Published online Feb 16, 2018. doi: 10.4253/wjge.v10.i2.56
Peer-review started: October 16, 2017
First decision: November 7, 2017
Revised: December 28, 2017
Accepted: January 15, 2018
Article in press: January 15, 2018
Published online: February 16, 2018
Processing time: 115 Days and 16.7 Hours
Hepatocellular carcinoma constitutes over 90% of the primary liver tumors, the rest being cholangiocarcinoma. It has an insidious presentation, which is responsible for the delayed presentation. Hence, the management strategy relies on screening to diagnose it an early stage for curative resection and/or treatment with local ablative techniques or chemotherapy. However, even with different screening programs, more than 60% of tumors are still detected at an advanced stage, leading to an unchanged mortality rate, thereby implying a room for improvement in the screening and diagnostic process. In the last few years, there has been evolution of utility of endoscopy, specifically endoscopic ultrasonography along with Fine needle aspiration, for this purpose, which we comprehensively review in this article.
Core tip: Hepatocellular carcinoma (HCC) constitutes the commonest primary liver cancer, and if diagnosed at an early stage, has better prognosis. Of late, there has been evolution of utility of endoscopic techniques, specifically endoscopic ultrasonography (EUS) with fine needle aspiration, for this purpose. EUS is superior over computed tomography in detecting hepatic lesions smaller than 1cm, and also allows FNA for accurate histopathological diagnosis. This strategy is particularly useful for indeterminate nodules, with non-specific imaging characteristics. Role of EUS in diagnosis and management of HCC are the focus of this article. In addition, other endoscopic techniques, including esophagogastroduodenoscopy and endoscopic retrograde cholangio-pancreatography, are of immense use in management of complications of HCC, which are also briefly discussed in this review.
- Citation: Girotra M, Soota K, Dhaliwal AS, Abraham RR, Garcia-Saenz-de-Sicilia M, Tharian B. Utility of endoscopic ultrasound and endoscopy in diagnosis and management of hepatocellular carcinoma and its complications: What does endoscopic ultrasonography offer above and beyond conventional cross-sectional imaging? World J Gastrointest Endosc 2018; 10(2): 56-68
- URL: https://www.wjgnet.com/1948-5190/full/v10/i2/56.htm
- DOI: https://dx.doi.org/10.4253/wjge.v10.i2.56
Hepatocellular carcinoma (HCC) constitutes the most common primary liver cancer. It is the fifth most frequent cancer and the second commonest cause of cancer related death worldwide[1], the occurrence of which is more in men and increases with age. Over 75% of HCC in the United States is due to hepatitis C and B viral infections. Successful management strategy relies on screening with the help of imaging techniques to diagnose it at an early stage for curative resection and/or treatment with local ablative techniques or chemotherapy. Tumors diagnosed at an early stage have a better prognosis with a five-year survival rate up to 80%[2]. However, more than 60% of tumors are still detected at an advanced stage, thereby implying that there is a room for improvement in the screening and diagnostic process[3]. In the last few years, there have been few studies evaluating the utility of endoscopy, and specifically endoscopic ultrasonography (EUS) along with fine needle aspiration (FNA), for this purpose, which we attempt to review in this article.
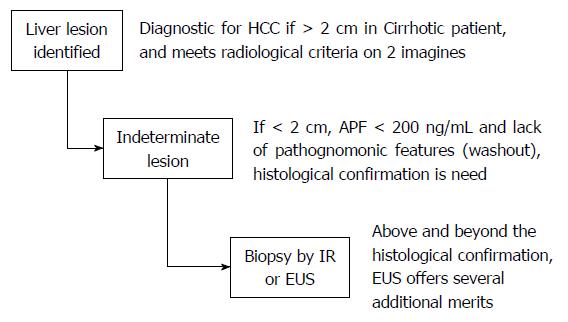
Diagnosis of HCC is often difficult and usually occurs late in the course of chronic liver disease because of absence of any pathognomonic signs or symptoms[4]. Even with the current screening strategies, vast majority of cases of HCC are diagnosed at an advanced stage and hence palliative treatments are the only available options[5,6]. Current guidelines for diagnosis of HCC involve frequent monitoring of liver nodules < 1 cm with abdominal ultrasound and advanced imaging for nodules > 1 cm. Imaging options include liver ultrasound (US), computed tomography (CT), or magnetic resonance imaging (MRI) scans[7,8]. Barcelona convention of HCC experts agreed on non-invasive establishment of a radiological diagnosis requiring a > 2 cm focal hepatic lesion in cirrhotic patient confirmed by 2 different imaging techniques. However, for lesions < 2 cm, histological confirmation was deemed necessary[9,10] (Figure 1). The American Association for Study of liver Disease guidelines recommend biopsy of lesions under 2 cm only if there is no pathognomonic imaging (wash out) and an alfa-feto protein (AFP) below 200 ng/mL. This is where EUS may be potentially used safely and effectively to obtain cytology or histology from the lesion, even in patients with cirrhosis.
Traditional esophagogastroduodenoscopy is useful prior to EUS in evaluating the grade of esophageal or gastric varices if any, as lot of these patients have HCC complicating cirrhosis. EUS combines two different investigations-endoscopy and ultrasound-into one, to acquire images from the digestive tract and surrounding organs. Considering its proximity to the surrounding organs, it is more accurate than traditional US. EUS further combined with fine needle aspiration (FNA) or fine needle biopsy (FNB) offers further evaluation of a suspected lesion often with rapid on-site evaluation (ROSE). Currently, it is mostly used for diagnosis and staging of pancreatic cancer and for staging of esophageal cancer[11,12]. However, over the last decade, there have been efforts to define role of EUS in evaluation of liver lesions-particularly metastatic lesions and HCC[13-14], which forms the focus of our discussion.
The superiority of EUS over CT in detecting hepatic lesions smaller than 1cm was demonstrated as early as 1999[14]. Imaging with CT scans and MRI may have a high miss rate in the diagnosis of HCC. Several studies have reported a sensitivity of 60%-68% with CT scans, based on the type of study and the technique of CT scan, for diagnosis of HCC[15,16]. Awad et al[17] studied the role of EUS in pre-operative evaluation of HCC, compared it with CT and found EUS to be a feasible investigation for the purpose examined. EUS diagnosed hepatic lesions between 0.3-14 cm, detected new/additional lesions in 28% of patients (all lesions < 0.5 cm), could more reliably detect smaller lesions and successfully differentiated hemangiomas among lesions which appeared suspicious for HCC on CT. In this study, EUS led to change of management in around 67% of patients. In the study by DeWitt et al[18], EUS diagnosed malignancy in over 40% with previously negative traditional imaging, having an impact on management in 84% either by making new diagnoses or by upstaging thus avoiding un-necessary surgery. Singh et al[19] conducted a prospective study to compare the accuracy of EUS and EUS-FNA with CT for detection of HCC. They found that for diagnosis of HCC, EUS had a sensitivity of 100% as compared to 71% for CT although the specificity and positive predictive values were much lower (25% vs 67% and 60% vs 71%, respectively)[19]. However, when combined with FNA, EUS had accuracy of 94% in comparison to 69% with CT scan[19]. EUS was able to detect significantly more lesions in the left lobe of the liver, sample hilar nodes (non-feasible by traditional imaging methods)[20] and characterize lesions that were too small and indeterminate for HCC on a CT scan. They proposed a diagnostic algorithm for evaluating high-risk patients with negative imaging. Choudhary et al[21] in their prospective study of over 50 patients showed that EUS-FNA of lymph nodes detected in patients with HCC confirmed metastasis and hence precluded transplantation in over a third of the patient cohort.
MRI with angiography has been shown to be better than CT for diagnosis of HCC, the benefit being mostly for detection of nodules between 10-20 mm[22,23]. At present, it is considered the gold standard for staging of HCC prior to surgery[22,24]. The accuracy of EUS alone for accurate diagnosis of liver lesions may only be 65%, but it increases to ~ 94% when combined with FNA which is similar to that of MRI[19]. However, in the same study EUS was found to detect a significantly higher number of nodular lesions than MRI (P = 0.04)[19]. In addition, few other reports have also supported the use of FNA to identify lesions missed by CT scan[25-27], which may be of clinical significance only if their size is over 1 cm, in which case, biopsy could be accomplished at the time of EUS.
Lai et al[25], Storch et al[26] and Michael et al[27], independently demonstrated the safety of EUS-FNA to diagnose HCC as the cause of portal vein thrombosis. In all three reports, CT scan showed portal vein thrombosis without any definite hepatic mass, but FNA of the thrombus was used to diagnose malignant HCC, thus proving it to be a tumor thrombus rather than a bland one, thereby changing the management. In addition to the role of EUS to provide tissue diagnosis, it also provides better visualization of the portal vein and the FNA needle also has to travel a short distance only[28,29]. Doppler ability of EUS helps choose an avascular trajectory for the needle.
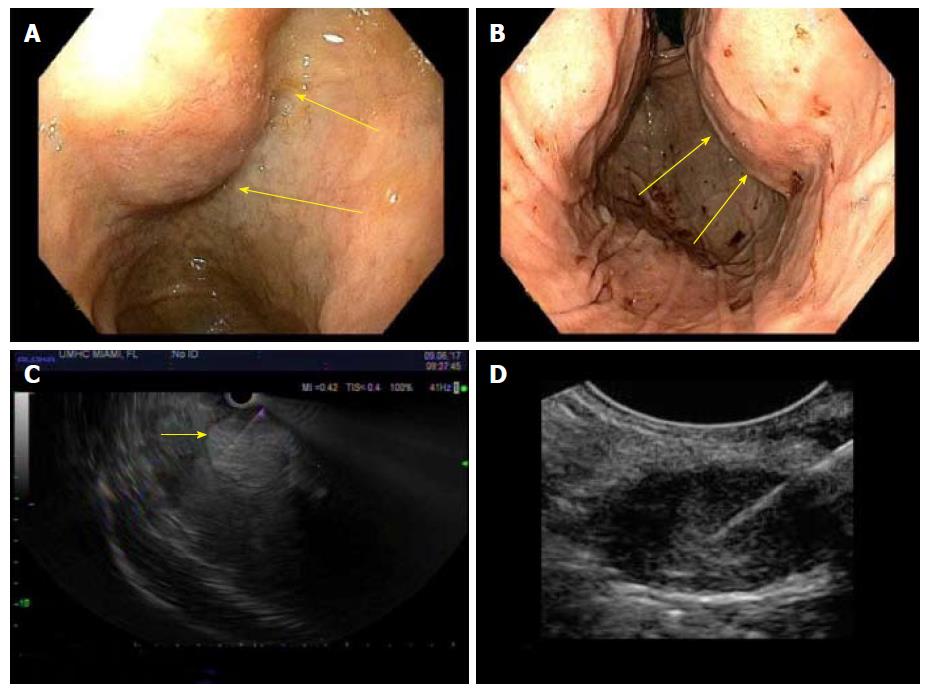
Data on utility of EUS in primary diagnosis of hepatic lesions and HCC is limited (Figure 2). One of the earliest studies looking at role of EUS in liver lesions was conducted by Nguyen et al[14] who prospectively evaluated the livers of 574 patients with history or suspicion of malignancy with EUS. Hepatic lesions were found in 14 patients, only 3 of who had a lesion previously detected by CT scan. Moreover, amongst these 14 patients, while 7 carried a diagnosis of malignancy, the other 50% received the initial diagnosis with help of EUS-FNA. Since this study did not include any patients with primary HCC, its results cannot be fully extrapolated to HCC patients. Similar studies were conducted by DeWitt et al[18] in 2003, Prasad et al[30] in 2004 and Crowe et al[31] in 2006, underscoring the benefits of EUS and EUS-FNA in diagnosis of liver metastasis (Figure 3). More recently, Fujii-Lau et al[32] proposed EUS-derived criteria for distinguishing benign from malignant metastatic solid hepatic masses, but are not specific for HCC. The authors suggested using 7 EUS features, which had fair-moderate inter-observer agreement among expert endosonographers, and yielded an area under the receiver-operating curve (AUC) of 0.92, and overall positive predictive value of 88%.
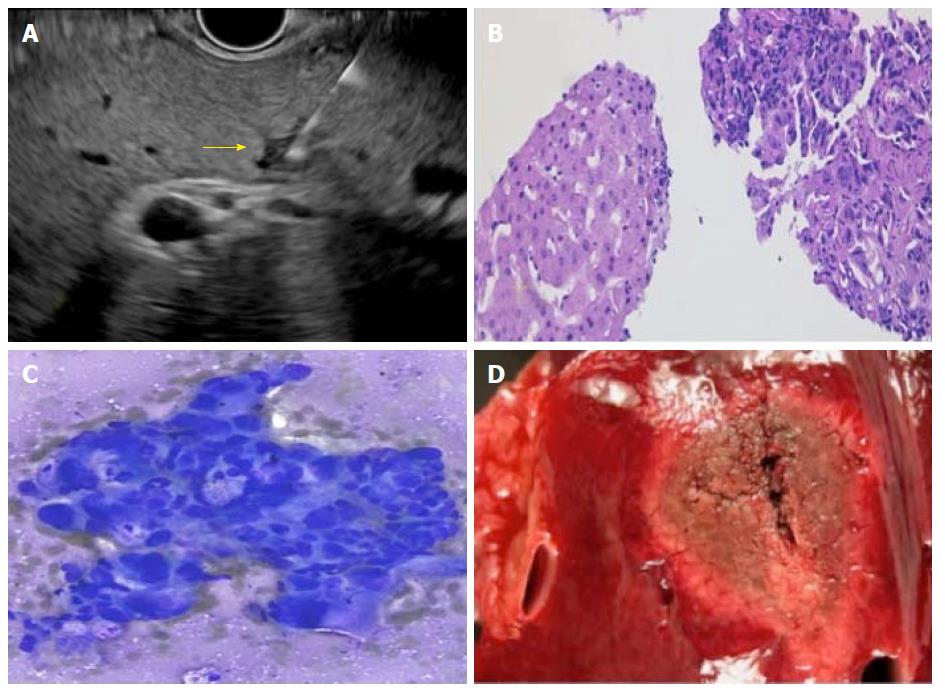
Previous studies have upheld that diagnosis of HCC is highly dependent on size of the lesion. For lesions < 2 cm, accurate diagnosis requires presence of histologic confirmation, which is currently achieved using CT-guided percutaneous route[33]. EUS with FNA/FNB is an alternate strategy. In their prospective study of 17 patients (10 with malignancy), Singh et al[19] found 5 lesions < 2 cm, of which three were diagnosed to be malignant using EUS-FNA and the rest two were benign with the smallest lesion to undergo FNA being 4 mm in size. In cirrhosis, hyperintense non-dysplastic nodules are commonly seen on MRI, which may be indistinguishable from dysplastic lesions[34]. This happens more frequently in cases of smaller lesions, which makes it even more difficult to evaluate[35]. Smaller lesions are difficult to characterize by either CT or traditional US as HCC can present with hypoechoic, hyperechoic or isoechoic lesions. Targeted EUS-FNA can be performed on these lesions to obtain histologic confirmation. A multi-centric study reported EUS-FNA to diagnose malignancy in 89% patients, after traditional US guided FNA was non-diagnostic[36]. It is also well known that presence of dysplastic nodules in liver is a major risk factor for development of HCC, especially in presence of HBsAg and anti-hepatitis C virus antibodies[37-39].
There exist various staging systems for HCC, including American joint committee on Cancer (AJCC) TNM (which does not incorporate hepatic function and reserve, which are major determinants of outcome), Cancer of the Liver Italian Program (CLIP) score, Japanese Staging System and Japan Integrated Staging (JIS) score (which includes TNM + Child-Pugh score), GRoupe d’Etude et de Traitement du Carcinoma Hépatocellulaire (GRETCH) system (which includes serum AFP + liver function parameters and portal vein thrombosis) and Chinese University Prognostic Index (CUPI) (combines conventional TNM + serum AFP + liver function parameters). The Barcelona Clinic Liver Cancer (BCLC) staging system is a comprehensive scheme using variables related to tumor size, number of nodules, liver functional status, physical status, and cancer-related symptoms, and links the five stages described with a treatment algorithm. The purpose of BCLC was to stratify patients into treatment groups according to the extent of the disease and the predicted prognosis[40]. The consensus statement of the American Hepato-Pancreato-Biliary Association, updated in 2010, recommends the use of the TNM system to predict outcomes following resection or liver transplantation and the BCLC scheme for patients with advanced HCC who are not candidates for surgery[41]. Since we know that EUS/EUS-FNA may detect smaller liver tumors more effectively than available imaging, sample the lymph nodes and metastatic lesions, we hypothesize that EUS/EUS-FNA may play a strong role in the diagnosis of indeterminate lesions.
In addition to EUS improving staging and diagnostic yield of HCC, other endoscopic modalities might play a role in comprehensive management of these patients. HCC develops on a background of cirrhotic liver in most circumstances; hence all the clinical manifestations of a cirrhotic liver may be seen, ranging from splenomegaly, ascites, and jaundice to formation of varices and variceal bleeding due to portal hypertension (PHT). Esophageal varices are treated with band ligation and rarely with sclerotherapy. However gastric varices may be managed either with endoscopic injection of cyanoacrylate glue or EUS guided coil embolization and glue injection[42]. EUS could also be used to confirm obliteration of the varices, perforators and peri/paraesophageal varices presence of which might predict recurrence[43]. Along with this, occult gastrointestinal bleeding (OGIB) can also be observed in patients with HCC either due to small bowel varices, portal hypertensive enteropathy, metastasis or mucosal erosions which are observed with a higher frequency in patients with HCC[44]. These can be diagnosed with capsule endoscopy (CE) and managed with deep enteroscopy (spiral, single or double balloon). Kunizaki et al[45] described a case where a patient with metastatic HCC developed OGIB secondary to a small bowel metastatic lesion diagnosed with double balloon enteroscopy (DBE).
Jaundice occurs infrequently in HCC, and only 1%-12% of patients manifest with obstructive jaundice as the initial complaint[46]. Cholestasis occurs due to bile duct occlusion, which may be from benign causes (blood clots, pus, or sludge), malignant causes (primary intra-biliary malignant tumors, HCC with invasion to bile ducts, or metastatic cancer with bile duct invasion) or combination or progressive terminal liver failure (advanced underlying cirrhosis). Furthermore, trans-catheter arterial chemoembolization (TACE) can also increase probability of tumor thrombi obstructing the biliary tree[47]. The ominous features indicative of malignant obstruction are high level of serum AFP, history of cholangitis with dilation of intrahepatic bile duct, aggravating jaundice and rapidly deteriorating liver function[46].
Various endoscopic techniques find utility in such scenarios. Choledochoscopy and bile duct brush cytology are useful endoscopic techniques in differentiating obstruction due to intraluminal mass, infiltrating ductal lesions or extrinsic compression[48-49]. Besides technical difficulties in accessing the bile duct with tumor fragments or protrusion and possible strictures, endoscopic biliary drainage (EBD) is frequently debated because of the short survival of these patients. EBD, achieved via bilio-nasal and bilio-duodenal drainage, could be considered for palliation in these patients with obstructive jaundice caused by tumor fragments and/or protruding into the CBD lumen. Endoscopic retrograde cholangiography (ERC) may be diagnostic as well as therapeutic in such cases as it can relieve jaundice via biliary stenting[50]. In terms of choice of stent, metal stent is preferred for palliation of malignant biliary obstruction due to larger lumen ensuring patency over longer period of time[51-54]. Plastic stents would be more cost effective if the life expectancy is under 3 mo. Cho et al[55] evaluated the effects of biliary drainage on clinical outcomes in patients with obstructive jaundice secondary to HCC. They observed that patients with bilirubin >13 mg/dL and Child-Turcotte-Pugh class C did not have effective biliary drainage, which correlated with decreased mean survival time in such patients[55]. Choi et al[56] also showed that presence of portal vein thrombosis is also correlated with ineffective drainage of the biliary tree along with the above-mentioned factors. A recent study also advocated that while there was no statistical difference in rate of successful drainage with ERC compared to percutaneous cholangiography (PTC), ERC had longer duration of drainage patency, thus the first choice for palliative biliary drainage[57]. More recently, EUS guided biliary drainage is increasingly being used when endoscopic retrograde cholangio-pancreatography fails[58]. Thus, the combination of palliative endoscopic methods may relieve jaundice, ensure a good quality of life and possibly prolong survival of this type of HCC patients.
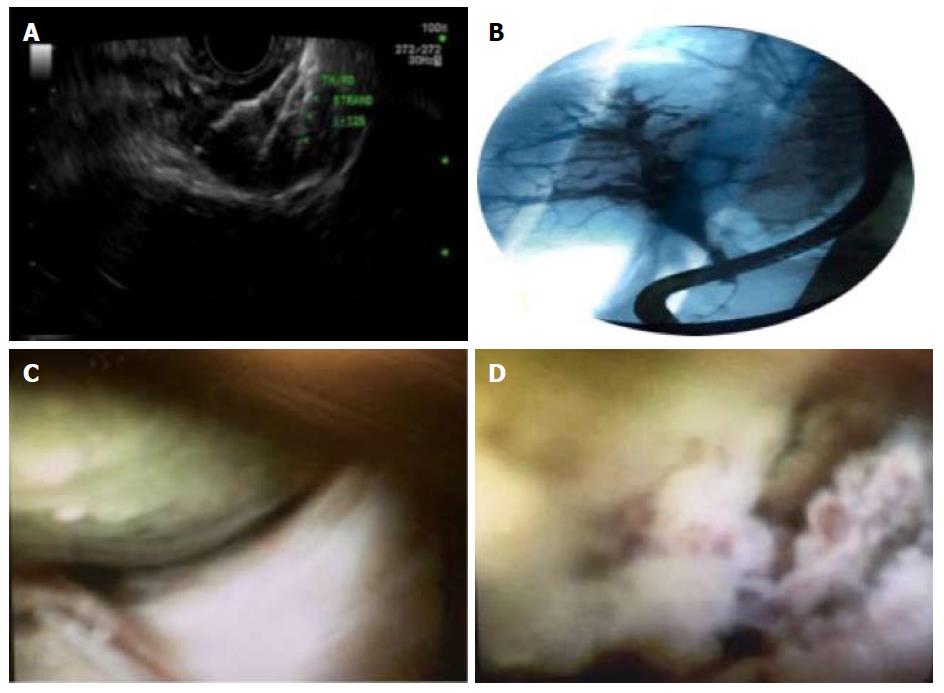
HCC tends to stay within the liver, with late occurrence of distant metastases. Hence, early diagnosis and an effective local therapy can have a great impact on the course of the disease and outcome. Curative liver resection and orthotopic liver transplant (OLT) are the best modalities of treatment, however, only 10%-50% of patients get to them due to impaired liver function and the delay in diagnosis[59]. For these reasons, local therapy to treat such subset of patients has garnered a lot of interest, with the modalities including radiofrequency ablation (RFA), microwave ablation (MWA), percutaneous ethanol injection (PEI), transarterial chemoembolization (TACE), radioembolization (TARE), cryoablation, brachytherapy, stereotactic radiotherapy (Figure 4), systemic and molecularly targeted therapies (Sorafenib).
Local ablation (using RFA or PEI) is the standard of care for BCLC stage 0-A not suitable for surgery. This can be administered by various routes including laparoscopy, percutaneous route or endoscopy. The latter is still mostly at experimental stage, though there has been progress made over the years. Before the advent of RFA, PEI was the most widely accepted, minimally invasive method for treating such patients. However, RFA has been proven to be a very safe procedure with a reported mortality rate of less than 1% and a complication rate of 3%-7%[60-61]. It is utilized in patients who do not meet the criteria for surgical resection of the tumor or OLT, and has been studied in patients with HCC ≤ 4 cm[62]. Recent meta-analysis suggests that overall and disease-free survival rates continue to improve with RFA, despite an increase in the size and numbers of tumors treated[63]. RFA has been shown to be more efficacious with higher tissue necrosis rate, decreased local recurrence and higher cancer free survival rates, than other local ablative techniques like PEI[61,64-66]. These facts have been demonstrated in studies evaluating RFA over a long period of time, which make RFA one of the established treatments for local and small HCC at present[67-71]. However, it is associated with more complications than PEI, especially when RFA is performed on a lesion in proximity to a large vessel or another visceral organ, and may lead to bleeding, hemothorax or gastric perforation[54,72]. RFA can be delivered by either a percutaneous, endoscopic or surgical approach, latter being most invasive[73]. Percutaneous approach is less invasive but is more often associated with tumor seeding[74-75]. On the other hand, open surgical approach is highly invasive with an increased rate of complications. RFA using thoracoscopy or laparoscopy is associated with less blood loss, post-operative complications and duration of surgery while delivering similar success rates for treatment of HCC as open RFA[76-78]. It can also be combined with other local therapies for HCC like TACE which is better than RFA alone[79,80]. With these merits, it’s the first line treatment for small sized HCCs that are not suitable for surgical resection and can act as a bridging therapy before liver transplantation. EUS-guided Nd: YAG laser ablation of caudate lobe HCC and EUS-guided ethanol ablation of deep HCC closer to IVC have also been reported[81-82]. Nakaji et al[83] showed that twelve patients with early stage HCC of the caudate lobe, who underwent EUS guided ethanol injection, had overall survival rates of 91.7%, 75% and 53% at 1, 2 and 3 years respectively. Recurrence was seen in 2 cases after 3 and 9 mo respectively[83]. Laser ablation via percutaneous route has been shown to be useful[84,85]. Di Matteo et al[81] successfully delivered Nd:YAG laser ablation via EUS in a patient with HCC to obtain encouraging results. Hybrid approach may be an alternative to consider, using percutaneous ablation for deep seated, while endoscopic ablation for superficial HCC[86]. Another example of successful hybrid technique was laparoscopic RFA while cooling bile ducts via endoscopic nasobiliary drainage tube, to prevent bile duct injury, to manage HCC located adjacent to the Glisson’s capsule in the hilar region in two patients[87]. Conceptually, EUS may also have similar use, especially for easily accessible lesions.
Among patients with large multifocal HCC or noncurative (inoperable/non-ablatable) tumor characteristics, TACE is used as first-line, especially for BCLC stage B multinodular asymptomatic tumors without vascular invasion or extra-hepatic spread. This technique involves the injection into the arteries feeding the tumor, a mixture of a chemotherapeutic agent (doxorubicin, cisplatin, mitomycin, and epirubicin) and embolic material, to potentially obtain higher intra-tumoral drug concentrations compared with intravenous therapy, with occlusion of the blood vessel causing infarction and necrosis, thus causing shrinkage. In future, EUS guided intra-tumoral administration of chemotherapy may be considered. Artifon et al[88] utilized EUS to deliver intra-arterial chemotherapy for liver metastases in colon cancer patients. The authors reported a statistically significant decrease in the median hospital stay after such a procedure, while maintaining the safety profile and response rates. This technology is still in a nascent phase.
Microwave ablation (MWA) is a newer loco-regional therapy for HCC, especially in patients who are not candidates for surgical resection. Currently, RFA is the most popular loco-regional therapeutic modality throughout the world, but has significant limitations including higher complication rates, especially in HCC lesions located close to the gallbladder, liver capsule, and diaphragm, or near large vessels, which may be associated with incomplete ablation due to the “heat-sink” effect[89-91]. These situations may render at least 10%-25% of patients with HCC ineligible for RFA[89]. In such difficult to treat tumors, MWA can be offered as an alternative ablation strategy, since it provides a homogeneous and more predictable ablation zone[92-94]. MWA also offers improved efficacy for perivascular tumors, since the faster heating and higher temperatures provided by microwave energy allow heat-sink effect reduction[93,95]. Shibata et al[96] demonstrated statistically comparable local control rates of 89% for MWA as compared to 96% for RFA in a randomized study. The survival benefit remains similar in both the techniques with fewer complications associated with MWA as compared to RFA. Shi et al[97] reported MWA to be as effective as surgical resection for solitary HCC ≤ 3 cm. The overall 1-, 3-, and 5-year survival rates were 94%, 70%, 52% for the MWA group and 94%, 72%, 60% for the resection group[97].
Transarterial radioembolization (TARE) delivers microspheres impregnated with the radioisotope yttrium-90 (Y90, 90Y) through the hepatic vasculature directly to the target tumor, thus allowing for safe administration of high radiation doses to the tumor burden. This strategy is usually utilized in patients with unresectable HCC, deemed not to be good candidates for TACE, or those with failed prior TACE procedures[98]. TARE is delivered in a lobar fashion, rather than segmental fashion as is TACE, and can target more lesions at the same time. Thus, most of the patients undergoing TARE have much more advanced disease, as compared to those undergoing TACE. Currently, two Y90 products are commercially available: TheraSphere_ glass microspheres (BTG, Canada) and SIR-Spheres_resin microspheres (Sirtex Medical, Woburn, MA, United States). Salem et al[99] showed a trend towards a higher response rate for patients who underwent TARE as compared to TACE (49% vs 36%, respectively, P = 0.104). Also, time-to-progression was longer following TARE than TACE (13.3 mo vs 8.4 mo, respectively, P = 0.046), although median survival times were not statistically different[99]. TARE is associated with fewer side effects, and is considered as an outpatient procedure, as opposed to TACE, which typically requires post-procedure hospitalization. TARE may also be used in patients with portal vein thrombosis and has been shown to downstage patients outside of transplant criteria from UNOS stage T3 to T2, helping them undergo transplant[100,101].
Contrast-enhanced ultrasound (CE-US) has been demonstrated to have superiority over CE-CT for detection of residual tumors after TACE[102]. With improved imaging technique with EUS, availability of contrast-enhanced option in EUS and its inherent ability to detect smaller lesions, contrast-enhanced EUS (CE-EUS) is emerging as a newer technique to assess the treatment effects of TACE on HCC in the caudate lobe of the liver[103], previously difficult to assess with CE-US.
Treatment of HCC by local therapies can result in several serious complications, which may be managed endoscopically. TACE can lead to formation of biliary stricture, variceal bleeding, bile leak and hepatoduodenal fistulae; all of which have been reported to be managed endoscopically[104-107]. A hepatoduodenal fistula is a rare complication, which ideally should be resected surgically. Recently, endoscopic closure of the fistula using histoacryl injection has been described in a case report[104]. Similarly, use of RFA has been associated with the formation of biliocutaneous fistula that can be managed endoscopically[105] and infected biloma drained via trans-gastric route[108]. RFA can also lead biliary stricture, which can further lead to sepsis and liver failure. This can be prevented by “cooling” the bile ducts using endoscopic nasobiliary drainage (ENBD) tube, during RFA[106]. Bile leak following TACE for HCC has been successfully managed with choledochoscope-assisted fibrin glue[107].
EUS guided portal vein interventions in HCC: EUS based portal vein interventions are emerging as newer diagnostic and therapeutic techniques in HCC. EUS-FNA can be used to differentiate between a bland vs tumor thrombus in the portal vein, which can help us in the correct staging of HCC. The approach for this technique is trans-duodenal (25 gauge needle) which has been shown to cause less sampling errors, thus leading to fewer false positive or negative results, as compared to trans-hepatic approach with US/CT. This technique has also shown to cause less biliary and vascular injury as well. EUS-FNA has also been shown in several case reports in diagnosing tumor thrombus in the portal vein from HCC without visualization of any hepatic mass on the imaging[25-27].
There are few experimental animal studies being performed to assess the efficacy of EUS-guided portal vein chemotherapy injections in anaesthetized pig models. The advantage of this technique will be a higher hepatic and lower systemic chemotherapy drug levels. Thus, it is hypothesized that it may lead to lower systemic toxicities in patients with diffuse liver metastasis[109]. In another animal model, selective PV embolization has been demonstrated for causing the contralateral hypertrophy of the liver lobe. This helps in resection in hepatic malignancies without compromising the liver function[110]. Further human studies are needed to validate the therapeutic benefits of EUS guided portal vein interventions suggested by these animal studies.
In spite of the zeal generated by these studies, like every technique, EUS also comes with its share of controversies and limitations[111,112]. The major criticism is that large-scale studies and randomized controlled trials evaluating the role of EUS in the management of HCC are still lacking. In addition, it may also be associated with multiple technical problems like difficulty to visualize and sample right lobe lesions that need a transduodenal approach[14]. Although sensitivity is high in reported cases, EUS may also miss smaller lesions, especially if farther from the probe, which may also pose challenges when attempting to perform FNA. While lesions in peri-hepatic region, hilum, caudate lobe, left lobe and part of right lobe in proximity to the falciform ligament (Liver segments 1, 2, 3 and 4) may be easy to evaluate with EUS, the remainder of the right lobe (segments 5-8) may pose a technical challenge. There are no studies yet to evaluate which hepatic segments are consistently seen by EUS. Furthermore, not only does EUS add to the overall cost of the work-up, it also involves a long and complex learning curve for the operator, which is yet another Understand factor in this conundrum. This limits its availability and accessibility.
An additional debate in the utility of EUS-FNA for evaluation of liver cancers is the potential for tumor seeding along the needle track and peritoneal spillage, which are known to occur with the more traditional radiologic approach. This has already been reported in pancreatic cancer; but in liver biopsy the increased vascularity of the tumor and the distance between the gut wall and the liver capsule theoretically increases the risk[113]. EUS-guided tissue acquisition is not feasible if an avascular trajectory cannot be obtained when viewed under Doppler. Moreover, pneumobilia, calcification, metal stents, fatty infiltration and fibrosis could interfere with the image quality. Furthermore, HCC being a much more vascular tumor than pancreatic cancer, further augments the potential risk of tumor spillage. However, needle tracking has been observed in less than 2% of cases of percutaneous biopsy and is more common in lesions > 2 cm[114,115], although, analogous data for EUS-FNA is lacking. Complications are more in those individuals with moderate ascites and decompensated liver disease. Though intravenous contrast is not used unlike traditional cross sectional imaging, newer technology like power doppler, tissue harmonic imaging, real time elastography and contrast enhanced imaging offer promise in differentiating various lesions.
Due to the mechanical properties of large echoendoscope, with longer fixed segment at the tip, coupled with learning curve of therapeutic endoscopists, the adverse events with EUS are greater than standard upper or lower endoscopic procedures, perforation being the most feared. Esophageal perforation was noted in 8 of almost 85000 diagnostic EUS in Germany[116], and 16 of almost 44000 EUS in United States[117], half of which were by endosonographers during early learning phase. Duodenal perforation is more common, accounting for 6 of 10 GI perforations reported in a prospective United States registry of almost 14000 EUS[118]. Overall mortality with EUS is reported around 0.02%[118], and another study attributed 73% of all EUS-related mortalities (13/18) to duodenal tears with retroperitoneal perforations[119]. Cognizance of these occurrences is essential for therapeutic endoscopist attempting any of the above-discussed EUS maneuvers. For liver lesions in particular, the overall rate of complications was noted to be approximately 1% in a multi-centric international survey, including biliary sepsis (0.6%), local bleeding (0.6%), fever (1.2%) and pain (1.2%)[36].
In summary, we have reviewed the current literature on the utility of endoscopic techniques, with a special focus on EUS, in management of HCC, especially as an adjunct to traditional imaging. The current HCC staging systems and diagnostic guidelines do not yet utilize EUS, as most of the literature on its use is either from retrospective studies or small prospective analysis, without any dedicated randomized controlled trials. However, we have presented the data on the increasing role of EUS in the diagnosis of indeterminate and small lesions, and highlighted the settings where lesions can be better visualized with EUS for diagnosis and treatment. If incorporated on a more regular basis, EUS/EUS-FNA can potentially further help in the accurate staging of HCC, thereby impacting management strategies in selected patients, especially with indeterminate nodules. Other endoscopic modalities find their potential role in the treatment of HCC itself and management of complications as result of current approved treatments. This is an evolving field, and we anticipate greater use of endoscopy in these scenarios with further progression of research in this field, leading to improved clinical care.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: El Eneen Khattab MA, Lopez V S- Editor: Cui LJ L- Editor: A E- Editor: Li RF
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25533] [Article Influence: 1823.8] [Reference Citation Analysis (7)] |
| 2. | Yoo HY, Patt CH, Geschwind JF, Thuluvath PJ. The outcome of liver transplantation in patients with hepatocellular carcinoma in the United States between 1988 and 2001: 5-year survival has improved significantly with time. J Clin Oncol. 2003;21:4329–4335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 3. | Singal AG, Nehra M, Adams-Huet B, Yopp AC, Tiro JA, Marrero JA, Lok AS, Lee WM. Detection of hepatocellular carcinoma at advanced stages among patients in the HALT-C trial: where did surveillance fail? Am J Gastroenterol. 2013;108:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Kew MC, Dos Santos HA, Sherlock S. Diagnosis of primary cancer of the liver. Br Med J. 1971;4:408-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 100] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 5. | de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56 Suppl 1:S75-S87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 483] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 6. | Burak KW, Kneteman NM. An evidence-based multidisciplinary approach to the management of hepatocellular carcinoma (HCC): the Alberta HCC algorithm. Can J Gastroenterol. 2010;24:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Yu NC, Chaudhari V, Raman SS, Lassman C, Tong MJ, Busuttil RW, Lu DS. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Szklaruk J, Silverman PM, Charnsangavej C. Imaging in the diagnosis, staging, treatment, and surveillance of hepatocellular carcinoma. AJR Am J Roentgenol. 2003;180:441-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6562] [Article Influence: 468.7] [Reference Citation Analysis (1)] |
| 10. | European Association for the Study of the Liver. European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4515] [Article Influence: 347.3] [Reference Citation Analysis (2)] |
| 11. | Yasuda I, Iwashita T, Doi S, Nakashima M, Moriwaki H. Role of EUS in the early detection of small pancreatic cancer. Dig Endosc. 2011;23 Suppl 1:22-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Shimpi RA, George J, Jowell P, Gress FG. Staging of esophageal cancer by EUS: staging accuracy revisited. Gastrointest Endosc. 2007;66:475-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Bissonnette J, Paquin S, Sahai A, Pomier-Layrargues G. Usefulness of endoscopic ultrasonography in hepatology. Can J Gastroenterol. 2011;25:621-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Nguyen P, Feng JC, Chang KJ. Endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration (FNA) of liver lesions. Gastrointest Endosc. 1999;50:357-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 123] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Miller WJ, Baron RL, Dodd GD 3rd, Federle MP. Malignancies in patients with cirrhosis: CT sensitivity and specificity in 200 consecutive transplant patients. Radiology. 1994;193:645-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 106] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 16. | Peterson MS, Baron RL, Marsh JW Jr, Oliver JH 3rd, Confer SR, Hunt LE. Pretransplantation surveillance for possible hepatocellular carcinoma in patients with cirrhosis: epidemiology and CT-based tumor detection rate in 430 cases with surgical pathologic correlation. Radiology. 2000;217:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 148] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Awad SS, Fagan S, Abudayyeh S, Karim N, Berger DH, Ayub K. Preoperative evaluation of hepatic lesions for the staging of hepatocellular and metastatic liver carcinoma using endoscopic ultrasonography. Am J Surg. 2002;184:601-604; discussion 604-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | DeWitt J, LeBlanc J, McHenry L, Ciaccia D, Imperiale T, Chappo J, Cramer H, McGreevy K, Chriswell M, Sherman S. Endoscopic ultrasound-guided fine needle aspiration cytology of solid liver lesions: a large single-center experience. Am J Gastroenterol. 2003;98:1976-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Singh P, Erickson RA, Mukhopadhyay P, Gopal S, Kiss A, Khan A, Ulf Westblom T. EUS for detection of the hepatocellular carcinoma: results of a prospective study. Gastrointest Endosc. 2007;66:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Jones K, Biederman L, Draganova-Tacheva R, Solomides C, Bibbo M. Diagnostic Yield of Endoscopic Ultrasound-Guided Fine-Needle Aspiration Cytology of Porta Hepatis Lesions: A Retrospective Study. Acta Cytol. 2016;60:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Choudhary NS, Puri R, Saigal S, Bhangui P, Saraf N, Shah V, Nasa M, Sarin H, Guleria M, Sud R. Impact of endoscopic ultrasound-guided fine-needle aspiration in prospective liver transplant recipients with hepatocellular carcinoma and lymphadenopathy. Indian J Gastroenterol. 2016;35:465-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, Caralt T, Ayuso JR, Solé M, Sanchez M. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Rode A, Bancel B, Douek P, Chevallier M, Vilgrain V, Picaud G, Henry L, Berger F, Bizollon T, Gaudin JL. Small nodule detection in cirrhotic livers: evaluation with US, spiral CT, and MRI and correlation with pathologic examination of explanted liver. J Comput Assist Tomogr. 2001;25:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 24. | Hanna RF, Kased N, Kwan SW, Gamst AC, Santosa AC, Hassanein T, Sirlin CB. Double-contrast MRI for accurate staging of hepatocellular carcinoma in patients with cirrhosis. AJR Am J Roentgenol. 2008;190:47-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Lai R, Stephens V, Bardales R. Diagnosis and staging of hepatocellular carcinoma by EUS-FNA of a portal vein thrombus. Gastrointest Endosc. 2004;59:574-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Storch I, Gomez C, Contreras F, Schiff E, Ribeiro A. Hepatocellular carcinoma (HCC) with portal vein invasion, masquerading as pancreatic mass, diagnosed by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). Dig Dis Sci. 2007;52:789-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Michael H, Lenza C, Gupta M, Katz DS. Endoscopic Ultrasound -guided Fine-Needle Aspiration of a Portal Vein Thrombus to Aid in the Diagnosis and Staging of Hepatocellular Carcinoma. Gastroenterol Hepatol (NY). 2011;7:124-129. [PubMed] |
| 28. | Cedrone A, Rapaccini GL, Pompili M, Aliotta A, Trombino C, De Luca F, Caturelli E, Caputo S, Gasbarrini G. Portal vein thrombosis complicating hepatocellular carcinoma. Value of ultrasound-guided fine-needle biopsy of the thrombus in the therapeutic management. Liver. 1996;16:94-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Kantsevoy S, Thuluvath PJ. Utility and Safety of EUS-guided Portal Vein FNA. Gastroenterol Hepatol (NY). 2011;7:129-131. [PubMed] |
| 30. | Prasad P, Schmulewitz N, Patel A, Varadarajulu S, Wildi SM, Roberts S, Tutuian R, King P, Hawes RH, Hoffman BJ. Detection of occult liver metastases during EUS for staging of malignancies. Gastrointest Endosc. 2004;59:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Crowe A, Knight CS, Jhala D, Bynon SJ, Jhala NC. Diagnosis of metastatic fibrolamellar hepatocellular carcinoma by endoscopic ultrasound-guided fine needle aspiration. Cytojournal. 2011;8:2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Fujii-Lau LL, Abu Dayyeh BK, Bruno MJ, Chang KJ, DeWitt JM, Fockens P, Forcione D, Napoleon B, Palazzo L, Topazian MD. EUS-derived criteria for distinguishing benign from malignant metastatic solid hepatic masses. Gastrointest Endosc. 2015;81:1188-1196.e1-e7. [PubMed] |
| 33. | Talwalkar JA, Gores GJ. Diagnosis and staging of hepatocellular carcinoma. Gastroenterology. 2004;127:S126-S132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Krinsky GA, Israel G. Nondysplastic nodules that are hyperintense on T1-weighted gradient-echo MR imaging: frequency in cirrhotic patients undergoing transplantation. AJR Am J Roentgenol. 2003;180:1023-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Krinsky GA, Lee VS, Theise ND, Weinreb JC, Rofsky NM, Diflo T, Teperman LW. Hepatocellular carcinoma and dysplastic nodules in patients with cirrhosis: prospective diagnosis with MR imaging and explantation correlation. Radiology. 2001;219:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 230] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 36. | tenBerge J, Hoffman BJ, Hawes RH, Van Enckevort C, Giovannini M, Erickson RA, Catalano MF, Fogel R, Mallery S, Faigel DO. EUS-guided fine needle aspiration of the liver: indications, yield, and safety based on an international survey of 167 cases. Gastrointest Endosc. 2002;55:859-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 113] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 37. | Anthony PP, Vogel CL, Barker LF. Liver cell dysplasia: a premalignant condition. J Clin Pathol. 1973;26:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 217] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Kobayashi M, Ikeda K, Hosaka T, Sezaki H, Someya T, Akuta N, Suzuki F, Suzuki Y, Saitoh S, Arase Y. Dysplastic nodules frequently develop into hepatocellular carcinoma in patients with chronic viral hepatitis and cirrhosis. Cancer. 2006;106:636-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Borzio M, Bruno S, Roncalli M, Mels GC, Ramella G, Borzio F, Leandro G, Servida E, Podda M. Liver cell dysplasia is a major risk factor for hepatocellular carcinoma in cirrhosis: a prospective study. Gastroenterology. 1995;108:812-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 140] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 40. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2866] [Article Influence: 110.2] [Reference Citation Analysis (1)] |
| 41. | Vauthey JN, Dixon E, Abdalla EK, Helton WS, Pawlik TM, Taouli B, Brouquet A, Adams RB; American Hepato-Pancreato-Biliary Association; Society of Surgical Oncology; Society for Surgery of the Alimentary Tract. Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford). 2010;12:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 42. | Binmoeller KF, Weilert F, Shah JN, Kim J. EUS-guided transesophageal treatment of gastric fundal varices with combined coiling and cyanoacrylate glue injection (with videos). Gastrointest Endosc. 2011;74:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 169] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 43. | Hammoud GM, Ibdah JA. Utility of endoscopic ultrasound in patients with portal hypertension. World J Gastroenterol. 2014;20:14230-14236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Yamada A, Watabe H, Obi S, Sugimoto T, Kondo S, Ohta M, Togo G, Ogura K, Yamaji Y, Okamoto M. Surveillance of small intestinal abnormalities in patients with hepatocellular carcinoma: a prospective capsule endoscopy study. Dig Endosc. 2011;23:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Kunizaki M, Hidaka S, Isomoto H, Takeshita H, Nanashima A, Sawai T, Yasutake T, Nagayasu T. Diagnosis of small-bowel metastasis of hepatocellular carcinoma by double-balloon enteroscopy. Int J Surg Case Rep. 2012;3:263-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Qin LX, Tang ZY. Hepatocellular carcinoma with obstructive jaundice: diagnosis, treatment and prognosis. World J Gastroenterol. 2003;9:385-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 109] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 47. | Spahr L, Frossard JL, Felley C, Brundler MA, Majno PE, Hadengue A. Biliary migration of hepatocellular carcinoma fragment after transcatheter arterial chemoembolization therapy. Eur J Gastroenterol Hepatol. 2000;12:243-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Dusenbery D. Biliary stricture due to hepatocellular carcinoma: diagnosis by bile duct brushing cytology. Diagn Cytopathol. 1997;16:55-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 49. | Jan YY, Chen MF. Obstructive jaundice secondary to hepatocellular carcinoma rupture into the common bile duct: choledochoscopic findings. Hepatogastroenterology. 1999;46:157-161. [PubMed] |
| 50. | Martin JA, Slivka A, Rabinovitz M, Carr BI, Wilson J, Silverman WB. ERCP and stent therapy for progressive jaundice in hepatocellular carcinoma: which patients benefit, which patients don’t? Dig Dis Sci. 1999;44:1298-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Yoshioka T, Uchida H, Kitano S, Makutani S, Maeda M, Taoka T, Ohishi H. Long-term palliative treatment of hepatocellular carcinoma extending into the portal vein and bile duct by chemoembolization and metallic stenting. Cardiovasc Intervent Radiol. 1997;20:390-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Okazaki M, Mizuta A, Hamada N, Kawamura N, Nakao K, Kikuchi T, Osada T. Hepatocellular carcinoma with obstructive jaundice successfully treated with a self-expandable metallic stent. J Gastroenterol. 1998;33:886-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Lee BH, Choe DH, Lee JH, Kim KH, Chin SY. Metallic stents in malignant biliary obstruction: prospective long-term clinical results. AJR Am J Roentgenol. 1997;168:741-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 103] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 54. | Huang JW, Hernandez-Alejandro R, Croome KP, Yan LN, Wu H, Chen ZY, Prasoon P, Zeng Y. Surgical vs percutaneous radiofrequency ablation for hepatocellular carcinoma in dangerous locations. World J Gastroenterol. 2011;17:123-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Cho HC, Lee JK, Lee KH, Lee KT, Paik S, Choo SW, Do YS, Choo IW. Are endoscopic or percutaneous biliary drainage effective for obstructive jaundice caused by hepatocellular carcinoma? Eur J Gastroenterol Hepatol. 2011;23:224-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 56. | Choi J, Shim JH, Park DH, Lee SS, Seo DW, Lee SK, Kim MH, Kim KM, Lim YS, Chung YH. Clinical usefulness of endoscopic palliation in patients with biliary obstruction caused by hepatocellular carcinoma. Digestion. 2013;88:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 57. | Choi J, Ryu JK, Lee SH, Ahn DW, Hwang JH, Kim YT, Yoon YB, Han JK. Biliary drainage for obstructive jaundice caused by unresectable hepatocellular carcinoma: the endoscopic versus percutaneous approach. Hepatobiliary Pancreat Dis Int. 2012;11:636-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Khashab MA, Messallam AA, Penas I, Nakai Y, Modayil RJ, De la Serna C, Hara K, El Zein M, Stavropoulos SN, Perez-Miranda M. International multicenter comparative trial of transluminal EUS-guided biliary drainage via hepatogastrostomy vs. choledochoduodenostomy approaches. Endosc Int Open. 2016;4:E175-E181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 59. | Lee JG, Kang CM, Park JS, Kim KS, Yoon DS, Choi JS, Lee WJ, Kim BR. The actual five-year survival rate of hepatocellular carcinoma patients after curative resection. Yonsei Med J. 2006;47:105-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Galandi D, Antes G. Radiofrequency thermal ablation versus other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev. 2004;CD003046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 61. | Lau WY, Leung TW, Yu SC, Ho SK. Percutaneous local ablative therapy for hepatocellular carcinoma: a review and look into the future. Ann Surg. 2003;237:171-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 179] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 62. | Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Radiofrequency ablation improves prognosis compared with ethanol injection for hepatocellular carcinoma < or =4 cm. Gastroenterology. 2004;127:1714-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 437] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 63. | Tiong L, Maddern GJ. Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2011;98:1210-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 202] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 64. | Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, Ishikawa T, Koike Y, Yoshida H, Kawabe T. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 634] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 65. | Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, Frings H, Laubenberger J, Zuber I, Blum HE. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radio-frequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228:235-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 674] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 66. | Weis S, Franke A, Mössner J, Jakobsen JC, Schoppmeyer K. Radiofrequency (thermal) ablation versus no intervention or other interventions for hepatocellular carcinoma. Cochrane Database Syst Rev. 2013;CD003046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 67. | Lencioni R, Cioni D, Crocetti L, Franchini C, Pina CD, Lera J, Bartolozzi C. Early-stage hepatocellular carcinoma in patients with cirrhosis: long-term results of percutaneous image-guided radiofrequency ablation. Radiology. 2005;234:961-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 628] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 68. | Raut CP, Izzo F, Marra P, Ellis LM, Vauthey JN, Cremona F, Vallone P, Mastro A, Fornage BD, Curley SA. Significant long-term survival after radiofrequency ablation of unresectable hepatocellular carcinoma in patients with cirrhosis. Ann Surg Oncol. 2005;12:616-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 69. | Machi J, Bueno RS, Wong LL. Long-term follow-up outcome of patients undergoing radiofrequency ablation for unresectable hepatocellular carcinoma. World J Surg. 2005;29:1364-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 70. | Choi D, Lim HK, Rhim H, Kim YS, Lee WJ, Paik SW, Koh KC, Lee JH, Choi MS, Yoo BC. Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol. 2007;17:684-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 71. | Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, Fujishima T, Yoshida H, Kawabe T, Omata M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 582] [Cited by in RCA: 594] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 72. | Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, Mine N, Kondo Y, Kawabe T, Omata M. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006;43:1101-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 73. | Yoon WJ, Brugge WR. Endoscopic ultrasonography-guided tumor ablation. Gastrointest Endosc Clin N Am. 2012;22:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 74. | Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, Ganau S, Sala M, Pagès M, Ayuso C, Solé M, Rodés J, Bruix J; Barcelona Clínic Liver Cancer (BCLC) Group. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001;33:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 583] [Cited by in RCA: 529] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 75. | Livraghi T, Lazzaroni S, Meloni F, Solbiati L. Risk of tumour seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2005;92:856-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 76. | Tanaka S, Shimada M, Shirabe K, Taketomi A, Maehara S, Tsujita E, Ito S, Kitagawa D, Maehara Y. Surgical radiofrequency ablation for treatment of hepatocellular carcinoma: an endoscopic or open approach. Hepatogastroenterology. 2009;56:1169-1173. [PubMed] |
| 77. | Topal B, Hompes D, Aerts R, Fieuws S, Thijs M, Penninckx F. Morbidity and mortality of laparoscopic vs. open radiofrequency ablation for hepatic malignancies. Eur J Surg Oncol. 2007;33:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 78. | Sakoda M, Ueno S, Iino S, Minami K, Ando K, Kawasaki Y, Kurahara H, Mataki Y, Maemura K, Shinchi H. Endoscopic versus open radiofrequency ablation for treatment of small hepatocellular carcinoma. World J Surg. 2013;37:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 79. | Yan S, Xu D, Sun B. Combination of radiofrequency ablation with transarterial chemoembolization for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci. 2012;57:3026-3031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 80. | Ni JY, Liu SS, Xu LF, Sun HL, Chen YT. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol. 2013;19:3872-3882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 81. | Di Matteo F, Grasso R, Pacella CM, Martino M, Pandolfi M, Rea R, Luppi G, Silvestri S, Zardi E, Costamagna G. EUS-guided Nd:YAG laser ablation of a hepatocellular carcinoma in the caudate lobe. Gastrointest Endosc. 2011;73:632-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Nakaji S, Hirata N, Iwaki K, Shiratori T, Kobayashi M, Inase M. Endoscopic ultrasound (EUS)-guided ethanol injection for hepatocellular carcinoma difficult to treat with percutaneous local treatment. Endoscopy. 2012;44 Suppl 2:E380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | Nakaji S, Hirata N, Mikata R, Kobayashi M, Shiratori T, Ogasawara S, Ooka Y, Tsuyuguchi T, Yamaguchi T, Yokosuka O. Clinical outcomes of endoscopic ultrasound-guided ethanol injection for hepatocellular carcinoma in the caudate lobe. Endosc Int Open. 2016;4:E1111-E1115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 84. | Ishikawa T, Zeniya M, Fujise K, Hokari A, Toda G. Clinical application of Nd:YAG laser for the treatment of small hepatocellular carcinoma with new shaped laser probe. Lasers Surg Med. 2004;35:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 85. | Pacella CM, Bizzarri G, Francica G, Forlini G, Petrolati A, Valle D, Anelli V, Bianchini A, Nuntis SD, Pacella S. Analysis of factors predicting survival in patients with hepatocellular carcinoma treated with percutaneous laser ablation. J Hepatol. 2006;44:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Masuda T, Beppu T, Mizumoto T, Ishiko T, Chikamoto A, Hayashi H, Okabe H, Otao R, Horlad H, Takamori H. Hybrid ablation using percutaneous and endoscopic approach for multi-nodular hepatocellular carcinomas. Hepatogastroenterology. 2012;59:836-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 87. | Nakata Y, Haji S, Ishikawa H, Yasuda T, Nakai T, Takeyama Y, Shiozaki H. Two cases of hepatocellular carcinoma located adjacent to the Glisson’s capsule treated by laparoscopic radiofrequency ablation with intraductal chilled saline perfusion through an endoscopic nasobiliary drainage tube. Surg Laparosc Endosc Percutan Tech. 2010;20:e189-e192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 88. | Artifon EL, Cunha MAB, Da Silveira EB, Gupta K, Furuya CK, Aparicio DP, Paione J. 349 EUS-Guided or Interventional Radiology to Hepatic Intra-Arterial Chemotherapy: a Prospective Trial. Gastrointestinal Endoscopy. 2013;77:AB142-AB143. [DOI] [Full Text] |
| 89. | Thandassery RB, Goenka U, Goenka MK. Role of local ablative therapy for hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4:S104–S111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 90. | Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, Sayre J. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 358] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 91. | Lucchina N, Tsetis D, Ierardi AM, Giorlando F, Macchi E, Kehagias E, Duka E, Fontana F, Livraghi L, Carrafiello G. Current role of microwave ablation in the treatment of small hepatocellular carcinomas. Ann Gastroenterol. 2016;29:460-465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 92. | Simo KA, Sereika SE, Newton KN, Gerber DA. Laparoscopic-assisted microwave ablation for hepatocellular carcinoma: safety and efficacy in comparison with radiofrequency ablation. J Surg Oncol. 2011;104:822-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 93. | Lu DS, Raman SS, Vodopich DJ, Wang M, Sayre J, Lassman C. Effect of vessel size on creation of hepatic radiofrequency lesions in pigs: assessment of the “heat sink” effect. AJR Am J Roentgenol. 2002;178:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 357] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 94. | Lin SM. Local ablation for hepatocellular carcinoma in taiwan. Liver Cancer. 2013;2:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 95. | Poggi G, Tosoratti N, Montagna B, Picchi C. Microwave ablation of hepatocellular carcinoma. World J Hepatol. 2015;7:2578-2589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (2)] |
| 96. | Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 391] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 97. | Shi J, Sun Q, Wang Y, Jing X, Ding J, Yuan Q, Ren C, Shan S, Wang Y, Du Z. Comparison of microwave ablation and surgical resection for treatment of hepatocellular carcinomas conforming to Milan criteria. J Gastroenterol Hepatol. 2014;29:1500-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 98. | Kallini JR, Gabr A, Salem R, Lewandowski RJ. Transarterial Radioembolization with Yttrium-90 for the Treatment of Hepatocellular Carcinoma. Adv Ther. 2016;33:699-714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 99. | Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, Sato KT, Gupta R, Nikolaidis P, Miller FH. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497-507.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 503] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 100. | Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, Maccauro M, Marchianò A, Bongini M, Lanocita R. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 397] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 101. | Kulik LM, Atassi B, van Holsbeeck L, Souman T, Lewandowski RJ, Mulcahy MF, Hunter RD, Nemcek AA Jr, Abecassis MM, Haines KG 3rd, Salem R. Yttrium-90 microspheres (TheraSphere) treatment of unresectable hepatocellular carcinoma: downstaging to resection, RFA and bridge to transplantation. J Surg Oncol. 2006;94:572-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 252] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 102. | Liu M, Lin MX, Lu MD, Xu ZF, Zheng KG, Wang W, Kuang M, Zhuang WQ, Xie XY. Comparison of contrast-enhanced ultrasound and contrast-enhanced computed tomography in evaluating the treatment response to transcatheter arterial chemoembolization of hepatocellular carcinoma using modified RECIST. Eur Radiol. 2015;25:2502-2511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 103. | Nakaji S, Hirata N. Evaluation of the viability of hepatocellular carcinoma in the caudate lobe using contrast-enhanced endoscopic ultrasonography after transarterial chemoembolization. Endosc Ultrasound. 2016;5:390-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Jeon J, Ahn J, Yoo H, Park TK, Je D, Jeong H, Lee KH. Hepatoduodenal fistula formation following transcatheter arterial chemoembolization and radiotherapy for hepatocellular carcinoma: treatment with endoscopic Histoacryl injection. Korean J Intern Med. 2014;29:101-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 105. | Tsujino T, Sasahira N, Hirano K, Tateishi R, Isayama H, Tada M, Shiina S, Yoshida H, Kawabe T, Omata M. Endoscopic management of biliocutaneous fistula after percutaneous radiofrequency ablation therapy for hepatocellular carcinoma. Dig Endosc. 2010;22:53-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 106. | Ogawa T, Kawamoto H, Kobayashi Y, Nakamura S, Miyatake H, Harada R, Tsutsumi K, Fujii M, Kurihara N, Kato H. Prevention of biliary complication in radiofrequency ablation for hepatocellular carcinoma-Cooling effect by endoscopic nasobiliary drainage tube. Eur J Radiol. 2010;73:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 107. | Cennamo V, Fuccio L, Giampalma E, Terzi E, Eusebi LH, Mosconi C, Piscaglia F. Choledochoscope-assisted percutaneous fibrin glue sealing of bile leak complicating transarterial chemoembolization of hepatocellular carcinoma after liver transplantation. Endoscopy. 2011;43 Suppl 2:E238-E239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 108. | Eso Y, Marusawa H, Tsumura T, Okabe Y, Osaki Y. Endoscopic ultrasonography-guided transgastric drainage of infectious biloma following radiofrequency ablation for hepatocellular carcinoma. Dig Endosc. 2012;24:390. [PubMed] [DOI] [Full Text] |
| 109. | Faigel D, Lake D, Landreth T, Kelman C, Marler R. Endoscopic ultrasonography guided portal injection chemotherapy for hepatic metastases. Endosc Ultrasound. 2014;3:S1. [PubMed] |
| 110. | Vazquez-Sequeiros E, Olcina JR. Endoscopic ultrasound guided vascular access and therapy: A promising indication. World J Gastrointest Endosc. 2010;2:198-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 111. | Shah KN, Clary BM. Endoscopic and percutaneous approaches to the treatment of biliary tract and primary liver tumors: controversies and advances. Surg Oncol Clin N Am. 2014;23:207-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 112. | Minami Y, Kudo M. Hepatocellular carcinoma with obstructive jaundice: endoscopic and percutaneous biliary drainage. Dig Dis. 2012;30:592-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 113. | Maheshwari A, Kantsevoy S, Jagannath S, Thuluvath PJ. Endoscopic ultrasound and fine-needle aspiration for the diagnosis of hepatocellular carcinoma. Clin Liver Dis. 2010;14:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 114. | Ganne-Carrié N, Chastang C, Chapel F, Munz C, Pateron D, Sibony M, Dény P, Trinchet JC, Callard P, Guettier C. Predictive score for the development of hepatocellular carcinoma and additional value of liver large cell dysplasia in Western patients with cirrhosis. Hepatology. 1996;23:1112-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 128] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 115. | Hollerbach S, Willert J, Topalidis T, Reiser M, Schmiegel W. Endoscopic ultrasound-guided fine-needle aspiration biopsy of liver lesions: histological and cytological assessment. Endoscopy. 2003;35:743-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 116. | Jenssen C, Faiss S, Nürnberg D. Complications of endoscopic ultrasound and endoscopic ultrasound-guided interventions - results of a survey among German centers. Z Gastroenterol. 2008;46:1177-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 117. | Das A, Sivak MV Jr, Chak A. Cervical esophageal perforation during EUS: a national survey. Gastrointest Endosc. 2001;53:599-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 118. | Jenssen C, Alvarez-Sánchez MV, Napoléon B, Faiss S. Diagnostic endoscopic ultrasonography: assessment of safety and prevention of complications. World J Gastroenterol. 2012;18:4659-4676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 80] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 119. | Lachter J. Fatal complications of endoscopic ultrasonography: a look at 18 cases. Endoscopy. 2007;39:747-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 120. | Kimura H, Matsubayashi H, Fukutomi A, Asakura K, Sasaki K, Yamaguchi Y, Ono H. Lymph node metastasis diagnosed by EUS-FNA in four cases with hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2011;35:237-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 121. | Yoon WJ, Brugge WR. Endoscopic ultrasonography-guided tumor ablation. Gastrointest Endosc Clin N Am. 2012;22:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 122. | George NE, Raghavapuram S, Banerjee D, Al-Shoha M, Fedda F, Tharian B. Ectopic Hepatocellular Carcinoma within a Choledochal Cyst Diagnosed Using Single-Operator Digital Cholangioscopy. Am J Gastroenterol. 2017;112:1347-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |