and neovascularization are important factors in tumor invasion, and as mucosal and submucosal vessels are not visible to the naked eye, but after intravenous injection of a fluorophore and illumination by infrared light, profound structures can be visualized.
Fluorescence endoscopy can be used within the detection the early development of neoplasms and tumors, adenomas, assessment of tumor invasion within the gastrointestinal tract. Those qualities are a part of the recent advances within the field of fluorescence-guided surgery and cancer-specific imaging.
The research method was a data analysis. We followed the PRISMA guidelines for this systematic review. The research covered five databases; PubMed, Scopus, Web of Science, Embase, and the Cochrane Collection. Authors screened title and abstract for inclusion, subsequently full-text for inclusion according to eligibility criteria listed in the protocol. The risk of bias was assessed for all studies according to the Newcastle-Ottawa Scale. The authors extracted the data and reported the results in both text and tables.
We included seven studies in the systematic review after screening a total of 2769 papers. Four studies evaluated the usefulness of fluorescence endoscopy in assessing tumor invasion. Three of the four studies reported an exceptional diagnostic accuracy in assessing tumor invasion, thus representing better visualization and more correct diagnosis by fluorescence endoscopy compared with the conventional endoscopy. The relationship between the endoscopic findings, tumor invasion, and tumor vascularity was evaluated in two studies showing a significant correlation. The use of fluorescence endoscopy is a promising method.
This systematic review explored the diagnostic and therapeutic value of fluorescence endoscopy. This study proposes fluorescence endoscopy as a method, which can increase those values, in the context of what is already known. This systematic review reflects a high clinical applicability, and fluorescence endoscopy is a method, that builds on the approach of tumor vascularity. This is the hypothesis of this systematic review and how this cooperate with the diagnostic and therapeutic value.
More studies are needed to utilize the feasibility of fluorescence endoscopy compared with other endoscopic methods exploring the diagnostic and therapeutic value in different clinical issues.
Christian Dam Lütken, research scholar at the Department of Surgical Gastroenterology, Rigshospitalet helped and supported through the process; Brandon R. Schulz reviewed the manuscript according to English orthography.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Denmark
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Quadros LGD, Wan Q S-Editor: Zhang L L-Editor: A P-Editor: Wang LL
| 1. | Hellier MD, Williams JG. The burden of gastrointestinal disease: implications for the provision of care in the UK. Gut. 2007;56:165-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53206] [Cited by in F6Publishing: 51049] [Article Influence: 8508.2] [Reference Citation Analysis (122)] |
| 3. | Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 578] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 4. | Lee JH, Wang TD. Molecular endoscopy for targeted imaging in the digestive tract. Lancet Gastroenterol Hepatol. 2016;1:147-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Davis ID, Ho M, Hupertz V, Avner ED. Survival of childhood polycystic kidney disease following renal transplantation: the impact of advanced hepatobiliary disease. Pediatr Transplant. 2003;7:364-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Goetz M, Wang TD. Molecular imaging in gastrointestinal endoscopy. Gastroenterology. 2010;138:828-33.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, Venermo M, Välisuo P. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging. 2012;2012:940585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 682] [Cited by in F6Publishing: 769] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 8. | Frangioni JV. In vivo near-infrared fluorescence imaging. Curr Opin Chem Biol. 2003;7:626-634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1974] [Cited by in F6Publishing: 1794] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 9. | Tsai SR, Hamblin MR. Biological effects and medical applications of infrared radiation. J Photochem Photobiol B. 2017;170:197-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 10. | Folkman J. Angiogenesis. Annu Rev Med. 2006;57:1-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 980] [Cited by in F6Publishing: 962] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 11. | Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5115] [Cited by in F6Publishing: 5659] [Article Influence: 106.8] [Reference Citation Analysis (1)] |
| 12. | Ishihara R, Uedo N, Iishi H, Ogiyama S, Yamada T, Higashino K, Narahara H, Tatsuta M, Iseki K, and Ishiguro S. Recent development and usefulness of infrared endoscopic system for diagnosis of gastric cancer. Digest Endosc. 2006;18:45-48. [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Liu G, Zhao Y. In Vivo Near-Infrared Fluorescence Imaging, Nanotechnology Characterization Tools for Biosensing and Medical Diagnosis. Springer, Berlin, Heidelberg 2018: 67-125. [DOI] [Cited in This Article: ] |
| 14. | Lamberts LE, Koch M, de Jong JS, Adams ALL, Glatz J, Kranendonk MEG, Terwisscha van Scheltinga AGT, Jansen L, de Vries J, Lub-de Hooge MN, Schröder CP, Jorritsma-Smit A, Linssen MD, de Boer E, van der Vegt B, Nagengast WB, Elias SG, Oliveira S, Witkamp AJ, Mali WPTM, Van der Wall E, van Diest PJ, de Vries EGE, Ntziachristos V, van Dam GM. Tumor-Specific Uptake of Fluorescent Bevacizumab-IRDye800CW Microdosing in Patients with Primary Breast Cancer: A Phase I Feasibility Study. Clin Cancer Res. 2017;23:2730-2741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 154] [Cited by in F6Publishing: 183] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 15. | Hartmans E, Tjalma JJJ, Linssen MD, Allende PBG, Koller M, Jorritsma-Smit A, Nery MESO, Elias SG, Karrenbeld A, de Vries EGE, Kleibeuker JH, van Dam GM, Robinson DJ, Ntziachristos V, Nagengast WB. Potential Red-Flag Identification of Colorectal Adenomas with Wide-Field Fluorescence Molecular Endoscopy. Theranostics. 2018;8:1458-1467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6253] [Cited by in F6Publishing: 7039] [Article Influence: 469.3] [Reference Citation Analysis (0)] |
| 17. | National Institute for Health Research. PROSPERO: International prospective register of systematic reviews. [Assessed August 2019]. Available from: https://www.crd.york.ac.uk/PROSPERO/. [Cited in This Article: ] |
| 18. | Qatar Computing Research Institute. Rayyan QCRI. [Assessed October 2019]. Available from: https://rayyan.qcri.org/welcome. [Cited in This Article: ] |
| 19. | Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5711] [Cited by in F6Publishing: 7811] [Article Influence: 976.4] [Reference Citation Analysis (0)] |
| 20. | Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M, and Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis., 2000. Assessed October 2019; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Cited in This Article: ] |
| 21. | Mataki N, Nagao S, Kawaguchi A, Matsuzaki K, Miyazaki J, Kitagawa Y, Nakajima H, Tsuzuki Y, Itoh K, Niwa H, Miura S. Clinical usefulness of a new infrared videoendoscope system for diagnosis of early stage gastric cancer. Gastrointest Endosc. 2003;57:336-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Okamoto K, Muguruma N, Kimura T, Yano H, Imoto Y, Takagawa M, Kaji M, Aoki R, Sato Y, Okamura S, Kusaka Y, Ito S. A novel diagnostic method for evaluation of vascular lesions in the digestive tract using infrared fluorescence endoscopy. Endoscopy. 2005;37:52-57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Kimura T, Muguruma N, Ito S, Okamura S, Imoto Y, Miyamoto H, Kaji M, Kudo E. Infrared fluorescence endoscopy for the diagnosis of superficial gastric tumors. Gastrointest Endosc. 2007;66:37-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Ortiz-Fernandez-Sordo J, Sami SS, Mansilla-Vivar R, Subramanian V, Mannath J, Telakis E, Ragunath K. Evaluation of a novel infra-red endoscopy system in the assessment of early neoplasia in Barretts esophagus: pilot study from a single center. Dis Esophagus. 2018;31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Iseki K, Tatsuta M, Iishi H, Sakai N, Yano H, Ishiguro S. Effectiveness of the near-infrared electronic endoscope for diagnosis of the depth of involvement of gastric cancers. Gastrointest Endosc. 2000;52:755-762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Baiocchi GL, Diana M, Boni L. Indocyanine green-based fluorescence imaging in visceral and hepatobiliary and pancreatic surgery: State of the art and future directions. World J Gastroenterol. 2018;24:2921-2930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 86] [Cited by in F6Publishing: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 27. | Trivedi PJ, Braden B. Indications, stains and techniques in chromoendoscopy. QJM. 2013;106:117-131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Yao K, Yao T, Matsui T, Iwashita A, Oishi T. Hemoglobin content in intramucosal gastric carcinoma as a marker of histologic differentiation: a clinical application of quantitative electronic endoscopy. Gastrointest Endosc. 2000;52:241-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Honmyo U, Misumi A, Murakami A, Mizumoto S, Yoshinaka I, Maeda M, Yamamoto S, Shimada S. Mechanisms producing color change in flat early gastric cancers. Endoscopy. 1997;29:366-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Kogure K, Choromokos E. Infrared absorption angiography. J Appl Physiol. 1969;26:154-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Hope-Ross M, Yannuzzi LA, Gragoudas ES, Guyer DR, Slakter JS, Sorenson JA, Krupsky S, Orlock DA, Puliafito CA. Adverse reactions due to indocyanine green. Ophthalmology. 1994;101:529-533. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 406] [Cited by in F6Publishing: 394] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 32. | Benya R, Quintana J, Brundage B. Adverse reactions to indocyanine green: a case report and a review of the literature. Cathet Cardiovasc Diagn. 1989;17:231-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 160] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | FDA. Professional Drug Information: Indocyanine Green. 2018. [Assessed November 2019]. Available from: https://www.drugs.com/pro/indocyanine-green.html. [Cited in This Article: ] |
| 34. | Kelly K, Alencar H, Funovics M, Mahmood U, Weissleder R. Detection of invasive colon cancer using a novel, targeted, library-derived fluorescent peptide. Cancer Res. 2004;64:6247-6251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 35. | van Oosten M, Crane LM, Bart J, van Leeuwen FW, van Dam GM. Selecting Potential Targetable Biomarkers for Imaging Purposes in Colorectal Cancer Using TArget Selection Criteria (TASC): A Novel Target Identification Tool. Transl Oncol. 2011;4:71-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Schwegmann K, Bettenworth D, Hermann S, Faust A, Poremba C, Foell D, Schäfers M, Domagk D, Lenz P. Detection of Early Murine Colorectal Cancer by MMP-2/-9-Guided Fluorescence Endoscopy. Inflamm Bowel Dis. 2016;22:82-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Ito S, Muguruma N, Kusaka Y, Tadatsu M, Inayama K, Musashi Y, Yano M, Bando T, Honda H, Shimizu I, Ii K, Takesako K, Takeuchi H, Shibamura S. Detection of human gastric cancer in resected specimens using a novel infrared fluorescent anti-human carcinoembryonic antigen antibody with an infrared fluorescence endoscope in vitro. Endoscopy. 2001;33:849-853. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Laerum OD, Ovrebo K, Skarstein A, Christensen IJ, Alpízar-Alpízar W, Helgeland L, Danø K, Nielsen BS, Illemann M. Prognosis in adenocarcinomas of lower oesophagus, gastro-oesophageal junction and cardia evaluated by uPAR-immunohistochemistry. Int J Cancer. 2012;131:558-569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Noh H, Hong S, Huang S. Role of urokinase receptor in tumor progression and development. Theranostics. 2013;3:487-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 40. | Alpízar-Alpízar W, Christensen IJ, Santoni-Rugiu E, Skarstein A, Ovrebo K, Illemann M, Laerum OD. Urokinase plasminogen activator receptor on invasive cancer cells: a prognostic factor in distal gastric adenocarcinoma. Int J Cancer. 2012;131:E329-E336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Christensen A, Kiss K, Lelkaitis G, Juhl K, Persson M, Charabi BW, Mortensen J, Forman JL, Sørensen AL, Jensen DH, Kjaer A, von Buchwald C. Urokinase-type plasminogen activator receptor (uPAR), tissue factor (TF) and epidermal growth factor receptor (EGFR): tumor expression patterns and prognostic value in oral cancer. BMC Cancer. 2017;17:572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Christensen A, Juhl K, Persson M, Charabi BW, Mortensen J, Kiss K, Lelkaitis G, Rubek N, von Buchwald C, Kjær A. uPAR-targeted optical near-infrared (NIR) fluorescence imaging and PET for image-guided surgery in head and neck cancer: proof-of-concept in orthotopic xenograft model. Oncotarget. 2017;8:15407-15419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 43. | Rønn JH, Nerup N, Strandby RB, Svendsen MBS, Ambrus R, Svendsen LB, Achiam MP. Laser speckle contrast imaging and quantitative fluorescence angiography for perfusion assessment. Langenbecks Arch Surg. 2019;404:505-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Nerup N, Andersen HS, Ambrus R, Strandby RB, Svendsen MBS, Madsen MH, Svendsen LB, Achiam MP. Quantification of fluorescence angiography in a porcine model. Langenbecks Arch Surg. 2017;402:655-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 45. | Nerup N, Knudsen KBK, Ambrus R, Svendsen MBS, Thymann T, Ifaoui IBR, Svendsen LB, Achiam MP. Reproducibility and Reliability of Repeated Quantitative Fluorescence Angiography. Surg Technol Int. 2017;31:35-39. [PubMed] [Cited in This Article: ] |