Published online Apr 16, 2019. doi: 10.4253/wjge.v11.i4.298
Peer-review started: February 20, 2019
First decision: February 26, 2019
Revised: March 15, 2019
Accepted: March 26, 2019
Article in press: March 26, 2019
Published online: April 16, 2019
Endoscopic retrograde cholangiopancreatography (ERCP) is preferred for managing biliary obstruction in patients with bilio-enteric anastomotic strictures (BEAS) and calculi. In patients whose duodenal anatomy is altered following upper gastrointestinal (UGI) tract surgery, ERCP is technically challenging because the biliary tree becomes difficult to access by per-oral endoscopy. Advanced endoscopic therapies like balloon-enteroscopy or rendevous-ERCP may be considered but are not always feasible. Biliary sepsis and comorbidities may also make these patients poor candidates for surgical management of their biliary obstruction.
We present two 70-year-old caucasian patients admitted as emergencies with obstructive cholangitis. Both patients had BEAS associated with calculi that were predominantly extrahepatic in Patient 1 and intrahepatic in Patient 2. Both patients were unsuitable for conventional ERCP due to surgically-altered UGl anatomy. Emergency biliary drainage was by percutaneous transhepatic cholangiography (PTC) in both cases and after 6-weeks’ maturation, PTC tracts were dilated to perform percutaneous transhepatic cholangioscopy and lithotripsy (PTCSL) for duct clearance. BEAS were firstly dilated fluoroscopically, and then biliary stones were flushed into the small bowel or basket-retrieved under visualization provided by the percutaneously-inserted video cholangioscope. Lithotripsy was used to fragment impacted calculi, also under visualization by video cholangioscopy. Satisfactory duct clearance was achieved in Patient 1 after one PTCSL procedure, but Patient 2 required a further procedure to clear persisting intrahepatic calculi. Ultimately both patients had successful stone clearance confirmed by check cholangiograms.
PTCSL offers a pragmatic, feasible and safe method for biliary tract clearance when neither ERCP nor surgical exploration is suitable.
Core tip: The purpose of this case report is to highlight the feasibility of percutaneous transhepatic cholangioscopy and lithotripsy (PTCSL) as therapy for biliary obstruction in patients with surgically altered anatomy which makes them unsuitable for conventional endoscopic retrograde cholangiopancreatography. Percutaneous transhepatic cholangiography used for emergency biliary drainage provides the access required for PTCSL, so it is reasonable to consider PTCSL in such patients. PTCSL attractively combines radiological and endoscopic techniques already established in most Hepato-Pancreato-Biliary units. Advanced endoscopic options are not widely available, and surgical options are limited as such patients are poor surgical candidates. We review the literature to compare our cases to previously reported cases of PTCSL.
- Citation: Alabraba E, Travis S, Beckingham I. Percutaneous transhepatic cholangioscopy and lithotripsy in treating difficult biliary ductal stones: Two case reports. World J Gastrointest Endosc 2019; 11(4): 298-307
- URL: https://www.wjgnet.com/1948-5190/full/v11/i4/298.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i4.298
Per-oral endoscopic access to the biliary tree is difficult after surgical procedures which alter the upper gastrointestinal (UGI) anatomy. These procedures include Billroth II distal gastrectomy, total gastrectomy, Roux-en-Y reconstruction gastric bypass, and, those involving bilio-enteric anastomotic strictures (BEAS) such as pancreaticoduodenectomy and hepaticojejunostomy. BEAS could be particularly problematic as they promote cholestasis and their sutures generate foreign body reaction, thus forming calculi that cause further biliary strictures[1-4].
Biliary tree calculi in these patients with altered upper GI anatomy can be managed by advanced endoscopic procedures such as balloon-assisted endoscopic retrograde cholangiopancreatography (ERCP)[5], or, rendezvous ERCP facilitated by Endoscopic Ultrasound (EUS)-guided or percutaneous biliary tree puncture[6]. Enteroscopes used for balloon-assisted ERCP have smaller working channels than those used for conventional ERCP and so may not permit use of adjuncts such as lithotripsy[5]. EUS-guided procedures are technically challenging and not widely available.
Another option is surgical bile duct exploration but patients presenting with cholangitis on a background of BEAS and calculi are usually poor surgical candidates due to their comorbidities. Re-operation can also be difficult due to adhesions from previous surgery. Surgical treatment options are thus limited by the potential for increased morbidity and mortality.
Current guidance recommends percutaneous radiological stone extraction for the small number of patients in whom endoscopic techniques are unsuccessful or impossible[7]. Biliary access is usually achieved by inserting a percutaneous transhepatic cholangiography (PTC) drain via which catheter interventions are performed under fluoroscopic guidance. In this context, a less commonly reported procedure is percutaneous transhepatic cholangioscopy and lithotripsy (PTCSL) which uses PTC biliary access for duct clearance under video cholangioscopy guidance with lithotripsy as an adjunct for stone fragmentation[7]. PTCSL is more established in East Asia where primary choledocholithiasis and hepatolithiasis are more prevalent, and PTCSL is used in their management. There are very few reports of PTCSL from other parts of the world; mostly case reports[8-10] and one single-centre case series[11]. In this first report of PTCSL from a UK centre, we describe our first 2 cases of PTCSL in patients with symptomatic BEAS associated with ductal stones.
Chief complaint: Patient 1 is a 70-year-old man who was admitted with acute cholangitis. He previously underwent curative total gastrectomy for gastro-oesophageal cancer 3-years prior, with reconstruction by Roux-en-Y oeso-phagojejunostomy. He underwent open repair of an incarcerated hiatus hernia two years later at which time symptomatic choledocholithiasis was treated by surgical common bile duct (CBD) exploration and side-to-side choledochoduodenostomy. His other co-morbidities were atrial fibrillation for which he was anticoagulated with warfarin, previous myocardial infarction, and limited mobility due to Paget's disease affecting both of his hips. He was malnourished and significantly underweight as a result of his previous surgeries.
Diagnostic evaluation: Physical examination of Patient 1 revealed a tachycardia of 120 bpm, and pyrexia of 38.5 °C. Abdominal exam demonstrated multiple previous surgical scars but a soft and non-distended abdomen. Laboratory work-up showed raised circulating white blood cell count of 19.6 × 109/L and raised bilirubin of 204 μmol/L.
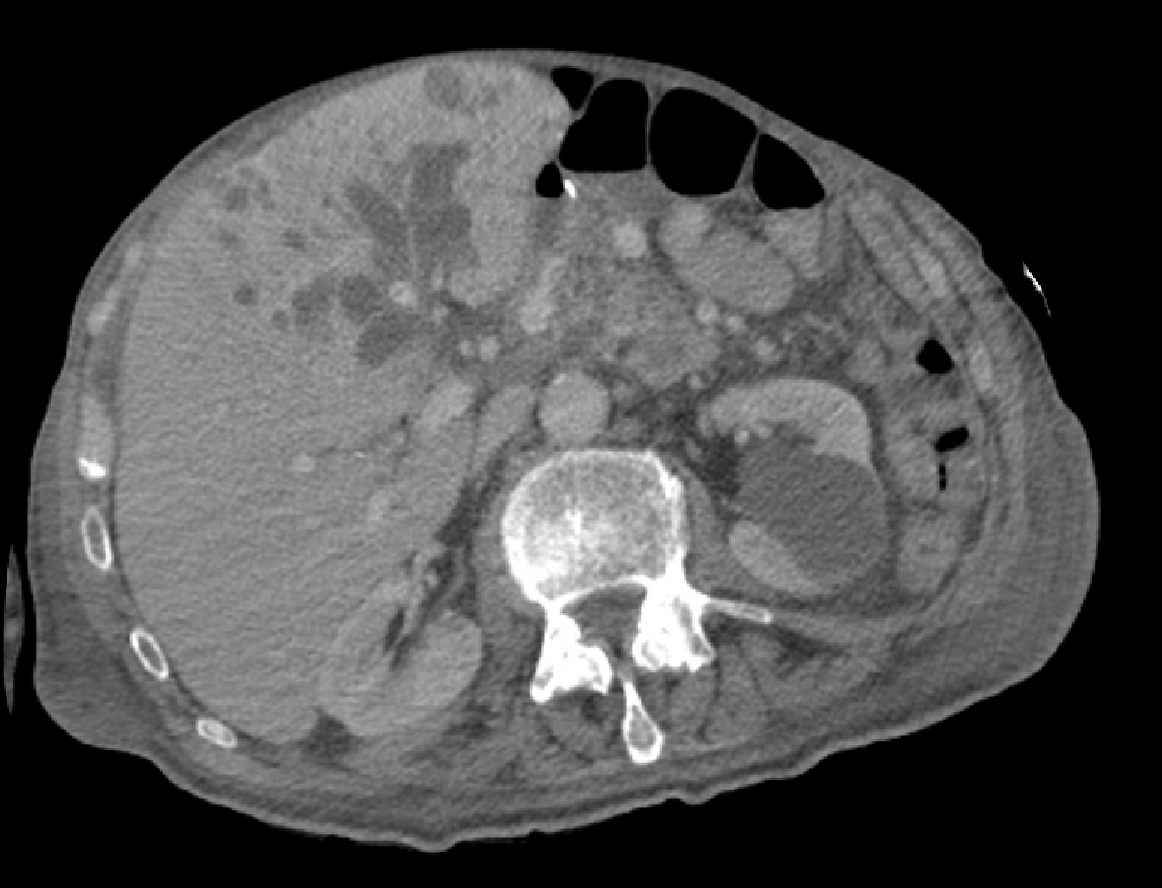
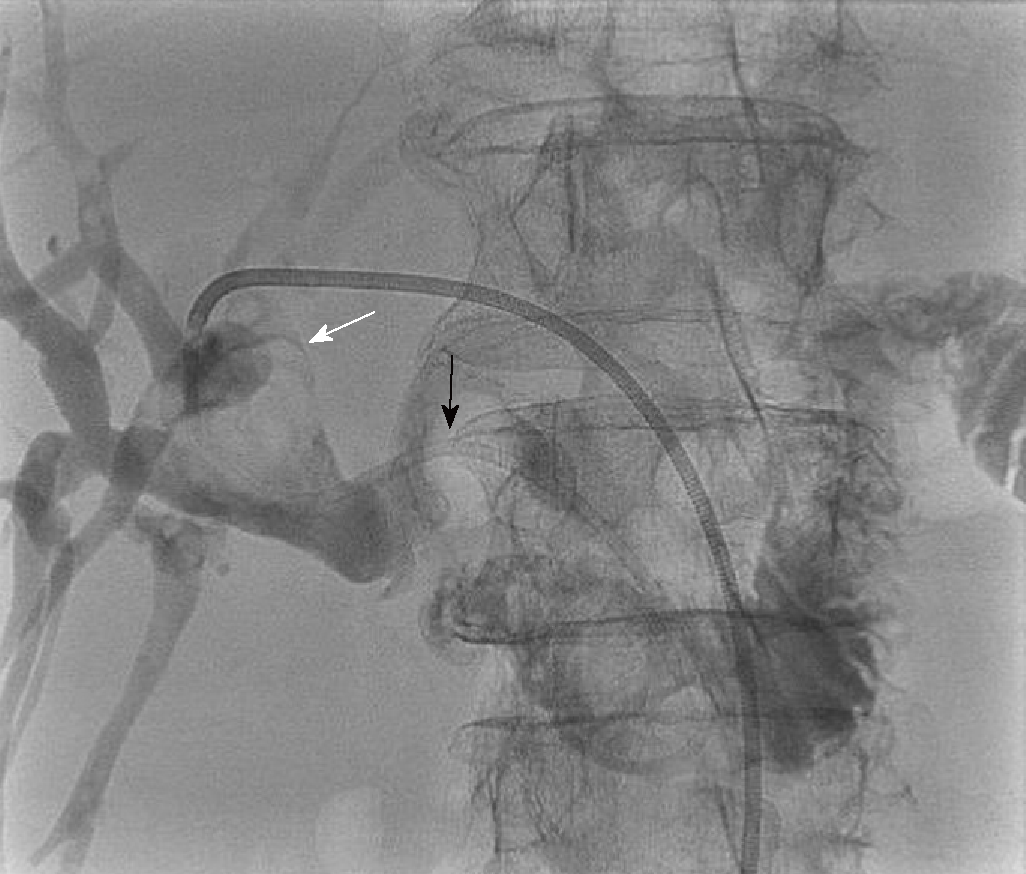
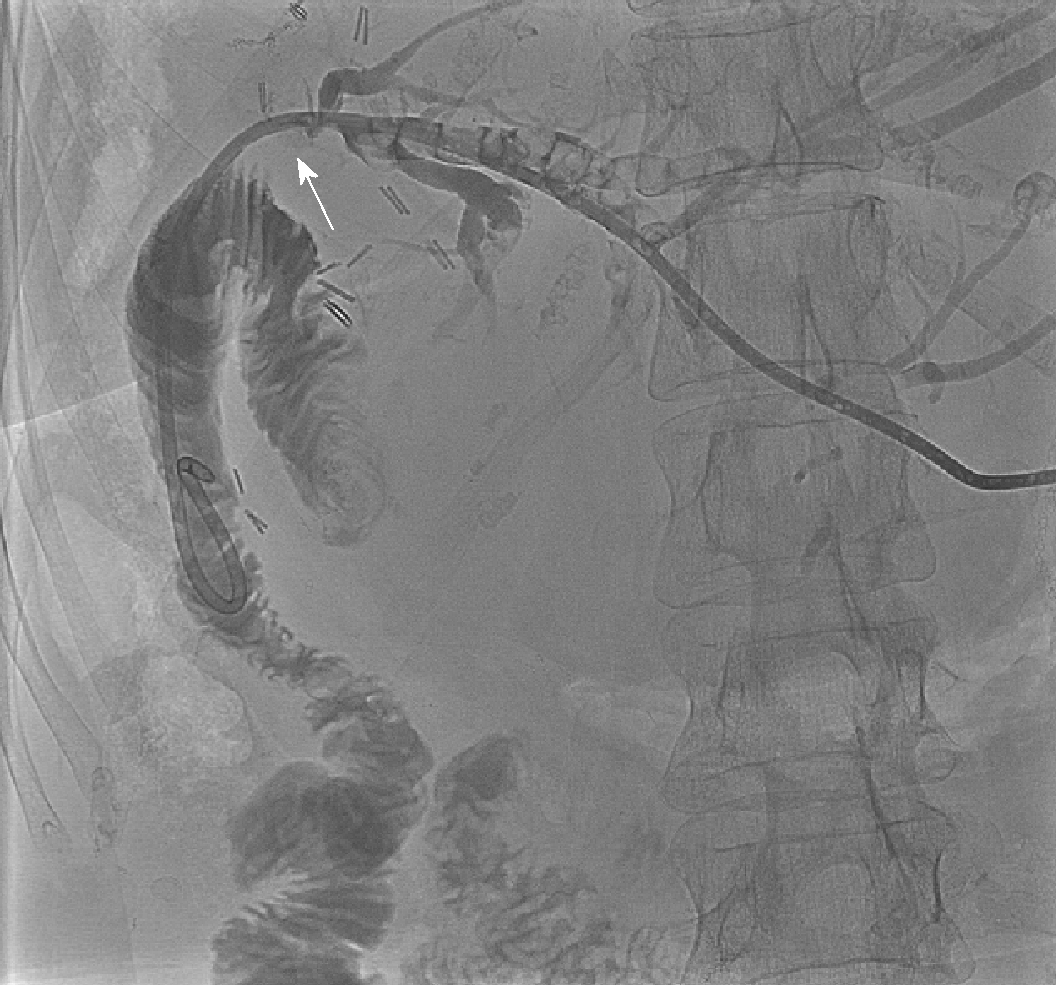
Computerised tomography (CT) scanning in Patient 1, after broad-spectrum antibiotics and intravenous fluid resuscitation, showed dilated left intrahepatic ducts Figure 1. USS-guided PTC (PTC) showed a large extrahepatic calculus and choledochoduodenostomy stricture Figure 2.
Chief complaint: Patient 2 is a 70-year-old female who was admitted as an emergency with acute cholangitis. She underwent curative subtotal-gastrectomy, en bloc right hemihepatectomy, and reconstruction by roux-en-y hepaticojejunostomy 4-years prior for metastatic gastric GIST down-staged with Imatinib. She also previously had left breast cancer treated with curative wide local excision and axillary node clearance 1-year prior. Her regular medications were Imatinib and Letrozole.
Diagnostic evaluation: Physical examination of Patient 2 revealed tachycardia of 115 bpm, pyrexia of 38.7 °C, soft non-distended abdomen and previous abdominal surgical scars. Her circulating white blood cell count and bilirubin were raised at 17.8 × 109/L and 163 μmol/L respectively.
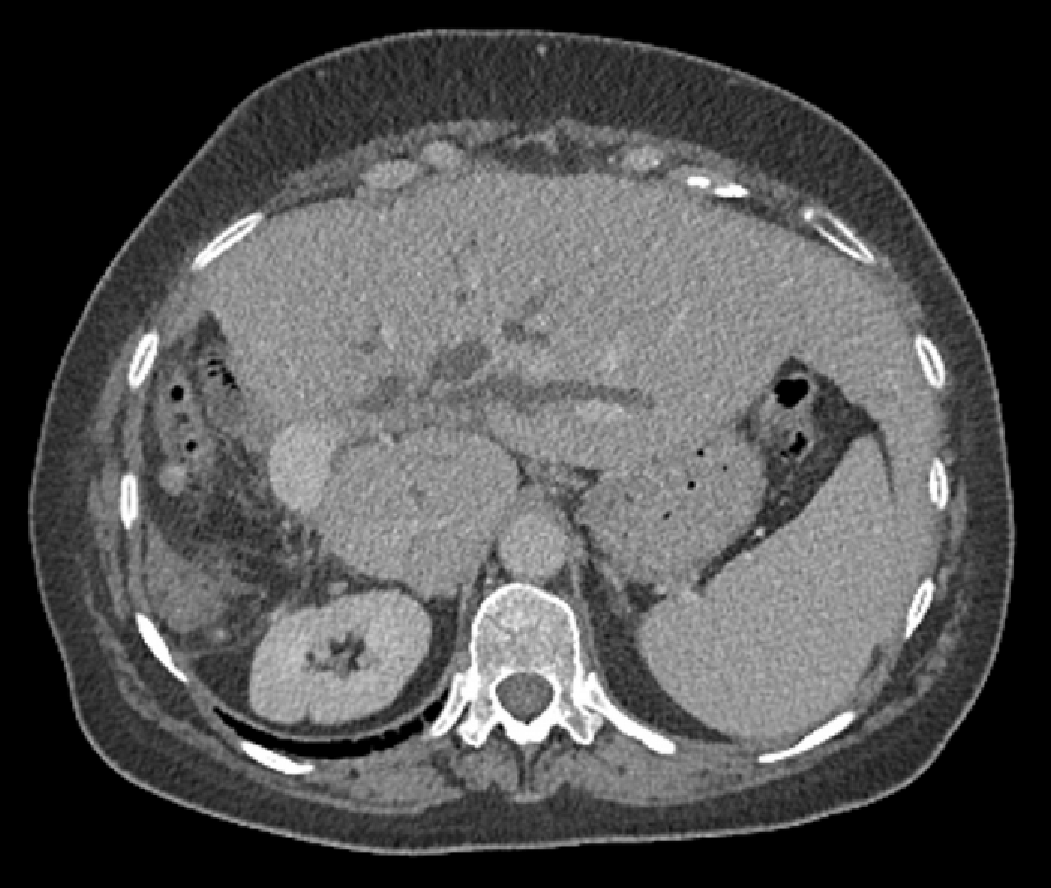
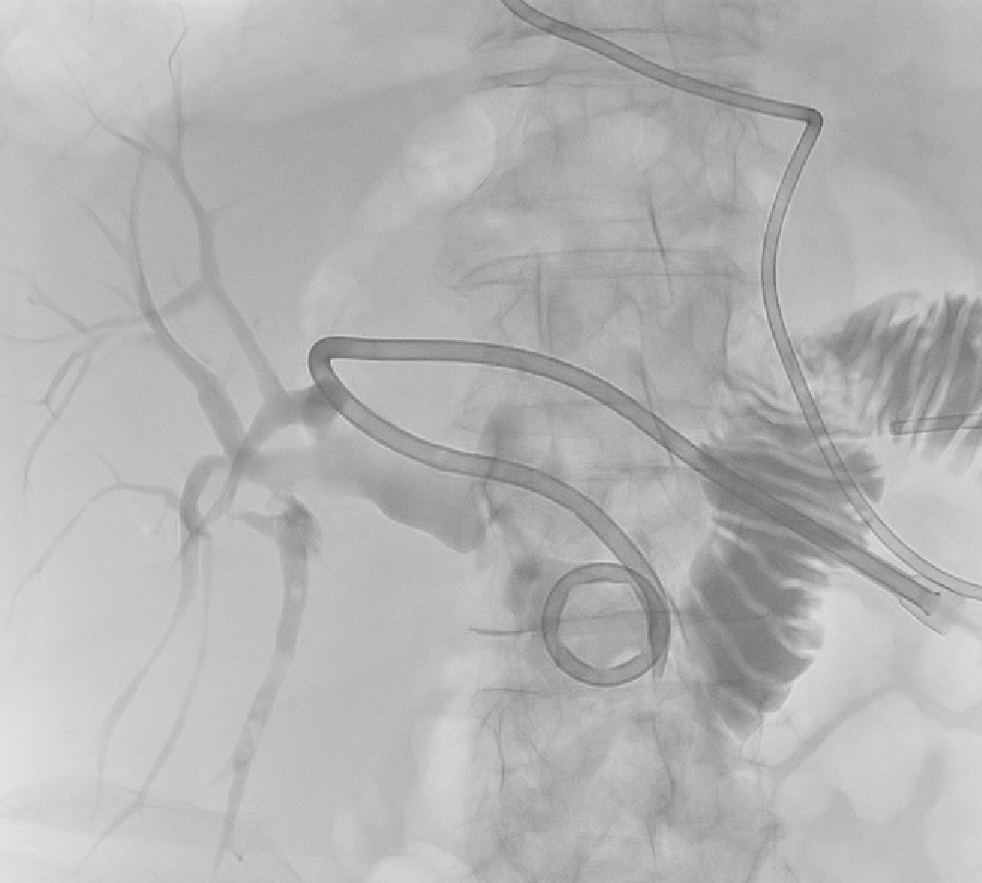
Patient 2 received broad-spectrum antibiotics and intravenous fluid resuscitation, then CT scanning showed dilated left intrahepatic ducts Figure 3. USS-guided PTC in Patient 2 showed multiple calculi in the dilated left intrahepatic ducts Figure 4 and a hepaticojejunostomy stricture.
Patient 1 had a BEAS of his choledochoduodenostomy and biliary calculi causing symptomatic biliary obstruction.
Patient 2 had a symptomatic hepaticojejunostomy stricture and biliary calculi.
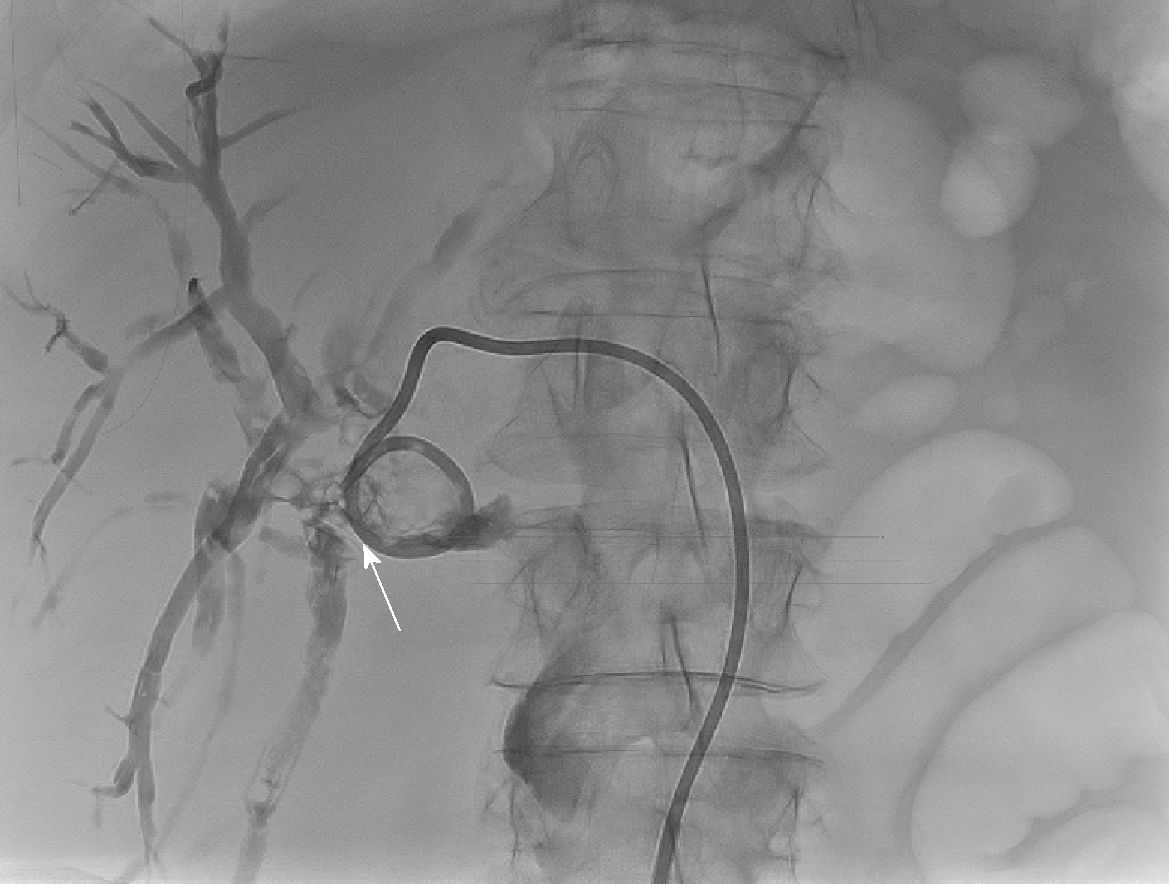
Emergency biliary drainage was achieved with an 8Fr locking pigtail external biliary drain inserted into the left hepatic ducts at diagnostic PTC in Patient 1 Figure 2. When the patient was clinically improved, the drain was internalised 6 d later Figure 5 with the internal component crossing the choledochoduodenostomy stricture. Jaundice resolved and cytology from PTC brushings did not show malignancy. Patient 1 was unsuitable for ERCP due to surgically altered anatomy and was a poor surgical candidate, so he was treated by PTCSL (procedure described in section titled “Treatment: PTCSL procedure”).
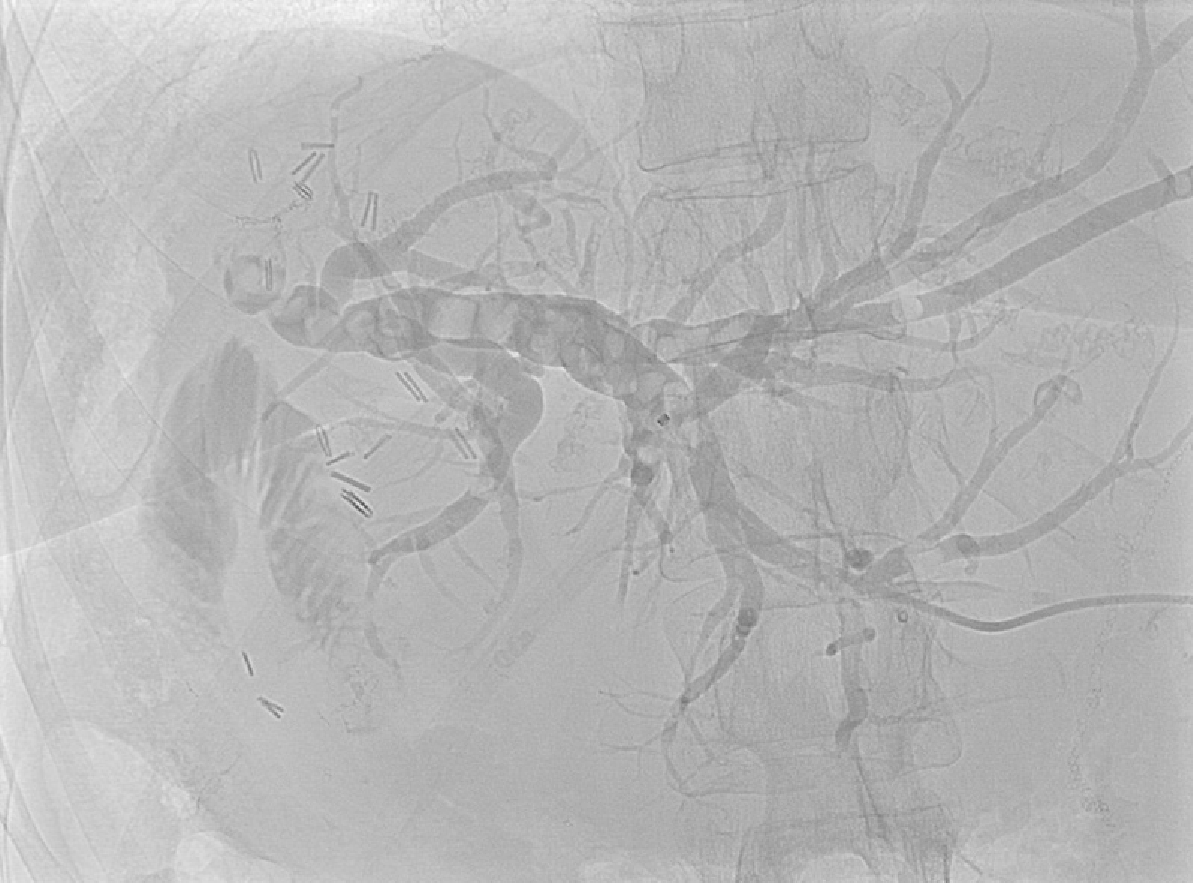
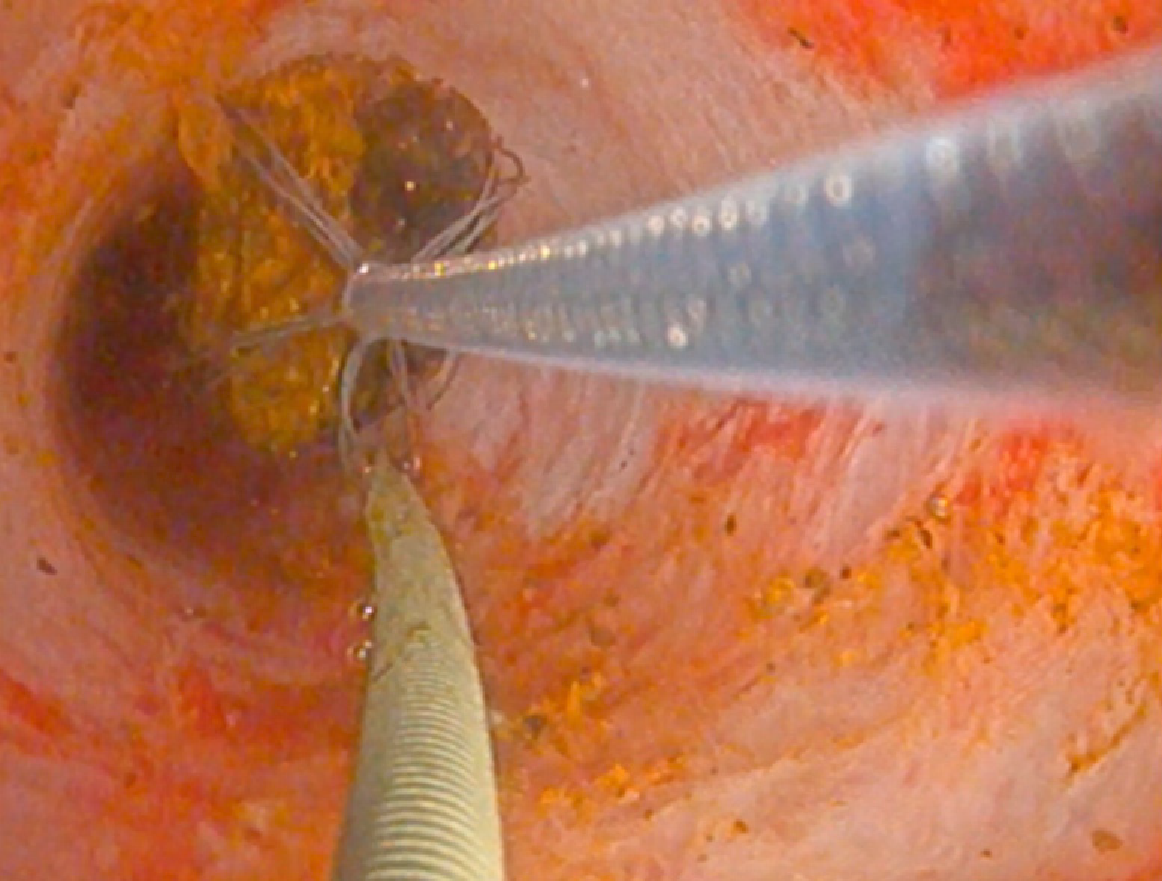
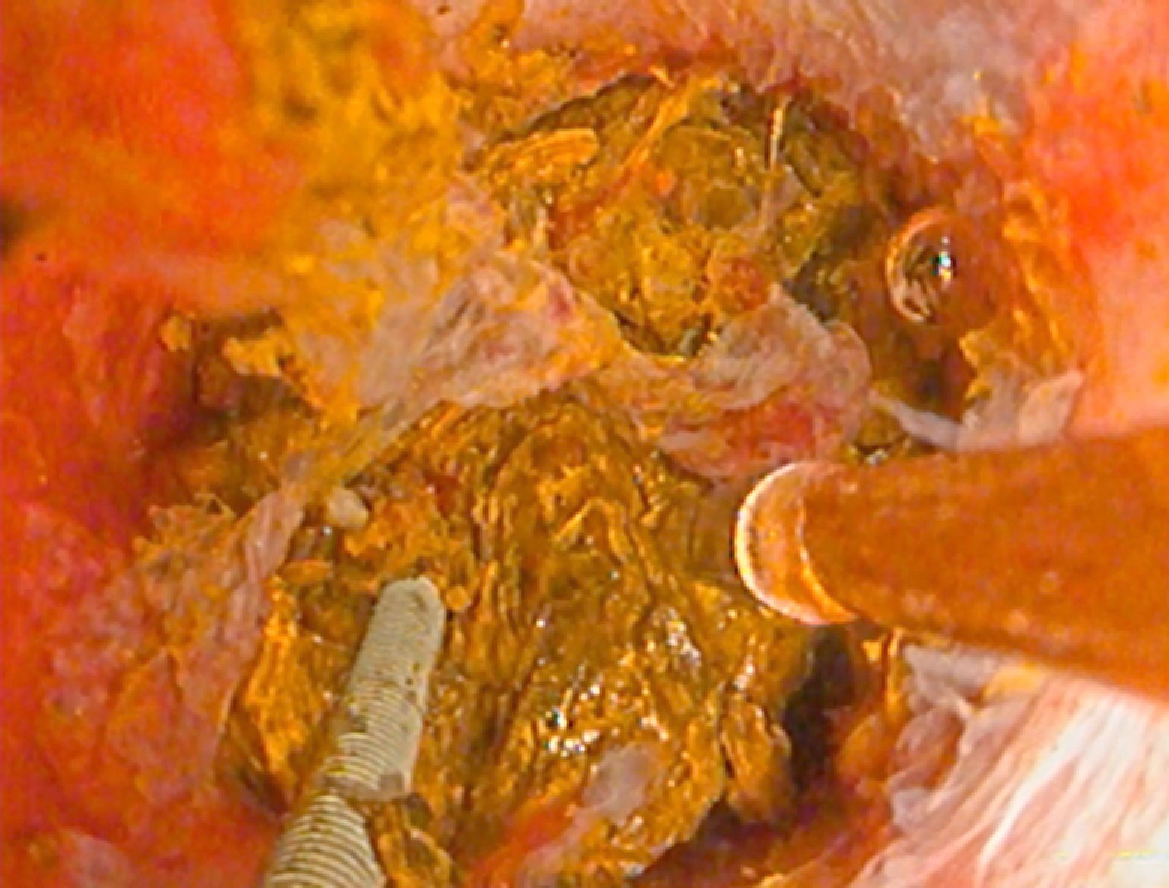
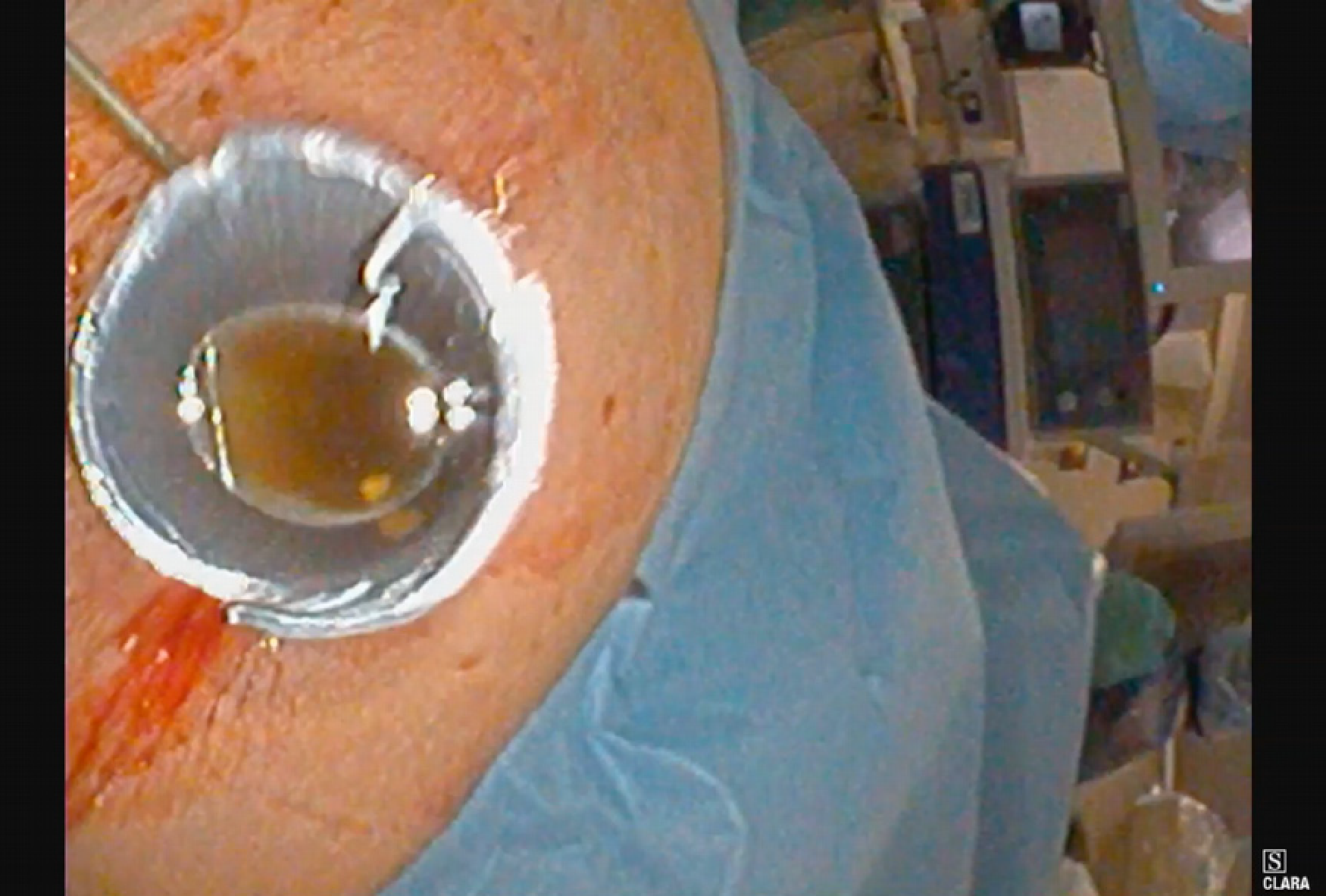
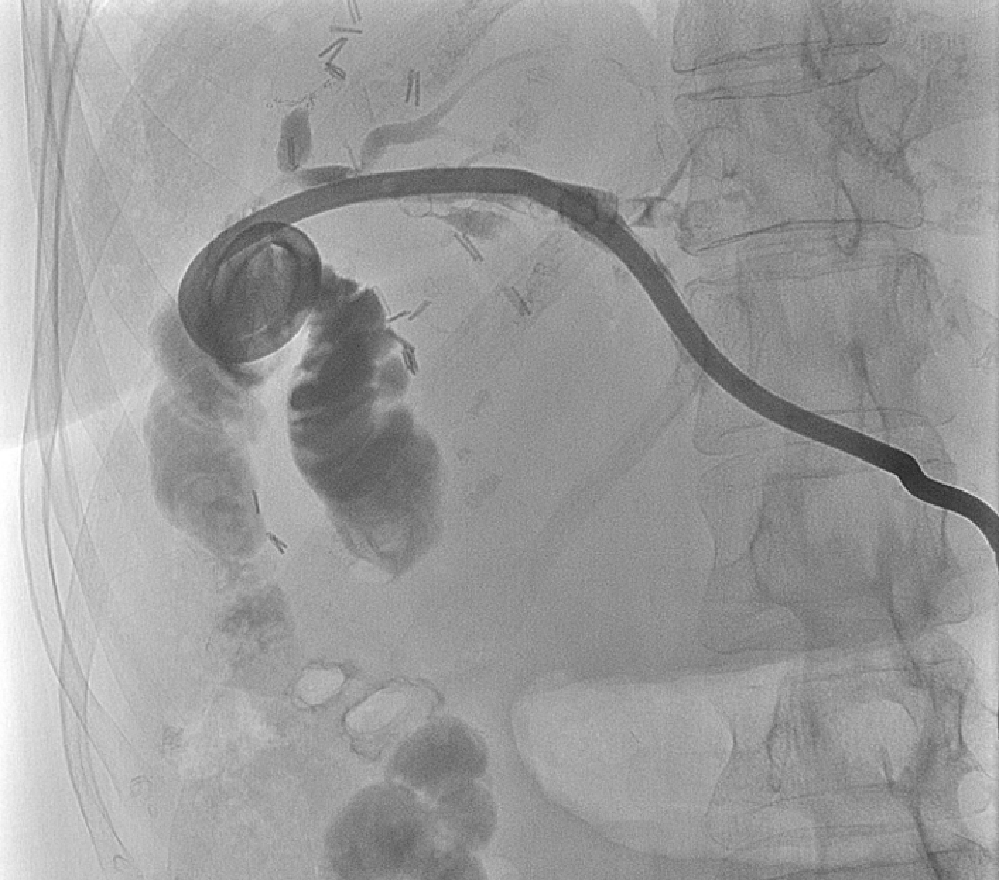
PTC tracts were allowed to mature for 6-wk; then PTCSL procedure performed aseptically under general anaesthesia administering prophylactic Co-Amoxiclav targeting Enterococci and gram-negative bacilli. Normal blood clotting was also ensured and the PTCSL procedure performed as follows: (1) PTC drains were exchanged over stiff wires (Terumo, Tokyo) for 23 cm 8F vascular sheaths (Cordis Milpitas, California) that were cannulated through the BEAS to serve as PTCSL access; (2) The vascular sheaths were exchanged for stiff Amplatz (Boston Scientific, Washington, United States) and standard J tipped (Kimal, Droitwich, United Kingdom) wires over which the tracts were dilated for insertion of 20F vascular sheaths. The sheaths were attached to skin with heavy silk sutures and trimmed to leave 20 French conduits for access into the left hepatic duct; (3) BEAS were dilated with 10mm Mustang angioplasty balloons (Boston Scientific, Washington) and some stones pushed into the small bowel; (4) Under direct visualisation with a 16F Choledochoscope (Karl Storz, Tuttlingen, Germany), stones were basket-retrieved Figure 6. Impacted stones were first fragmented using a Wolf 2280 Riwolith electrohydraulic lithotriptor (Richard Wolf, Knittliger, Germany) with a 5F flexible probe Figures 7 and 8; (5) At the end of the PTCSL procedure, the 20F sheaths were exchanged for 14F Flexima locking pigtail drains (Boston Scientific, Washington) over stiff Amplatz wires. Additional side holes were cut in the intra-biliary segment of the pigtail drains to create internal-external drains; the distal loops locked in the small bowel; (6) Drain position was confirmed fluoroscopically before securing with skin-suture and RevolutionTM catheter securement device (Merit Medical, Utah, United States); and (7) PTC drains were clamped after 24 h free-drainage and check cholangiogram performed 1-2 wk later.
Emergency biliary drainage was achieved with an 8Fr internal-external biliary drain inserted into the left hepatic ducts at diagnostic PTC in Patient 2 Figure 9. Jaundice resolved and cytology from PTC brushings did not show malignancy. Surgically altered anatomy made ERCP unfeasible and she was high-risk for surgery, so had PTCSL (procedure described in section titled “Treament: PTCSL procedure”).
Patient 1 made a good recovery with ward-based care following PTCSL. He had nasojejunal (NJ) feeding nutritional support. Check cholangiogram Figure 10 showed four small non-obstructing calculi in peripheral ducts of Segment 6 but good flow of contrast into the duodenum. The internal-external drain was removed, and the tract sealed with AviteneTM microfibrillar collagen haemostat (Bard, Rhode Island, United States), and he was discharged from hospital to continue NJ feeding at home. Patient 1 was clinically well and reported no further episodes of symptomatic biliary obstruction at 6-mo follow-up.
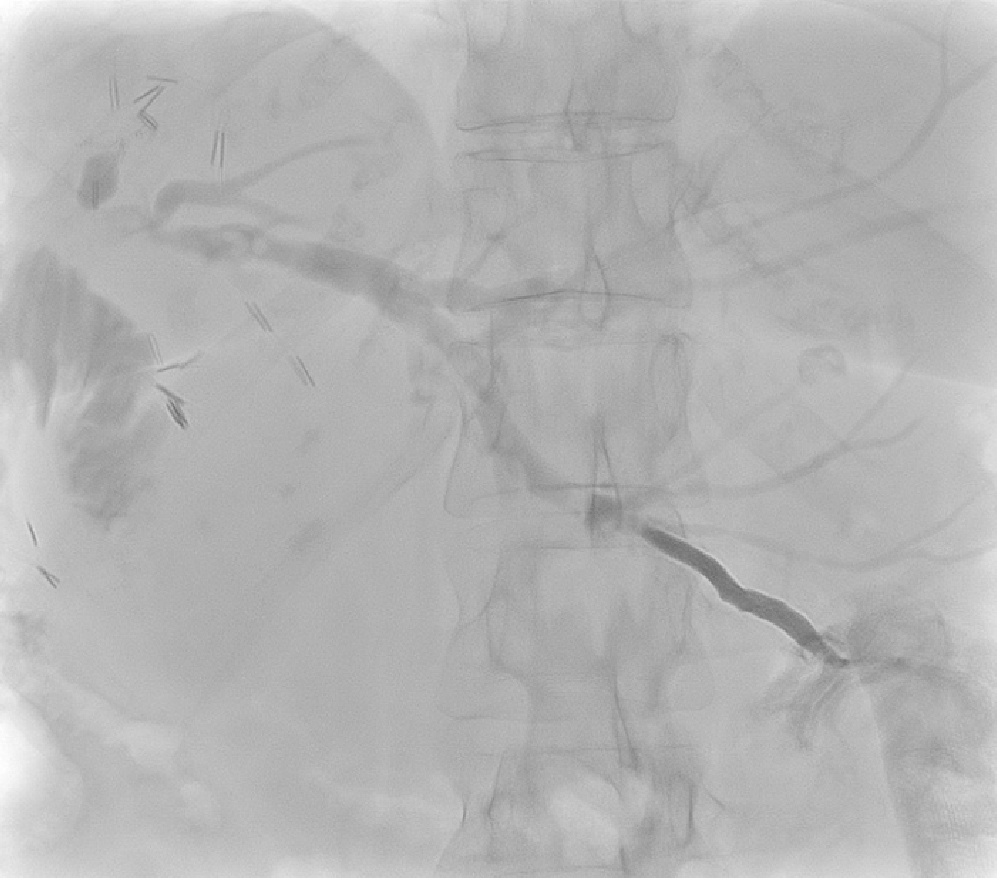
Patient 2’s check cholangiogram showed remnant stones in segment 2 but contrast flowing freely into jejunum Figure 11. She was discharged home with the PTC drain clamped. She was readmitted 4 wk later for flushing of PTC drain to remove remnant stones. Subsequent cholangiogram showed fewer intrahepatic biliary duct stones Figure 12. Her PTC drain was removed the day after, and the track sealed by AviteneTM. She was discharged home without complications and remained well with no further episodes of symptomatic biliary obstruction at 6-mo follow-up.
The attractiveness of this PTCSL is that it combines two techniques that are routinely used in most Hepato-Pancreato-Biliary (HPB) Centres, namely: PTC and endoscopic biliary cholangioscopy. This makes it more feasible to establish PTCSL as a treatment for difficult ductal calculi in most HPB Centres. Patients with acute cholangitis may require emergency biliary drainage which when not possible by ERCP, is done by PTC drain insertion. As in both of our cases, emergency biliary decompression in patients with surgically-altered UGI anatomy is usually be done by PTC which makes it pragmatic to subsequently adopt PTCSL for definitive biliary clearance. We uniquely describe the first report of PTCSL from a UK Centre.
Although percutaneous access for PTCSL is usually transhepatic, it is also possible to gain access via a small bowel access loop; a T-tube sinus tract; or trans-cholecystically. However, none of these alternative access routes were available in our cases. It is preferable to perform PTCSL under general anaesthesia as the procedure is painful due to its working sheath which traverses skin, intercostal muscles, and the liver capsule. The absence of enhanced sedation or general anaesthesia has been correlated with lack of therapeutic success in long and complex endoscopic procedures to manage difficult ductal calculi[12].
Complications occur in approximately 7% of patients treated with PTCSL, mainly biliary sepsis, haemobilia and bile duct injuries[13,14]. In order to reduce complication risk, PTC tracts must be allowed to mature and gradually dilated before use in PTCSL[3]. Tract maturation time vary depending on size of the final working sheath for PTCSL. Reported tract maturation times have been as short as ≤ 4 d for 8-10F access sheaths which are used for fluoroscopic mechanical lithotripsy without any video cholangioscopy[13,14]. Longer tract maturation times of up to 6 wk have been described for 16-18F working sheaths used for video cholangioscopy-guided interventions[15,16]. An average of 2 PTCSL treatments are required to clear CBD calculi[14,17] while 5 are needed for intrahepatic calculi[15,18]. Our case with mainly intrahepatic calculi required 2 treatment sessions with PTCSL while the case extrahepatic calculi required only 1 session. Comparative studies show clearance rates for calculi are better for extrahepatic than for intrahepatic stones[3,13].
Lithotripsy is a therapeutic adjunct that improves success rates in patients undergoing video cholangioscopy-guided clearance of biliary calculi[19]. The most commonly used modality is electrohydraulic lithotripsy (EHL) which fragments stones by generating shock waves from rapid expansion of surrounding fluid in response to short pulses of high-voltage electric sparks. Laser lithotripsy fragments stones by producing strong shockwaves using infrared light energy generated by the applied laser. Laser lithotripsy is more expensive and although randomized studies are lacking, systematic review shows it is more successful than EHL in treating impacted biliary tract calculi[20].
The recurrence rate for intrahepatic calculi post-PTCSL varies from 21%-40% after 10-years follow-up[15,18,21], bile duct strictures being the main risk factor[18]. The recurrence rate for CBD calculi post-PTCSL is 45% after 7-years follow-up[17]. The only 3 deaths reported due to PTCSL have been caused by biliary sepsis[14,15].
Our experience is that PTCSL is feasible and safe for biliary clearance when neither ERCP nor surgical exploration are suitable. PTCSL attractively combines PTC and video cholangioscopy which are techniques that are readily available in most HPB Centres, thus making it a pragmatic option for bile duct clearance when endoscopy is not feasible, and surgery is high-risk. Our report is limited by lack of long-term follow-up data.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Qayed E S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ
| 1. | Kim JH, Lee SK, Kim MH, Song MH, Park DH, Kim SY, Lee SS, Seo DW, Bae JS, Kim HJ, Han J, Sung KB, Min YI. Percutaneous transhepatic cholangioscopic treatment of patients with benign bilio-enteric anastomotic strictures. Gastrointest Endosc. 2003;58:733-738. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Hwang JH, Yoon YB, Kim YT, Cheon JH, Jeong JB. Risk factors for recurrent cholangitis after initial hepatolithiasis treatment. J Clin Gastroenterol. 2004;38:364-367. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Bonnel DH, Liguory CE, Cornud FE, Lefebvre JF. Common bile duct and intrahepatic stones: results of transhepatic electrohydraulic lithotripsy in 50 patients. Radiology. 1991;180:345-348. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Kondo S, Nimura Y, Hayakawa N, Kamiya J, Nagino M, Miyachi M, Kanai M. A clinicopathologic study of primary cholesterol hepatolithiasis. Hepatogastroenterology. 1995;42:478-486. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Inamdar S, Slattery E, Sejpal DV, Miller LS, Pleskow DK, Berzin TM, Trindade AJ. Systematic review and meta-analysis of single-balloon enteroscopy-assisted ERCP in patients with surgically altered GI anatomy. Gastrointest Endosc. 2015;82:9-19. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Jirapinyo P, Lee LS. Endoscopic Ultrasound-Guided Pancreatobiliary Endoscopy in Surgically Altered Anatomy. Clin Endosc. 2016;49:515-529. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Williams E, Beckingham I, El Sayed G, Gurusamy K, Sturgess R, Webster G, Young T. Updated guideline on the management of common bile duct stones (CBDS). Gut. 2017;66:765-782. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Trikudanathan G, Singh D, Shrestha P, Freeman ML. Percutaneous transhepatic cholangioscopy with intraductal electrohydraulic lithotripsy for management of choledocholithiasis in an inaccessible papilla. VideoGIE. 2017;2:152-154. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Franzini T, Cardarelli-Leite L, Figueira ERR, Morita F, Domingos FUG, Carnevale FC, de Moura EGH. SpyGlass percutaneous transhepatic cholangioscopy-guided lithotripsy of a large intrahepatic stone. Endoscopy. 2017;49:E292-E293. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Weigand K, Kandulski A, Zuber-Jerger I, Mueller M, Goessmann H. Cholangioscopy-guided electrohydraulic lithotripsy of large bile duct stones through a percutaneous access device. Endoscopy. 2018;50:E111-E112. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Cannavale A, Bezzi M, Cereatti F, Lucatelli P, Fanello G, Salvatori FM, Fanelli F, Fiocca F, Donatelli G. Combined radiological-endoscopic management of difficult bile duct stones: 18-year single center experience. Therap Adv Gastroenterol. 2015;8:340-351. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Kalaitzakis E, Webster GJ, Oppong KW, Kallis Y, Vlavianos P, Huggett M, Dawwas MF, Lekharaju V, Hatfield A, Westaby D, Sturgess R. Diagnostic and therapeutic utility of single-operator peroral cholangioscopy for indeterminate biliary lesions and bile duct stones. Eur J Gastroenterol Hepatol. 2012;24:656-664. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Ozcan N, Kahriman G, Mavili E. Percutaneous transhepatic removal of bile duct stones: results of 261 patients. Cardiovasc Intervent Radiol. 2012;35:621-627. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Kint JF, van den Bergh JE, van Gelder RE, Rauws EA, Gouma DJ, van Delden OM, Laméris JS. Percutaneous treatment of common bile duct stones: results and complications in 110 consecutive patients. Dig Surg. 2015;32:9-15. [PubMed] [DOI] [Cited in This Article: ] |
| 15. | Yeh YH, Huang MH, Yang JC, Mo LR, Lin J, Yueh SK. Percutaneous trans-hepatic cholangioscopy and lithotripsy in the treatment of intrahepatic stones: a study with 5 year follow-up. Gastrointest Endosc. 1995;42:13-18. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Ahmed S, Schlachter TR, Hong K. Percutaneous Transhepatic Cholangioscopy. Tech Vasc Interv Radiol. 2015;18:201-209. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Tsutsumi K, Kato H, Yabe S, Mizukawa S, Seki H, Akimoto Y, Uchida D, Matsumoto K, Tomoda T, Yamamoto N, Horiguchi S, Kawamoto H, Okada H. A comparative evaluation of treatment methods for bile duct stones after hepaticojejunostomy between percutaneous transhepatic cholangioscopy and peroral, short double-balloon enteroscopy. Therap Adv Gastroenterol. 2017;10:54-67. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Jan YY, Chen MF. Percutaneous trans-hepatic cholangioscopic lithotomy for hepatolithiasis: long-term results. Gastrointest Endosc. 1995;42:1-5. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Maydeo A, Kwek BE, Bhandari S, Bapat M, Dhir V. Single-operator cholangioscopy-guided laser lithotripsy in patients with difficult biliary and pancreatic ductal stones (with videos). Gastrointest Endosc. 2011;74:1308-1314. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Veld JV, van Huijgevoort NCM, Boermeester MA, Besselink MG, van Delden OM, Fockens P, van Hooft JE. A systematic review of advanced endoscopy-assisted lithotripsy for retained biliary tract stones: laser, electrohydraulic or extracorporeal shock wave. Endoscopy. 2018;50:896-909. [PubMed] [DOI] [Cited in This Article: ] |