Published online Nov 8, 2017. doi: 10.4254/wjh.v9.i31.1197
Peer-review started: August 27, 2017
First decision: September 15, 2017
Revised: September 17, 2017
Accepted: October 15, 2017
Article in press: October 15, 2017
Published online: November 8, 2017
This review considers the modern concepts of pathogenesis, diagnostic methods, and treatment principles of hepatic hydrothorax (HH). HH is the excessive (> 500 mL) accumulation of transudate in the pleural cavity in patients with decompensated liver cirrhosis but without cardiopulmonary and pleural diseases. It causes respiratory failure which aggravates the clinical course of liver cirrhosis, and the emergence of spontaneous bacterial pleural empyema may be the cause of death. The information was collected from the PubMed database, the Google Scholar retrieval system, the Cochrane reviews, and the reference lists from relevant publications for 1994-2016 using the keywords: “liver cirrhosis”, “portal hypertension”, “hepatic hydrothorax”, “pathogenesis”, “diagnostics”, and “treatment”. To limit the scope of this review, only articles dealing with uncomplicated hydrothorax in patients with liver cirrhosis were included. The analysis of the data showed that despite the progress of modern hepatology, the presence of HH is associated with poor prognosis and high mortality. Most patients suffering from it are candidates for orthotopic liver transplantation. In routine clinical practice, stratification of the risk for an adverse outcome and the subsequent determination of individual therapeutic strategies may be the keys to the successful management of the patient’s condition. The development of pathogenetic pharmacotherapy and optimization of minimally invasive treatment will improve the quality of life and increase the survival rate among patients with HH.
Core tip: This review considers the modern concepts of pathogenesis, diagnostic methods, and treatment principles of hepatic hydrothorax (HH). The analysis of the data showed that despite the progress of modern hepatology, the presence of HH is associated with poor prognosis and high mortality. Most patients suffering from it are candidates for orthotopic liver transplantation. In routine clinical practice, stratification of the risk for an adverse outcome and the subsequent determination of individual therapeutic strategies may be the keys to the successful management of the patient’s condition. The development of pathogenetic pharmacotherapy and minimally invasive treatment will improve the quality of life and increase the survival rate among patients with HH.
- Citation: Garbuzenko DV, Arefyev NO. Hepatic hydrothorax: An update and review of the literature. World J Hepatol 2017; 9(31): 1197-1204
- URL: https://www.wjgnet.com/1948-5182/full/v9/i31/1197.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i31.1197
Pleural effusion is a syndrome characterized by the accumulation of fluid in the pleural cavity, which has more than 50 causes associated with both pleural and pulmonary diseases, as well as the pathology of other organs and systems. Depending on its nature, it is classified into exudate and transudate. The criteria for their differentiation proposed in 1972 by Light et al are currently the “gold standard” of differential diagnosis (Table 1)[1]. Hepatic hydrothorax (HH) is the excessive (> 500 mL) accumulation of transudate in the pleural cavity in patients with decompensated liver cirrhosis (LC) but without cardiopulmonary and pleural diseases. Its localization is right-sided in approximately 85% of cases and left-sided in approximately 13%; whereas, only 2% of patients have fluid in the pleural cavity on both sides.
| Criteria | Exudate | Transudate |
| Effusion protein/serum protein ratio | > 0.5 | < 0.5 |
| Absolute LDH level in pleural fluid | > 200 IU/L | < 200 IU/L |
| Effusion LDH/serum LDH ratio | > 0.6 | < 0.6 |
HH is observed infrequently and, depending on the diagnostic method, may be found in almost 5%-10% of patients, constituting 2%-3% of all causes of pleural effusions. However, it causes respiratory failure which aggravates the clinical course of LC. The emergence of spontaneous bacterial pleural empyema may be the cause of death. Median survival in the presence of HH is 8-12 mo[2].
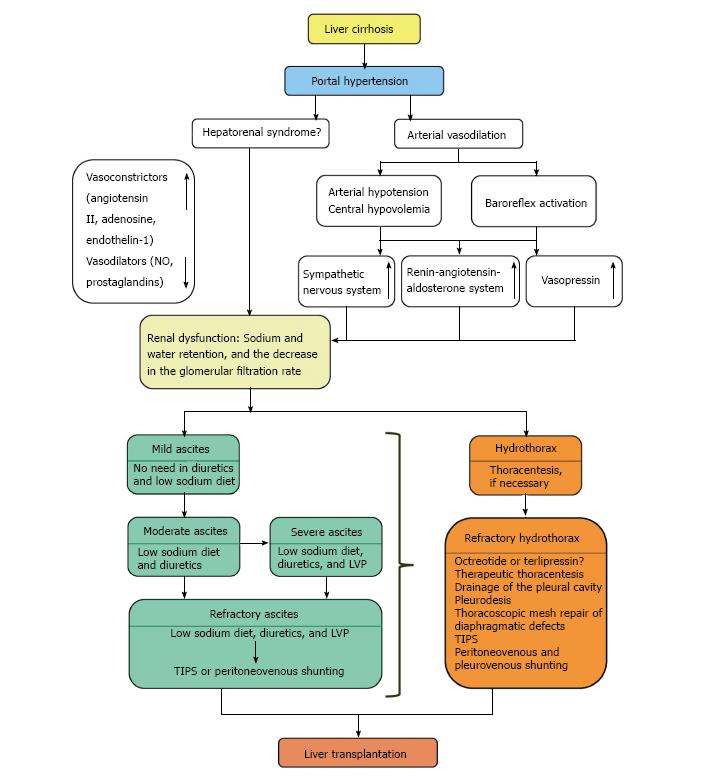
Several theories of HH pathogenesis in patients with LC are known, but the most probable one presupposes ascites formation, due to portal hypertension related pathophysiological disorders[3]. Concomitant splanchnic and systemic arterial vasodilation, along with the activation of various neurohormonal signaling pathways, cause kidney dysfunction and hence decrease Na+ and water excretion, as well as the glomerular filtration rate[4]. Ascitic fluid moves from the peritoneal cavity into the pleural space through small defects located mainly on the right side of the diaphragmatic tendon. The reason for this is negative intrathoracic pressure. Moreover, the liver in this situation may play the role of a piston[5]. Huang et al[6] classified diaphragmatic defects into four types: (1) Type 1 - no obvious defects; (2) Type 2 - blebs lying on the diaphragm; (3) Type 3 - broken defects (fenestrations) in the diaphragm; (4) Type 4 - multiple gaps in the diaphragm.
This theory of HH pathogenesis, first proposed in 1966, has been confirmed by numerous studies using air infused intraperitoneally, dyes, or radiolabeled material[7]. It does not contradict the phenomenon of isolated HH, in which the pleural reabsorption rate of ascites is equal to the ascites production in the abdominal cavity[8].
In patients suffering from LC with or without ascites, clinical examination may reveal pleural effusion and allow preliminary diagnosis of HH, which is often right-sided. Its left-sided localization, the presence of fever, and respiratory symptoms require the exclusion of other diseases, as well as spontaneous bacterial pleural empyema. For this purpose, a pleural puncture is performed at the first stage of the diagnosis to obtain and analyze fluid, which is inherently a transudate. This is similar to ascitic fluid, but the different absorption mechanisms from the pleural and abdominal cavities cause some distinctions between the two of them (Table 2)[9]. Radioisotope diagnostic techniques are used to make a decision in doubtful cases. Migration of intraperitoneally infused 99mTc-labeled microspheres of human serum albumin or a sulfur colloid into the pleural cavity confirms the diagnosis of HH, and the rate of isotope movement indicates the size of defects in the diaphragm[10]. Color Doppler and magnetic resonance imaging are used for their direct visualization[11].
| Criteria | Values |
| The number of white blood cells in pleural fluid | < 250/mm3 |
| Pleural effusion total protein level | < 25 g/L |
| Pleural effusion total protein/serum total protein ratio | < 0.5 |
| Pleural effusion LDH/serum LDH ratio | > 0.6 |
| Pleural effusion albumin/serum albumin ratio | > 1.1 |
| Pleural effusion bilirubin /serum bilirubin ratio | < 0.6 |
| pH | > 7.4 |
| Pleural effusion glucose level is equal to serum glucose level |
Currently, there are no evidence-based standards for the management of patients with HH. In usual clinical practice, the aim of therapeutic measures is to treat ascites and to provide thoracentesis if necessary (Figure 1). In case of adequate urine sodium concentration (> 30 mEq/24 h), this means completely eliminating a pleural effusion and thereby removing the signs of respiratory failure[12].
In accordance with modern clinical recommendations, the severity of ascites determines its treatment[13]. Accordingly, patients with LC and mild ascites do not need diuretics and a low-sodium diet. Since sodium excretion is slightly weakened in most patients with moderate ascites, the aim of the treatment is to reduce the intake of Na+ and stimulate its excretion by using diuretics and maintaining a usual drinking regimen. The consumption of Na+ should be reduced to 80-120 mmol/d, which corresponds to 4.6-6.9 g of salt per day.
In addition to this diet, patients should take either spironolactone or amiloride at an initial dose of 50-200 mg/d or 5-10 mg/d, respectively. The dose of spironolactone should be gradually increased by 100 mg every seven days. The maximum dose is 400 mg/d. The treatment is effective if there is a body weight reduction of at least 2 kg/wk. If monotherapy with spironolactone is inefficient or if hyperkalemia develops, furosemide should be taken at a starting dose of 40 mg/d with a gradual increase of 40 mg increments every seven days, with a maximum of 160 mg/d. In the case of hyperkalemia, the maximum permissible dose of spironolactone is 400 mg/d. The maximum acceptable weight loss for patients with ascites without peripheral edema is 0.5 kg/d. For patients with ascites and peripheral edema, it is 1 kg/d.
In patients with HH and tense ascites, the recommended procedure is to perform large-volume paracentesis (LVP) and albumin infusions in order to prevent associated circulatory disorders (8 g for each removed liter of ascitic fluid). The prospective study made by Angueira et al[14] has shown a statistically significant increase in the total lung capacity and functional residual capacity of the lungs with an improvement in the symptoms of HH within 2 h after LVP. It also contributed to an increase in PaO2/FiO2 and lung volume at the end of exhalation without hemodynamic disturbances, as noted in another prospective study of 31 mechanically ventilated patients with LC and acute lung injury[15].
Refractory ascites is defined as ascites that cannot be mobilized to at least first grade using diuretic treatment and dietary sodium restriction or as ascites which early recurrence after LVP cannot be satisfactorily prevented by medical therapy[16]. In this case, the first-line of treatment is LVP repeated every 2-3 wk in combination with albumin infusions and the prescription of diuretics to patients who have the urine sodium concentration > 30 mEq/d[17]. If this is ineffective, transjugular intrahepatic portosystemic shunt (TIPS) is recommended[18]. This operation reduces portal pressure, thereby improving the function of the cardiovascular system, which increases renal blood flow and the glomerular filtration rate[19]. Surgical intervention in refractory ascites may be considered in the case of LVP futility and when TIPS cannot be implemented due to technical, organizational, or anatomical problems. The operation of choice is peritoneovenous shunting[20].
In a multicenter, nonrandomized trial, Bellot et al[21] evaluated the efficacy of a device called “Automated Low Flow Pump System” (ALFApump system) in 40 patients with refractory ascites. It moves ascitic fluid from the abdominal cavity to the bladder through a system of catheters connected to a pump implanted beneath the skin. It was noted that 40% of patients had no need for LVP after its implantation, and 70% needed LVP less than once a month. Nevertheless, there is a high percentage of complications, which mainly involve migration and/or blockage of urinary or peritoneal catheters (22.5% and 12.5%, respectively). This fact currently excludes the introduction of this method into clinical practice and requires further study.
Refractory HH occurs under significant disturbance of Na+ excretion (< 10 mEq/d); therefore, measures aimed at treating ascites cannot eliminate it[22].
Pharmacotherapy: Despite the absence of specific pharmacotherapy for refractory HH, it is possible to use drugs recommended for the treatment of portal hypertension[23]. One of the publications demonstrated the good effects of octreotide, a synthetic analog of somatostatin, prescribed after ineffective use of diuretics with a low sodium diet, pleurodesis, and TIPS. It was infused intravenously at a dose of 25 μg/h on the first day, 50 μg/h on the second day, and 100 μg/h for the next five days. Then it was injected subcutaneously. The amount of fluid in the pleural cavity decreased after the fifth day. During a six-month period of observation, there was no relapse of HH[24]. The positive effect of octreotide can be explained by its ability to suppress the activation of the renin-angiotensin-aldosterone system induced by diuretics, as well as to increase the excretion of Na+ and water[25].
In another case, a positive result was achieved after a five-day course of terlipressin therapy in combination with albumin infusions given to a patient with decompensated LC who had type 1 hepatorenal syndrome along with HH[26].
Therapeutic thoracentesis: Repeated thoracentesis is the routine procedure to remove fluid from the pleural space in refractory HH. This procedure is relatively safe even in patients with an increased risk of bleeding[27]. Nevertheless, it is important to bear in mind the possibility of complications associated with it, such as pneumothorax, pleural empyema, purulent soft tissue infection of the chest wall, and air embolism, among others[28]. In addition, large-volume thoracentesis, which is necessary in a number of cases, may increase microvascular permeability and cause re-expansion pulmonary edema. It occurs due to inflammatory reaction accompanied by the production of reactive oxygen species and superoxide radicals in response to the rapid expansion of the initially collapsed lung. The key mediators of inflammation in this pathological situation may be interleukin 8, leukotriene B4, monocyte chemotactic and activating factor[29], tumor necrosis factor α, and interleukin 1 β[30] with the participation of Rho/ROCK signaling pathway[31]. Another possible factor is an increase in hydrostatic pressure in pulmonary blood vessels leading to a plasma leakage into the interstitial space[32].
To avoid re-expansion pulmonary edema, it is considered expedient to remove only one liter of transudate at a time. Meanwhile, a study by Feller-Kopman et al[33], which included 185 patients who underwent large-volume thoracentesis (from 1 to more than 3 L), did not find clinical and radiological signs of re-expansion pulmonary edema in many of them, and the appearance of this complication did not depend on the amount of removed fluid, pleural pressure, and pleural elasticity. The authors proposed to revise the recommendations for limiting the volume of thoracentesis and assumed that it should be stopped only if there are unpleasant sensations in the chest or a decrease in the pleural pressure to less than -20 mmH2O at the end of exhalation.
Drainage of the pleural cavity: The installation of tubular drains into the pleural cavity for prolonged aspiration of the contents is undesirable in refractory HH. First, it is fraught with the development of pneumothorax and pleural empyema. Second, a large loss of fluid may lead to renal dysfunction and electrolyte imbalance. Taken together, they significantly worsen the disease prognosis and increase the risk of death[34].
In this respect, catheters such as “Pigtail” or Pleurx® look safer[35]. So, the use of the first was successful in 48 out of 60 patients with refractory HH. The most significant complications were occlusion (3.3%) of the catheters and pain around their location (20%)[36]. The Pleurx® drainage system showed good results in five of the eight patients, who had it installed as a “bridge” before TIPS or liver transplantation. Pleural empyema developed in two patients, which required removal of the catheter in one case[37]. Since these catheters are not widely used, their expediency for the treatment of refractory HH cannot be determined, and further research is needed for final conclusions.
Pleurodesis: Pleurodesis may serve as a treatment method for refractory HH in the case of unsuccessful repeated thoracentesis. In most publications devoted to this problem, it was created by using chemical substances acting on visceral and parietal pleura and causing their aseptic inflammation and adhesion. Irritant agents were injected into the pleural cavity through the cannula or during therapeutic thoracoscopy. The most commonly used chemicals were talc, tetracycline, doxycycline, bleomycin, povidone-iodine, and picibanil (OK-432) with or without minocycline[38].
It is better to perform chemical pleurodesis after removal of ascitic fluid and transudate from the pleural cavity. In addition, some authors propose to combine it with constant positive pressure in the airways, which decreases the negative pressure in the pleural cavity. It prevents the ascitic fluid from moving there and leaves it dry for a longer period[39].
In a prospective study, which included 56 patients with refractory HH, 20 mL of a povidone-iodine 10% aqueous solution were administered through a cannula inserted into the pleural cavity under ultrasound control. This procedure was effective in 71.4% of all cases, and the success rate was 66.7% in massive effusion and 80% in moderate effusion. Twenty-eight patients had to undergo a repeat procedure after a week due to refractory HH relapse, of which 12 were successful[40]. Similar results were obtained in another prospective study after administration of 1 g of doxycycline diluted in 100 mL of saline[41].
In a randomized clinical trial, Helmy et al[42] evaluated the efficacy of chemical pleurodesis performed in 20 patients during therapeutic thoracoscopy without video assistance. For this purpose, the authors used povidone-iodine 10% aqueous solution (10 mL) in 8 cases, doxycycline (1 g) in 6 cases, and talc (2-3 g) in 6 cases. All drugs were diluted in 50 mL of saline. The observation lasted for three months and showed good results in 15 patients (75%): in 7 when using povidone-iodine (87.5%), in 4 out of 6 from the doxycycline group, and in 4 out of 6 from the talc group (66.7%). After the introduction of the talc suspension, one death was due to the development of the hepatic coma as a result of the LC progression.
The introduction of video-assisted thoracoscopic surgery (VATS) expanded the treatment options for patients with refractory HH. This makes it possible to perform not only a chemical, but also a combined pleurodesis with chemical, mechanical, and thermal effects on the pleura, the argon plasma coagulation, and the closure of diaphragmatic defects with fibrin glue, suturing, or synthetic materials[43].
In a systematic review with meta-analysis, Hou et al[44] evaluated the efficacy and safety of pleurodesis carried out by using different methods that included various drugs, as well as the closure of diaphragmatic defects with fibrin glue and their suturing during VATS in patients with refractory HH. They summarized the results of 20 clinical observations and 13 series of cases, including 26 and 180 people, respectively.
In clinical observations, the severity of hepatic dysfunction was indicated in 10 patients, 3 of whom had Child-Turcotte-Pugh (CTP) class B (30%), and 7 of whom had CTP class C (70%). Of the 26 patients, chemical pleurodesis during a therapeutic thoracoscopy without video assistance was performed in 12 cases (46.2%), and with VATS (19.2%) in 5 patients. The authors mainly used talc at a dose of 2-2.5 g (12/26, 46.2%) and OK-432 at a dose of 10 KE (7/26, 26.9%). The procedure was carried out once in 19 patients (73.1%), twice in 2 patients (7.7%), and thrice in 1 patient (3.9%). Information on the number of operations was missing in 4 cases (15.3%).
Chemical pleurodesis was effective in 17 patients (65.4%). Other methods were successfully applied to 4 patients from the group having negative results. One fatal outcome was associated with bleeding from the upper gastrointestinal tract and liver failure.
In this series of cases, the severity of liver dysfunction was indicated in 113 patients, two of whom had CTP class A (1.8%), 37 of whom had CTP class B (32.7%), and 74 of whom had CTP class C (65.5%). Pleurodesis during therapeutic thoracoscopy without video assistance was performed in 54 patients (30%), and during VATS in 126 (70%). Agents for chemical impact on the pleura were mainly talc at a dose of 2-2.5 g (115/180, 63.9%) and OK-432 at a dose of 10 KE in combination with minocycline or without it (19/180, 10.6%). Nine patients (5%) underwent mechanical pleurodesis (pleural abrasion, electrocautery), which was supplemented with talc applied to the pleural surface in 8 cases (4.4%). Pleurodesis was combined with the closure of diaphragmatic defects by using fibrin glue or suturing in 26 patients (20.6%) during VATS. No more than two sessions of pleurodesis were required to obtain a positive result, and only one procedure was needed in 80%-100% of cases. The complete response rate after pleurodesis was 72% (95%CI: 65%-79%).
The efficacy of pleurodesis performed with different methods was evaluated using meta-analyses of six and two studies including 90 and 16 patients respectively. They showed that the complete response rate was 78% (95%CI: 68%-87%) when performing therapeutic thoracoscopy without video assistance and 84% (95%CI: 64%-97%) when using VATS.
Meta-analyses of 7 and 2 studies including 114 and 19 patients respectively were performed to evaluate the efficacy of pleurodesis achieved with the use of various drugs. They showed that the complete response rate was 71% (95%CI: 63%-79%) when using talc and 93% (95%CI: 78%-100%) when using OK-432 with or without minocycline.
A meta-analysis of 6 studies including 63 patients showed that the complete complication rate was 82% (95%CI: 66%-94%). They included subfebrile temperature (47.6%), renal insufficiency (17.5%), pneumothorax (15.9%), hepatic encephalopathy (11.1%), pneumonia (9.5%), liver failure (9.5%), pleural empyema (6.4%), pleuro-cutaneous fistulas (4.8%), sepsis (3.2%), intraoperative bleeding (1.6%), and upper gastrointestinal bleeding (1.6%).
Therefore, the presented data suggest that despite a large percentage of complications, pleurodesis may be a promising method for treating refractory HH. Randomized controlled trials with a meta-analysis are necessary to confirm this.
Thoracoscopic mesh repair of diaphragmatic defects: Huang et al[45] published the results of thoracoscopic onlay reinforcement with Mersilene mesh to repair diaphragmatic defects in 63 patients with refractory HH (CTP class A - 12, B - 36, C - 15), which in 16 cases was combined with their suturing. The average observation period was 20.5 mo. Of the 4 patients who had a relapse of the disease, the pleural effusion was eliminated using thoracentesis (> 3 times) in 3 patients, and a second operation was required in 1 patient. The 30-d and 3-mo mortality rates were 9.5% and 25.4% respectively, and its main reasons were septic shock (37.5%), acute kidney damage (25%), and gastrointestinal bleeding (25%). The authors concluded that the method is effective in low-risk patients with adequate postoperative management. In their opinion, the most significant factors worsening the prognosis are the preoperative severity of liver and kidney dysfunction assessed according to the Model for End-Stage Liver Disease (MELD) and Acute Kidney Injury Network (AKIN) criteria.
ТIPS: In one of the few articles devoted to the use of TIPS in refractory HH, Ditah et al[46] provided a systematic review and a cumulative meta-analysis of 6 retrospective studies involving a total of 198 patients suffering from HH (CTP class A - 2, B - 82, C - 114). TIPS successfully eliminated the symptoms of refractory HH in 73% of cases. The early (45-d) and 1-year mortality rates were 18% and 50% respectively, with the most important predictors of an adverse outcome being old age (> 60-65 years), the initial severity of liver disease (CTP class C, MELD ≥ 15), and increased creatinine level and inefficiency of TIPS. Associated hepatic encephalopathy occurred in about 12% of all cases, and less frequently when expanded polytetrafluoroethylene-covered stents were used. The data obtained by the authors correlated well with the results of TIPS in other complications of portal hypertension[47].
Peritoneovenous and pleurovenous shunting: Pleurovenous shunting is a surgical operation described in 1975 for the treatment of malignant pleural effusion. It is rarely used in refractory HH, and the publications devoted to it are mainly presented as clinical observations and a series of cases. In one of them, Artemiou et al[48] performed it on six patients and showed that all the shunts were passable during 1-40 mo and no one needed a pleural puncture to remove transudate. Nevertheless, it is still too early to discuss the prospects of pleurovenous shunting in refractory HH due to the small sample size.
Liver transplantation: Since most of the patients with HH have a terminal stage of LC, they are potential candidates for orthotopic liver transplantation[49]. In the study by Xiol et al[50], it was performed in 28 patients with HH (CTP class B - 9, C - 19) without prior TIPS or drainage of the pleural cavity. HH was refractory in 5 cases and combined with ascites in 26 cases. Eleven patients had episodes of spontaneous bacterial pleural empyema. HH was resolved in all transplant patients for three months. At one lethal outcome, the average survival rate was 114 mo.
Sersté et al[51] compared the results of orthotopic liver transplantation without previously performed TIPS in three groups of patients at the terminal stage of LC: with refractory HH, tense ascites without HH, and without any of these complications. Patients with HH did not need therapeutic thoracentesis after transplantation. There were no significant differences in the duration of mechanical pulmonary ventilation, the duration of the stay in an intensive care unit and hospital, as well as the frequency of septic complications and early postoperative lethality. The one-year survival rate was similar.
Although HH is infrequent, its presence exacerbates the course of LC, and the occurrence of spontaneous bacterial pleural empyema may be the cause of death. Most patients, who suffer from it, are candidates for orthotopic liver transplantation. In routine clinical practice, the key to their successful management may be the stratification of the risk for an adverse outcome and the determination of individual therapeutic strategies. The development of pathogenetic pharmacotherapy and optimization of minimally invasive treatment will improve the quality of life and increase the survival rate among patients with HH.
The authors would like to thank Emily Thomas for her help in language editing and proofreading this paper.
Manuscript source: Invited Manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Russia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Fan XM, Ratnasari N, Sirin G S- Editor: Wei LJ L- Editor: A E- Editor: Lu YJ
| 1. | Porcel JM. Identifying transudates misclassified by Light’s criteria. Curr Opin Pulm Med. 2013;19:362-367. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Abbasi A, Bhutto AR, Alam MT, Aurangzaib M, Masroor M. Frequency of Hepatic Hydrothorax and its Association with Child Pugh Class in Liver Cirrhosis Patients. J Coll Physicians Surg Pak. 2016;26:566-569. [PubMed] [Cited in This Article: ] |
| 3. | Garbuzenko DV. Pathophysiological mechanisms and new directions of therapy of portal hypertension at liver cirrhosis. Klin Persp Gastrojen Gepatol. 2010;6:11-20. [Cited in This Article: ] |
| 4. | Pedersen JS, Bendtsen F, Møller S. Management of cirrhotic ascites. Ther Adv Chronic Dis. 2015;6:124-137. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Lazaridis KN, Frank JW, Krowka MJ, Kamath PS. Hepatic hydrothorax: pathogenesis, diagnosis, and management. Am J Med. 1999;107:262-267. [PubMed] [Cited in This Article: ] |
| 6. | Huang PM, Chang YL, Yang CY, Lee YC. The morphology of diaphragmatic defects in hepatic hydrothorax: thoracoscopic finding. J Thorac Cardiovasc Surg. 2005;130:141-145. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Singh A, Bajwa A, Shujaat A. Evidence-based review of the management of hepatic hydrothorax. Respiration. 2013;86:155-173. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Kim JS, Kim CW, Nam HS, Cho JH, Ryu JS, Lee HL. Hepatic hydrothorax without ascites as the first sign of liver cirrhosis. Respirol Case Rep. 2015;4:16-18. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Dinu M, Georgescu AC, Ciurea RN, Ştefan M. The role of cytology in the diagnosis of fluid collection syndromes associated with liver cirrhosis. Clinical, epidemiological, cytological and biochemical study of pleural effusion. Rom J Morphol Embryol. 2012;53:989-995. [PubMed] [Cited in This Article: ] |
| 10. | Hewett LJ, Bradshaw ML, Gordon LL, Rockey DC. Diagnosis of isolated hepatic hydrothorax using peritoneal scintigraphy. Hepatology. 2016;64:1364-1366. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Zenda T, Miyamoto S, Murata S, Mabuchi H. Detection of diaphragmatic defect as the cause of severe hepatic hydrothorax with magnetic resonance imaging. Am J Gastroenterol. 1998;93:2288-2289. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Cardenas A, Kelleher T, Chopra S. Review article: hepatic hydrothorax. Aliment Pharmacol Ther. 2004;20:271-279. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | Ivashkin VT, Mayevskaya MV, Pavlov CS, Fedosyina YA, Bessonova YN, Pirogova IY, Garbuzenko DV. Treatment of liver cirrhosis complications: Clinical guidelines of the Russian Scientific Liver Society and Russian gastroenterological association. Ros Zhurn Gastrojenterol, Gepatol, Koloproktol. 2016;26:71-102. [Cited in This Article: ] |
| 14. | Angueira CE, Kadakia SC. Effects of large-volume paracentesis on pulmonary function in patients with tense cirrhotic ascites. Hepatology. 1994;20:825-828. [PubMed] [Cited in This Article: ] |
| 15. | Levesque E, Hoti E, Jiabin J, Dellamonica J, Ichai P, Saliba F, Azoulay D, Samuel D. Respiratory impact of paracentesis in cirrhotic patients with acute lung injury. J Crit Care. 2011;26:257-261. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H, Schölmerich J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164-176. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Solà E, Solé C, Ginès P. Management of uninfected and infected ascites in cirrhosis. Liver Int. 2016;36 Suppl 1:109-115. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Thuluvath PJ, Bal JS, Mitchell S, Lund G, Venbrux A. TIPS for management of refractory ascites: response and survival are both unpredictable. Dig Dis Sci. 2003;48:542-550. [PubMed] [Cited in This Article: ] |
| 19. | Allegretti AS, Ortiz G, Cui J, Wenger J, Bhan I, Chung RT, Thadhani RI, Irani Z. Changes in Kidney Function After Transjugular Intrahepatic Portosystemic Shunts Versus Large-Volume Paracentesis in Cirrhosis: A Matched Cohort Analysis. Am J Kidney Dis. 2016;68:381-391. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Kuntz E, Kuntz H-D. Hepatology, Principles and Practice – History, Morphology, Biochemistry, Diagnostics, Clinic, Therapy, 2nd Edition. Heidelberg: Springer-Verlag 2006; 906 p. [Cited in This Article: ] |
| 21. | Bellot P, Welker MW, Soriano G, von Schaewen M, Appenrodt B, Wiest R, Whittaker S, Tzonev R, Handshiev S, Verslype C. Automated low flow pump system for the treatment of refractory ascites: a multi-center safety and efficacy study. J Hepatol. 2013;58:922-927. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Krok KL, Cárdenas A. Hepatic hydrothorax. Semin Respir Crit Care Med. 2012;33:3-10. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Garbuzenko DV. Contemporary concepts of the medical therapy of portal hypertension under liver cirrhosis. World J Gastroenterol. 2015;21:6117-6126. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Barreales M, Sáenz-López S, Igarzabal A, Muñoz-Yagüe T, Casis B, Alonso-Navas F, Solís-Herruzo JA. Refractory hepatic hydrothorax: successful treatment with octreotide. Rev Esp Enferm Dig. 2005;97:830-835. [PubMed] [Cited in This Article: ] |
| 25. | Kalambokis G, Economou M, Fotopoulos A, Bokharhii JA, Katsaraki A, Tsianos EV. Renal effects of treatment with diuretics, octreotide or both, in non-azotemic cirrhotic patients with ascites. Nephrol Dial Transplant. 2005;20:1623-1629. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | Ibrisim D, Cakaloglu Y, Akyuz F, Karadag A, Ozdil S, Besisik F, Mungan Z, Okten A. Treatment of hepatic hydrothorax with terlipressin in a cirrhotic patient. Scand J Gastroenterol. 2006;41:862-865. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Puchalski JT, Argento AC, Murphy TE, Araujo KL, Pisani MA. The safety of thoracentesis in patients with uncorrected bleeding risk. Ann Am Thorac Soc. 2013;10:336-341. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Castellote J, Xiol X, Cortés-Beut R, Tremosa G, Rodríguez E, Vázquez S. Complications of thoracentesis in cirrhotic patients with pleural effusion. Rev Esp Enferm Dig. 2001;93:566-575. [PubMed] [Cited in This Article: ] |
| 29. | Sherman SC. Reexpansion pulmonary edema: a case report and review of the current literature. J Emerg Med. 2003;24:23-27. [PubMed] [Cited in This Article: ] |
| 30. | Funakoshi T, Ishibe Y, Okazaki N, Miura K, Liu R, Nagai S, Minami Y. Effect of re-expansion after short-period lung collapse on pulmonary capillary permeability and pro-inflammatory cytokine gene expression in isolated rabbit lungs. Br J Anaesth. 2004;92:558-563. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Sawafuji M, Ishizaka A, Kohno M, Koh H, Tasaka S, Ishii Y, Kobayashi K. Role of Rho-kinase in reexpansion pulmonary edema in rabbits. Am J Physiol Lung Cell Mol Physiol. 2005;289:L946-L953. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Sue RD, Matthay MA, Ware LB. Hydrostatic mechanisms may contribute to the pathogenesis of human re-expansion pulmonary edema. Intensive Care Med. 2004;30:1921-1926. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Feller-Kopman D, Berkowitz D, Boiselle P, Ernst A. Large-volume thoracentesis and the risk of reexpansion pulmonary edema. Ann Thorac Surg. 2007;84:1656-1661. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Orman ES, Lok AS. Outcomes of patients with chest tube insertion for hepatic hydrothorax. Hepatol Int. 2009;3:582-586. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Bhatnagar R, Maskell NA. Indwelling pleural catheters. Respiration. 2014;88:74-85. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Sharaf-Eldin M, Bediwy AS, Kobtan A, Abd-Elsalam S, El-Kalla F, Mansour L, Elkhalawany W, Elhendawy M, Soliman S. Pigtail Catheter: A Less Invasive Option for Pleural Drainage in Egyptian Patients with Recurrent Hepatic Hydrothorax. Gastroenterol Res Pract. 2016;2016:4013052. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Kilburn JP, Hutchings J, Misselhorn D, Chen AC. Use of indwelling tunneled pleural catheters for the management of hepatic hydrothorax. Chest. 2010;138:418A. [Cited in This Article: ] |
| 38. | Rodríguez Suárez PM, Freixinet Gilart JL. Pleurodesis in the treatment of pneumothorax and pleural effusion. Monaldi Arch Chest Dis. 2013;79:81-86. [Cited in This Article: ] |
| 39. | Jung Y. Surgical Treatment of Hepatic Hydrothorax: A “Four-Step Approach”. Ann Thorac Surg. 2016;101:1195-1197. [PubMed] [DOI] [Cited in This Article: ] |
| 40. | Abdelhafeez AM, Zakaria MW, Fathalah WF, Omran D. Towards an easier pleurodesis: Ultrasound-guided iodopovidone sclerotherapy in cirrhotic patients with hepatic hydrothorax. Open J Gastroenterol. 2013;3:196-201. [Cited in This Article: ] |
| 41. | Abdelhafeez AM, Fathallah WF. Ultrasound-guided pleurodesis with doxycycline in patients with hepatic hydrothorax. Egypt J Bronchol. 2016;10:20-25. [Cited in This Article: ] |
| 42. | Helmy N, Akl Y, Kaddah S, Hafiz HA, Makhzangy HE. A case series: Egyptian experience in using chemical pleurodesis as an alternative management in refractory hepatic hydrothorax. Arch Med Sci. 2010;6:336-342. [PubMed] [DOI] [Cited in This Article: ] |
| 43. | Kiafar C, Gilani N. Hepatic hydrothorax: current concepts of pathophysiology and treatment options. Ann Hepatol. 2008;7:313-320. [PubMed] [Cited in This Article: ] |
| 44. | Hou F, Qi X, Guo X. Effectiveness and Safety of Pleurodesis for Hepatic Hydrothorax: A Systematic Review and Meta-Analysis. Dig Dis Sci. 2016;61:3321-3334. [PubMed] [DOI] [Cited in This Article: ] |
| 45. | Huang PM, Kuo SW, Chen JS, Lee JM. Thoracoscopic Mesh Repair of Diaphragmatic Defects in Hepatic Hydrothorax: A 10-Year Experience. Ann Thorac Surg. 2016;101:1921-1927. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Ditah IC, Al Bawardy BF, Saberi B, Ditah C, Kamath PS. Transjugular intrahepatic portosystemic stent shunt for medically refractory hepatic hydrothorax: A systematic review and cumulative meta-analysis. World J Hepatol. 2015;7:1797-1806. [PubMed] [DOI] [Cited in This Article: ] |
| 47. | Rössle M. TIPS: 25 years later. J Hepatol. 2013;59:1081-1093. [PubMed] [DOI] [Cited in This Article: ] |
| 48. | Artemiou O, Marta GM, Klepetko W, Wolner E, Müller MR. Pleurovenous shunting in the treatment of nonmalignant pleural effusion. Ann Thorac Surg. 2003;76:231-233. [PubMed] [Cited in This Article: ] |
| 49. | Krowka MJ, Wiesner RH, Heimbach JK. Pulmonary contraindications, indications and MELD exceptions for liver transplantation: a contemporary view and look forward. J Hepatol. 2013;59:367-374. [PubMed] [DOI] [Cited in This Article: ] |
| 50. | Xiol X, Tremosa G, Castellote J, Gornals J, Lama C, Lopez C, Figueras J. Liver transplantation in patients with hepatic hydrothorax. Transpl Int. 2005;18:672-675. [PubMed] [DOI] [Cited in This Article: ] |
| 51. | Sersté T, Moreno C, Francoz C, Razek WA, Paugham C, Belghitti J, Valla D, Moreau R, Durand F. The impact of preoperative hepatic hydrothorax on the outcome of adult liver transplantation. Eur J Gastroenterol Hepatol. 2010;22:207-212. [PubMed] [DOI] [Cited in This Article: ] |