Published online Feb 18, 2017. doi: 10.4254/wjh.v9.i5.270
Peer-review started: November 15, 2016
First decision: December 1, 2016
Revised: December 15, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: February 18, 2017
To determine whether addition of simvastatin could be an important pharmacological rescue therapy for carvedilol non-responders.
One hundred and two consecutive patients of cirrhosis of liver with significant portal hypertension were included. Hepatic venous pressure gradient (HVPG) was measured at the base line and after proper optimization of dose; chronic response was assessed at 3 mo. Carvedilol non-responders were given simvastatin 20 mg per day (increased to 40 mg per day at day 15). Carvedilol plus simvastatin was continued for 1 mo and hemodynamic response was again measured at 1 mo.
A total of 102 patients with mean age of 58.3 ± 6.6 years were included. Mean baseline HVPG was 16.75 ± 2.12 mmHg and after optimization of dose and reassessment of HVPG at 3 mo, mean reduction of HVPG from baseline was 5.5 ± 1.7 mmHg and 2.8 ± 1.6 mmHg among responders and non-responders respectively (P < 0.001). Addition of simvastatin to carvedilol non-responders resulted in significant response in 16 patients (42.1%) and thus overall response with carvedilol and carvedilol plus simvastatin was seen in 78 patients (80%). Two patients were removed in chronic protocol study with carvedilol and three patients were removed in carvedilol plus simvastatin study due to side effects.
Addition of simvastatin to carvedilol non-responders may prove to be an excellent rescue therapy in patients with portal hypertension.
Core tip: There is no pharmacological option available for treatment of carvedilol nonresponders in patients with portal hypertension. Addition of simvastatin could be an important pharmacological rescue therapy for carvedilol nonresponders. This study showed that addition of simvastatin to carvedilol non responders can increase overall response to around 80%, which is one of the best possible pharmacologically produced chronic response and it opens a new strategy for portal hypertension treatment.
- Citation: Wani ZA, Mohapatra S, Khan AA, Mohapatra A, Yatoo GN. Addition of simvastatin to carvedilol non responders: A new pharmacological therapy for treatment of portal hypertension. World J Hepatol 2017; 9(5): 270-277
- URL: https://www.wjgnet.com/1948-5182/full/v9/i5/270.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i5.270
The prevalence of esophageal varices in an asymptomatic compensated patient is around 40%[1]. While the incidence of variceal development is roughly 6% per year, it doubles if hepatic venous pressure gradient (HVPG) rises above 10 mmHg. Thus, cirrhotics with HVPG of > 10 mmHg represent higher risk group. HVPG > 10 mmHg also correlates with higher risk of decompensation and hepatocellular carcinoma (HCC)[2,3]. The result of a number of meta-analysis has shown that, prognosis of cirrhotic patients improve with significant decrease in portal pressure, i.e., when target decrease in HVPG (> 20% from baseline or to < 12 mmHg) is achieved[4,5]. In practice, cirrhotic patients complicated with varices should be treated except for Child-Pugh (C-P) class A patients with small varices without red color signs[6].
The role of non-selective beta blockers (NSBBs) and endoscopic variceal ligation (EVL) in the prevention of first variceal bleeding is conflicting. Analysis of a recent meta-analysis did not show any differences on mortality or bleeding rates between the two groups in trials with adequate bias control[7]. In contrast, another meta-analysis showed that compared with BBs, EVL reduced the risk of a first variceal bleed, although, there was no significant difference in survival[8]. Hence, the author concluded that EVL should be offered to patients with moderate to large oesphageal varices who are unlikely to comply or intolerant or who bleed while taking BB.
Still, the mainstream in pharmacological treatment of portal HTN (PHT) is NSBB like propranolol and nodolol which help in preventing first and recurrent variceal bleeding, gastropathy and spontaneous bacterial peritonitis (SBP)[9]. Drugs like isosorbide-5 mononitrate, prazosin or statins when added to NSBBs help in reducing the hepatic vascular tone and thus may turn many non-responders to responders[10,11]. Also, HVPG can be further decreased with these drugs. Recently our group has published a combined study on carvedilol in which 50% of the patients showed acute response and more than 60% of patients showed chronic response (please refer to definitions for details)[12]. We also showed in a separate study that, optimization of dose of carvedilol on chronic basis is an excellent policy for portal hypertension across different C-P class of liver disease[13].
Simvastatin improves liver generation of nitric oxide (NO) and hepatic endothelial dysfunction in patients with cirrhosis. Hence, it could be an effective therapy for portal hypertension. Recently, ideal drug for portal hypertension was pictured as one that should reduce portal pressure by decreasing intrahepatic vascular resistance while maintaining or enhancing hepatic blood flow[14,15]. Other desirable action would be an antifibrotic effect and a capacity to improve liver function. The drug that would be able to increase NO bioavailability in liver would fulfill many of the requirements[15-18]. However in patients with advanced cirrhosis, non-selective NO donors such as organic nitrates which enhance peripheral vasodilatation further decrease arterial pressure and activate endogenous vasoactive system. Thus, selectivity for hepatic circulation is a further requirement for vasodilators used to treat portal hypertension[19].
Recent experimental and human data[20,21] suggests that statins (3-hydroxy-3-methyl-COA reductase inhibitor) could decrease intra hepatic vascular resistance and improve flow mediated vasodilatation of liver vasculature in the cirrhotic liver. These effects are mediated by an up regulation of NO at the liver vasculature through an enhancement in endothelial NO synthetase activity[20]. Moreover, NO production in liver by statins is selective and could behave as true liver selective vasodilator.
Thus, the concept of our study was to assess the response of 3rd generation beta blocker carvedilol on chronic basis (after proper optimization of dose) and then to add simvastatin along with carvedilol, optimise dose in carvedilol non responders to have a new pharmacological approach and better rescue therapy.
We prospectively evaluated one hundred and two cirrhotic patients who were referred to our institution for hemodynamic evaluation from January, 2010 to December, 2014. The study was approved by the institutional review board (IRB) and all included patients gave informed consent for participation.
Diagnostic criteria for cirrhosis was based on clinical, biochemical, radiological and if needed on liver biopsy. The criteria for esophageal varices was based on quantitative grading used by Bavino consensus, i.e., esophageal varices less than 5 mm are small varices and esophageal varices equal to or greater than 5 mm are considered large varices. Criteria used to diagnose ascites was according to international ascites club 2003, i.e., grade I - mild (ultrasound based), Grade II - moderate, i.e., (symmetrical abdominal distension) and Grade III - gross with marked abdominal distension.
The inclusion criteria of the study include evidence of esophageal varices on upper gastrointestinal (GI) endoscopy, without a previous history of hemorrhage and a baseline HVPG of greater than 12 mmHg. Exclusion criteria were age < 18 years; severe liver failure INR > 2.5, or PT < 40% of control, bilirubin > 5 mg/dL; active alcohol consumption; IV drug abuse; renal failure, i.e., creatinine > 1.5 mg/dL; HCC; contraindication to NSBB; pre or post hepatic cause of PHT; pregnancy; previous surgical shunt or TIPPS; treatment with calcium channel blockers; treatment with (3-hydroxy-3-methyl-COA reductase inhibitor) in past three months; a known hypersensitivity to simvastatin and refusal to participate in study.
Baseline HVPG was measured for all included patients after 8 h of fasting. They were started on carvedilol 6.25 mg/d from the next day and dose was titrated by steps of 6.25 mg/wk. Dose of carvedilol was increased weekly until arterial systolic blood pressure (BP) was not less than < 90 mmHg and heart rate (HR) not less than < 55 bpm. Compliance with therapy was monitored by recording HR and BP during clinical visit.
Carvedilol non-responders were added simvastatin 20 mg/d for 15 d (then increased to 40 mg). Complete clinical examination and blood tests were performed at day 15, patients were interrogated specifically for muscle weakness, if no safe end point was met, dose was increased to 40 mg/d and continuing with continuation of carvedilol. Treatment was maintained for 1 mo and then repeat hemodynamic response was measured.
Acute response to carvedilol: Acute response to carvedilol is defined as “a drop in HVPG greater than 20% and or less than 12 mmHg from baseline at 90 min after administration of a single dose (12.5 mg) of carvedilol”.
Chronic response to carvedilol: Chronic response to carvedilol is defined as “a drop in HVPG greater than 20% and or less than 12 mmHg from baseline at 3 mo after proper optimization of dose of carvedilol”.
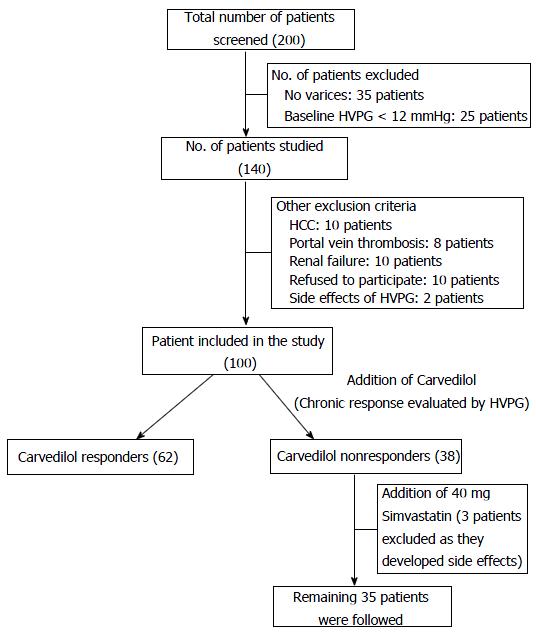
After 30 d of 40 mg simvastatin addition to carvedilol in carvedilol non responders, HVPG drop of greater than 20% from baseline and or less than 12 mmHg HVPG. The study design is illustrated in Figure 1. Dose optimization was done in all patients who were started with carvedilol. Once doses were optimized, weekly follow-up of each patient was done and HVPG was again measured at 3 mo of treatment. Patients were assessed for side effects; their BP and HR were measured on each follow-up visit. Carvedilol non responders were added with simvastatin 20 mg/d and after 15 d, all blood tests were taken for side effects of simvastatin and clinical history specifically muscle weakness was taken. With no clinical and biochemical evidence of adverse effects, patients were given 40 mg of simvastatin per day and continuing carvedilol for 1 mo, repeat hemodynamic assessment was done to see response in carvedilol non responders and thus overall response in the study group was seen.
Under fluoroscopic guidance, hepatic vein catherization was performed according to the standards described by Bosch et al[22]. A 7F balloon tipped catheter was advanced to main right hepatic vein to measure wedged hepatic venous pressure gradient (WHPG). HVPG was measured as the difference between WHPG and free hepatic pressure gradient (FHPG). Swangaz catheter was advanced to pulmonary artery for measurement of cardio pulmonary pressures like pulmonary artery pressure (PAP), wedged pulmonary pressures (WPP), right arterial pressure (RAP), etc. All measurements were repeated three times and tracing were noted. Mean arterial pressure was measured non-invasively by automatic sphygmomanometer. HR was derived by continuous ECG monitoring and systemic vascular resistance (SVR) as (MAP - RAP/CO × 80).
The statistical methods of this study were reviewed by Dr. Khan from Noora Multispeciality Hospital, Srinagar, India. Statistical analysis was performed by using statistical package for social sciences (SPSS) version 22.0. Descriptive statistics was presented as proportion, Mean ± standard deviation (SD) and median with inter-quartile range. Comparative analysis was done by utilizing student’s t-test and χ2 test. The univariate and multivariate logistic regression was used for finding the predictors. A P-value less 0.05 was considered significant.
A total of 68 patients (66.7%) had large varices and 34 patients (33.3%) had small varices on upper GI endoscopy and 63 (61.8%) patients had no ascites while others had ascites. The baseline parameters are shown in Table 1.
| Parameters | Description |
| Age (mean ± SD) | 58.35 ± 6.62 |
| Gender (male:female) | 63:39 |
| Child-Pugh class (A:B:C) | 43:32:27 |
| Etiology (Alcohol:Viral:NASH or Cryptogenic:AIH) | 31:37:29:5 |
| Oesophagea l Varices (small:large) | 34:68 |
| Ascites (No:Grade I:Grade II: Grade III) | 63:6:25:8 |
| Total bilirubin (mg/dL) | 1.96 ± 0.81 |
| Serum albumin (mg/dL) | 3.20 ± 0.49 |
| Prothrombin time | 14.13 ± 1.91 |
| International normalized ratio | 1.29 ± 0.16 |
After optimization of dose and reassessment of HVPG after 3 mo, total number of chronic responders was 62. However two patients discontinued treatment because of side effects. Mean duration of dose optimization was 15 ± 3 d. Mean reduction of HVPG from baseline and after 3 mo was 5.5 ± 1.7 mmHg and 2.8 ± 1.6 mmHg among responders and non-responders on chronic basis, respectively (P < 0.001). Mean dose of carvedilol was higher among non-responders (19.2 ± 5.7 mg) as compared to responders (18.7 ± 5.1 mg).
After assessing the chronic response at 3 mo with carvedilol, there were 38 patients who did not respond significantly to carvedilol and were thus called as carvedilol non-responder. In these 38 patients, simvastatin 20 mg/d was added initially for 15 d and at 15 d, side effects like muscle weakness along with biochemical parameters like CPK and ALT was seen. If CPK > 5 times and ALT > 3 times was found in any patient, they were withdrawn from the study. One patient developed CPK > 5 times with normal ALT was withdrawn from study on 15th day. Second patient developed hepatic encephalopathy and 3rd patient developed severe dizziness and both of these were withdrawn from study. Four patients developed minor side effects with normal CPK and ALT and were continued with treatment.
Among 38 carvedilol non responders, therefore, 35 patients continued carvedilol and simvastatin for 1 mo and then a repeat hemodynamic assessment was done. There were 16 responders and 19 non-responders at one month after adding simvastatin. Thus, overall carvedilol response in the study was 79.56% (78 patients). The pre baseline mean HVPG of carvedilol non responders was 16.429 mmHg which dropped to 13.029 mmHg, i.e., 3.4 mmHg drop (> 20%) after adding simvastatin. The post carvedilol HVPG (post chronic) in carvedilol non responders was 14.457 mmHg which dropped to 13.029 mmHg, i.e., 1.428 mmHg drop (9.87%) by adding simvastatin. It means that, simvastatin is responsible for HVPG drop of 9.87% in isolation.
Baseline and hemodynamic parameters of patients in whom simvastatin was added are shown in the Tables 2 and 3.
| Parameters | Description |
| Age (mean ± SD) | 58.45 ± 5.95 |
| Gender (male:female) | 21:17 |
| Child-Pugh class (A:B:C) | 14:13:11 |
| Etiology (Alcohol:Viral:NASH or Cryptogenic) | 12:15:11 |
| OesophagealVarices (small:large) | 12:26 |
| Ascites (No:Grade 1:Grade 2:Grade 3) | 21:4:8:5 |
| Total bilirubin (mg/dL) | 2.042 ± 0.77 |
| Serum albumin (mg/dL) | 3.203 ± 0.54 |
| Prothrombin time | 14.105 ± 2.16 |
| International normalized ratio | 1.318 ± 0.15 |
| Hemodynamic parameters | Baseline | Post chronic carvedilol (3 mo) | Post simvastatin |
| CO (L/min) | 7.525 ± 0.19 | 6.38 ± 0.13 | 6.195 ± 0.17 |
| HR (beats/min) | 79.45 ± 2.50 | 57.45 ± 2.44 | 55.053 ± 1.67 |
| MAP (mmHg) | 89.53 ± 2.42 | 75.54 ± 1.97 | 74.500 ± 1.48 |
| FHVP (mmHg) | 8.28 ± 1.85 | 9.45 ± 1.90 | 10.086 ± 1.68 |
| WHPG (mmHg) | 25.08 ± 2.55 | 22.04 ± 2.56 | 23.114 ± 2.32 |
| HVPG (mmHg) | 16.75 ± 2.12 | 12.60 ± 2.24 | 13.029 ± 1.56 |
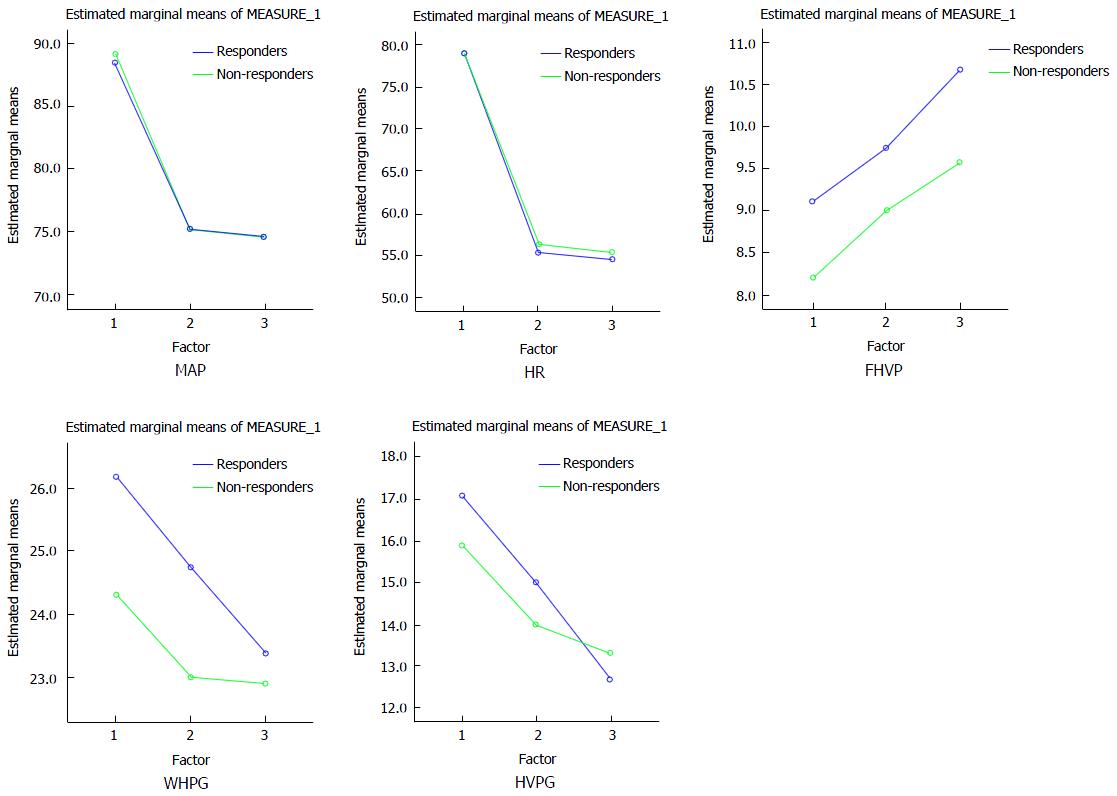
Gender, etiology, C-P class, ascites and variceal size were not seen to be statistically significant between responders and non-responders in simvastatin protocol. Among baseline hemodynamic parameters, only pre WHPG was significantly higher in responders as compared to non-responders (P = 0.01). HVPG was higher, though not statistically significant predictor of response. All hemodynamic parameters significantly decreased from baseline after treatment with simvastatin except FHVP which was significantly raised. All hemodynamic parameter were significantly decreased after treatment with simvastatin except FHVP which was significantly raised with respect to their values after chronic treatment with carvedilol (chronic protocol). Pre (baseline), post chronic (chronic carvedilol at 3 mo) and post simvastatin haemodynamic parameters in carvedilol non responders are shown as general linear model in Figure 2.
The mechanism of portal hypertension primarily involves an increase in resistance to portal outflow circulation. It leads to the formation of portosystemic collateral veins, of which esophageal varices have the highest clinical impact and the most severe complications. Other manifestations of portal hypertension include portal hypertensive gastropathy and large spontaneous shunts which refer to presence of patent paraumbilical vein, spleno-renal shunt, ano-rectosigmoid varices[23]. Recently, it has been showed that identifying cirrhotic patients with high blood ammonia concentrations could be clinically useful, as high levels would lead to suspicion of being in presence of collaterals[24]. The first line pharmacological therapy in portal hypertension is NSBB therapy. It decreases portal pressure through a reduction in portal venous inflow as a result of a decrease in cardiac output (β1-adrenergic blockade) and splanchnic blood flow (β2-adrenergic blockade). However, a major drawback of NSBBs is that not all patients respond to beta-blockers with a reduction of the HVPG.
Clinicians and researchers have always been looking for a more powerful portal hypotensive agent than propranolol and nodolol either administered alone or combination with nitrovasodilators. Advantages of medical therapy include safety and correction of detrimental systemic effects of portal hypertension. Our study tries to use best portal hypotensive agent, i.e., 3rd generation beta blocker (non-selective) with mild vasodilating properties, i.e., carvedilol which has been proven to show excellent hemodynamic response on chronic basis to the tune of 50%-72% of patients[25].
There are six studies which investigated chronic effects of carvedilol[26-28] with longest period of follow-up of 11 wk in one study. In another study by Stanley et al[27], seven of patients inclusively studied in acute protocol were unable to complete chronic administration of carvedilol as a result of side effects. This study suggests that, atleast for study group the administration of 25 mg without attempts to titrate according to response may not be ideal. Keeping in view the results of the above study, we used a titration or dose optimization based strategy for assessing chronic carvedilol response. It also studies difference of response between early liver disease and advanced liver disease, i.e., between C-P class A and B/C on chronic basis. Further this study looks into maximum dose tolerated by different C-P class of liver disease on chronic basis apart from looking into predictor of chronic response. Idea of our study was to further move to add an agent to carvedilol non responders which has no effects on MAP or peripheral vascular resistance and which behaves like a true liver selective vasodilator, i.e., simvastatin. Thus, it is the first study which has used a new pharmacological agent simvastatin in carvedilol non responders. Additive effects of simvastatin may markedly increase the number of patients who are protected effectively from portal hypertensive related complication. Such an effect is in agreement with liver perfusion studies in experimental model of cirrhosis which showed statins exert their hepatic vasodilating effect by upregulating endothelial NO production[29,30]. Our study shows that, chronic carvedilol non-responders were 62 (60%) which increased to overall response of nearly 80% once simvastatin was added to it. Thus around 42% of carvedilol non responders became responders by adding simvastatin.
In titration protocol on chronic basis, mean dose of carvedilol was 18.7 ± 5.1 mg and 19.7 ± 5.4 mg in responders and non-responders respectively. It was difficult to further increase the carvedilol dose in non-responders because of apprehension of hypotension and bradycardia. On multivariate analysis, absence of adverse events (OR = 11.3, 95%CI: 1.9-67.8) were the only independent predictors of chronic response (P < 0.05). Explanation for such results is that patients with less adverse events tolerated good dose to get good response. Major adverse events which resulted in drug discontinuation were hypotension in 2 patients and these patients could not be assessed further as shown in study design. Minor adverse events like fatigue, dyspnea, headache, temporary impotency, and dizziness were resolved without drug discontinuation. In addition, 2 patients had increase in ascites which resolved with escalation of diuretics. Further in our study, patients with C-P class A cirrhosis has shown better chronic response as compared to C-P class B and C but it was not statically significant.
Our studies showed that addition of simvastatin to carvedilol non responders can increase overall response to around 80%, which is one of the best possible pharmacologically produced chronic response and it opens a new strategy for portal hypertension treatment.
Etiology, C-P class, gender, ascites, adverse events, variceal size was not seen statically significant predictors of response for simvastatin protocol. Pre WHPG (baseline WHPG) was seen significantly higher among responders than non-responders and all hemodynamic parameters significantly decreased from baseline after treatment with simvastatin except FHVP which significantly raised. Similar results were observed after chronic treatment with carvedilol. In our study, HVPG after adding simvastatin decreased mainly because of increase in FHVP. Previous studies have shown that patients with cirrhosis have blood pooling in splanchnic region that correlates with degree of portal hypertension[20]. This might suggest that decreases in hepatic resistance by simvastatin could reduce splanchnic congestion and improving central blood volume[21] and alternatively simvastatin may have normalized venous compliance and by this mechanism can inverse venacaval and right arterial pressure and thus increase FHVP.
It is well known that simvastatin improves hepatic clearance, intrinsic clearance, and hepatic extraction of indocyanine green, parameters that reflect effective liver perfusion. Thus, an increase in intrahepatic bioavailability of NO might result in improvement in amount of blood that has functional contact with hepatocytes that explains the improvement in quantitative tests of liver function after simvastatin. We have not done these tests of liver function in our study as it is already a proven fact[11,12].
An important concern with the use of statins in patients with cirrhosis is potential for inducing liver toxicity. A number of studies have shown the safety of statins in patients with liver disease[31-33]. Our study particularly evaluated these issues in cirrhotic patients and our safety evaluation included Bil, ALP, GGT, ALT, AST, CPK and questionnaire for muscle weakness at 15th and 30th day of treatment. There was no major safety concern seen in our study. Some minor adverse events which were observed after addition of simvastatin are: (1) muscle weakness with CPK > 5 times in one patient and was withdrawn; (2) prurutis in one patient which settled and treatment continued; (3) diarrhea in one patient, self-settled and treatment continued; (4) severe dizziness and treatment withdrawn; and (5) hepatic encephalopathy in one patient and withdrawn from the study, not related to simvastatin likely part of disease.
However, whether safety profile is maintained after long term administration needs further long term studies especially with larger doses in advanced liver disease. Newer drugs like rovastatin have been shown to be safe in chronic liver disease also.
Overall, 7 patients had adverse events, 4 (57.1%) among responders, and 3 (42.9%) among non-responders with no statistical significance. Three patients were withdrawn due to side effects, first one because of increase in CPK > 5 times with muscle weakness, second one developed dizziness and 3rd patients developed hepatic encephalopathy not related to simvastatin. Liver function test after 30 d and CPK did not change and remained static and no further side effects were observed after 30 d.
Thus in conclusion, our study is first study which clearly shows that a sequential treatment strategy is an excellent policy in the pharmacological management of portal hypertension by which around 80% of response can be achieved. Further long term safety profile of statins with large doses particularly in advanced disease needs further studies and safe drugs like provastatin needs to be evaluated in future that can be used for adjuvant treatment along with carvedilol.
Carvedilol, a potent 3rd generation non-selective beta blocker (NSBB) has shown to be a promising therapy for reduction of portal hypertension. Although up to 60% of patients respond to carvedilol, options for carvedilol non responders in patients with portal hypertension is limited. Simvastatin improves liver generation of NO and hepatic endothelial dysfunction in patients with cirrhosis without affecting the hemodynamics such as heart rate and blood pressure. Hence, it could be used as an effective adjuvant therapy with carvedilol without causing any major side effects in patients with portal hypertension.
Current guidelines recommend using NSBB, such as propranolol or nadolol, with or without isosorbide-5-mononitrate to prevent variceal bleeding. Carvedilol, which blocks both α and β receptors, was shown to have better results than NSBBs by further reducing intrahepatic resistance and thus, could be used for propranolol non-responders. However, treatment option for carvedilol non-responders has not been studied yet.
Addition of simvastatin could be an important pharmacological rescue therapy for carvedilol nonresponders. This study showed that addition of simvastatin to carvedilol non responders can increase overall response to around 80%, which is one of the best possible pharmacologically produced chronic response and may open a new strategy for the treatment of portal hypertension.
Addition of simvastatin to carvedilol non-responders may prove to be an excellent therapy in patients with portal hypertension.
NSBB are very useful drugs in preventing first variceal bleeding and re-bleeding in patients with cirrhosis.
The observational study of Wani et al seems to be the first which demonstrate that a sequential treatment (carvedilol + simvastatin) strategy is an excellent policy in the pharmacological management of portal hypertension. The study is well designed and well presented.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sargsyants N, Sipos F, Tarantino G S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Groszmann RJ, Garcia-Tsao G, Bosch J, Grace ND, Burroughs AK, Planas R, Escorsell A, Garcia-Pagan JC, Patch D, Matloff DS. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254-2261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 714] [Cited by in F6Publishing: 612] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 2. | Ripoll C, Groszmann R, Garcia-Tsao G, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R, Patch D. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133:481-488. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 747] [Cited by in F6Publishing: 703] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 3. | Ripoll C, Groszmann RJ, Garcia-Tsao G, Bosch J, Grace N, Burroughs A, Planas R, Escorsell A, Garcia-Pagan JC, Makuch R. Hepatic venous pressure gradient predicts development of hepatocellular carcinoma independently of severity of cirrhosis. J Hepatol. 2009;50:923-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 4. | Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodés J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902-908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 395] [Cited by in F6Publishing: 342] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 5. | Albillos A, Bañares R, González M, Ripoll C, Gonzalez R, Catalina MV, Molinero LM. Value of the hepatic venous pressure gradient to monitor drug therapy for portal hypertension: a meta-analysis. Am J Gastroenterol. 2007;102:1116-1126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] [Cited in This Article: ] |
| 7. | Gluud LL, Klingenberg S, Nikolova D, Gluud C. Banding ligation versus beta-blockers as primary prophylaxis in esophageal varices: systematic review of randomized trials. Am J Gastroenterol. 2007;102:2842-2848; quiz 2841, 2849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Tripathi D, Graham C, Hayes PC. Variceal band ligation versus beta-blockers for primary prevention of variceal bleeding: a meta-analysis. Eur J Gastroenterol Hepatol. 2007;19:835-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 639] [Cited by in F6Publishing: 586] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 10. | Bureau C, Péron JM, Alric L, Morales J, Sanchez J, Barange K, Payen JL, Vinel JP. “A La Carte” treatment of portal hypertension: Adapting medical therapy to hemodynamic response for the prevention of bleeding. Hepatology. 2002;36:1361-1366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Abraldes JG, Albillos A, Bañares R, Turnes J, González R, García-Pagán JC, Bosch J. Simvastatin lowers portal pressure in patients with cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136:1651-1658. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 305] [Cited by in F6Publishing: 293] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 12. | Wani ZA, Bhat RA, Bhadori AS, Zargar SA, Shah AH, Maiwall R, Hameedand I, Syed B. A Haemodynamic Analysis to Assess the Safe Dose of Carvedilol across Different Child Class of Liver Disease. BJMMR. 2015;7:355-368. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Wani ZA, Baht RA, Bhadoria AS, Maiwall R, Majeed Y, Khan AA, Zargar SA, Shah MA, Khan KM. After proper optimization of carvedilol dose, do different child classes of liver disease differ in terms of dose tolerance and response on a chronic basis? Saudi J Gastroenterol. 2015;21:278-283. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 14. | Bosch J, Abraldes JG, Groszmann R. Current management of portal hypertension. J Hepatol. 2003;38 Suppl 1:S54-S68. [PubMed] [Cited in This Article: ] |
| 15. | Failli P, DeFRANCO RM, Caligiuri A, Gentilini A, Romanelli RG, Marra F, Batignani G, Guerra CT, Laffi G, Gentilini P. Nitrovasodilators inhibit platelet-derived growth factor-induced proliferation and migration of activated human hepatic stellate cells. Gastroenterology. 2000;119:479-492. [PubMed] [Cited in This Article: ] |
| 16. | Yu Q, Shao R, Qian HS, George SE, Rockey DC. Gene transfer of the neuronal NO synthase isoform to cirrhotic rat liver ameliorates portal hypertension. J Clin Invest. 2000;105:741-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 130] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Van de CM, Omasta A, Janssens S. In vivo gene transfer of endothelial nitric oxide synthase decreases portal pressure in anaesthetized carbon tetrachloride cirrhotic rats. Gut. 2002;51:440-445. [Cited in This Article: ] |
| 18. | Morales-Ruiz M, Cejudo-Martín P, Fernández-Varo G, Tugues S, Ros J, Angeli P, Rivera F, Arroyo V, Rodés J, Sessa WC. Transduction of the liver with activated Akt normalizes portal pressure in cirrhotic rats. Gastroenterology. 2003;125:522-531. [PubMed] [Cited in This Article: ] |
| 19. | Wiest R, Groszmann RJ. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology. 2002;35:478-491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 338] [Cited by in F6Publishing: 302] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 20. | Kiszka-Kanowitz M, Henriksen JH, Møller S, Bendtsen F. Blood volume distribution in patients with cirrhosis: aspects of the dual-head gamma-camera technique. J Hepatol. 2001;35:605-612. [PubMed] [Cited in This Article: ] |
| 21. | Huonker M, Schumacher YO, Ochs A, Sorichter S, Keul J, Rössle M. Cardiac function and haemodynamics in alcoholic cirrhosis and effects of the transjugular intrahepatic portosystemic stent shunt. Gut. 1999;44:743-748. [PubMed] [Cited in This Article: ] |
| 22. | Bosch J, Garcia-Pagán JC, Berzigotti A, Abraldes JG. Measurement of portal pressure and its role in the management of chronic liver disease. Semin Liver Dis. 2006;26:348-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 153] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 23. | Tarantino G, Citro V, Conca P, Riccio A, Tarantino M, Capone D, Cirillo M, Lobello R, Iaccarino V. What are the implications of the spontaneous spleno-renal shunts in liver cirrhosis? BMC Gastroenterol. 2009;9:89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Tarantino G, Citro V, Esposito P, Giaquinto S, de Leone A, Milan G, Tripodi FS, Cirillo M, Lobello R. Blood ammonia levels in liver cirrhosis: a clue for the presence of portosystemic collateral veins. BMC Gastroenterol. 2009;9:21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Bañares R, Moitinho E, Matilla A, García-Pagán JC, Lampreave JL, Piera C, Abraldes JG, De Diego A, Albillos A, Bosch J. Randomized comparison of long-term carvedilol and propranolol administration in the treatment of portal hypertension in cirrhosis. Hepatology. 2002;36:1367-1373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 79] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Tripathi D, Therapondos G, Lui HF, Stanley AJ, Hayes PC. Haemodynamic effects of acute and chronic administration of low-dose carvedilol, a vasodilating beta-blocker, in patients with cirrhosis and portal hypertension. Aliment Pharmacol Ther. 2002;16:373-380. [PubMed] [Cited in This Article: ] |
| 27. | Stanley AJ, Therapondos G, Helmy A, Hayes PC. Acute and chronic haemodynamic and renal effects of carvedilol in patients with cirrhosis. J Hepatol. 1999;30:479-484. [PubMed] [Cited in This Article: ] |
| 28. | Silkauskaitė V, Kupčinskas J, Pranculis A, Jonaitis L, Petrenkienė V, Kupčinskas L. Acute and 14-day hepatic venous pressure gradient response to carvedilol and nebivolol in patients with liver cirrhosis. Medicina (Kaunas). 2013;49:467-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Abraldes JG, Rodríguez-Vilarrupla A, Graupera M, Zafra C, García-Calderó H, García-Pagán JC, Bosch J. Simvastatin treatment improves liver sinusoidal endothelial dysfunction in CCl4 cirrhotic rats. J Hepatol. 2007;46:1040-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, Nevens F, Sauerbruch T, Heller J. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 223] [Article Influence: 13.1] [Reference Citation Analysis (2)] |
| 31. | Ritzel U, Leonhardt U, Näther M, Schäfer G, Armstrong VW, Ramadori G. Simvastatin in primary biliary cirrhosis: effects on serum lipids and distinct disease markers. J Hepatol. 2002;36:454-458. [PubMed] [Cited in This Article: ] |
| 32. | Chalasani N. Statins and hepatotoxicity: focus on patients with fatty liver. Hepatology. 2005;41:690-695. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 200] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 33. | Vuppalanchi R, Teal E, Chalasani N. Patients with elevated baseline liver enzymes do not have higher frequency of hepatotoxicity from lovastatin than those with normal baseline liver enzymes. Am J Med Sci. 2005;329:62-65. [PubMed] [Cited in This Article: ] |