Published online Mar 28, 2016. doi: 10.4254/wjh.v8.i9.446
Peer-review started: September 21, 2015
First decision: October 30, 2015
Revised: January 31, 2016
Accepted: March 9, 2016
Article in press: March 14, 2016
Published online: March 28, 2016
Processing time: 189 Days and 9.7 Hours
Incidentally found focal liver lesions are a common finding and a reason for referral to hepatobiliary service. They are often discovered in patients with history of liver cirrhosis, colorectal cancer, incidentally during work up for abdominal pain or in a trauma setting. Specific points should considered during history taking such as risk factors of liver cirrhosis; hepatitis, alcohol consumption, substance exposure or use of oral contraceptive pills and metabolic syndromes. Full blood count, liver function test and tumor markers can act as a guide to minimize the differential diagnosis and to categorize the degree of liver disease. Imaging should start with B-mode ultrasound. If available, contrast enhanced ultrasound is a feasible, safe, cost effective option and increases the ability to reach a diagnosis. Contrast enhanced computed tomography should be considered next. It is more accurate in diagnosis and better to study anatomy for possible operation. Contrast enhanced magnetic resonance is the gold standard with the highest sensitivity. If doubt still remains, the options are biopsy or surgical excision.
Core tip: Focal liver lesions are being found more commonly, which may need further investigations. History and physical examination is essential part of work up. Blood work is an important adjunct in the patient’s journey. There are different modalities of imaging (B-mode ultrasound, contrast enhanced ultrasound, contrast enhanced computed tomography and contrast enhanced magnetic resonance); each has advantages and disadvantages. The decision of biopsy or surgery is kept for the treating team.
- Citation: Algarni AA, Alshuhri AH, Alonazi MM, Mourad MM, Bramhall SR. Focal liver lesions found incidentally. World J Hepatol 2016; 8(9): 446-451
- URL: https://www.wjgnet.com/1948-5182/full/v8/i9/446.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i9.446
Focal liver lesions (FLLs) are a common reason for consultation to a hepatobiliary service, they often need further work up, and investigations. They are often discovered in patients with a cirrhotic liver or colorectal cancer but can be found incidentally during work up for abdominal pain and sometimes in the trauma setting.
Incidental liver lesions are being found more commonly due to advancement in imaging modalities. In some reports, incidental FLLs were found in up to 33% of radiological studies. In autopsy cases, it reached more than 50%[1,2].
Unfortunately, there is no clear pathway for work up and with a wide differential diagnosis; these lesions may need multiple imaging modalities to characterize whether they are benign or malignant.
A cornerstone in evaluating these patients is history and physical examination. A deferential diagnosis of metastasis vs hepatocellular carcinoma (HCC) should be considered for patients with family history of previous malignancies or chronic liver diseases. However, in a healthy population without significant medical background, the differential diagnosis should include wider possibilities, both benign and malignant.
Different modalities are being used to reach a definitive diagnosis. These include: B-mode ultrasound (B-US), contrast enhanced ultrasound (C-US), elastography, contrast enhanced computed tomography (C-CT) scan and contrast enhanced magnetic resonance (C-MR) imaging. Due to the lack of guidelines, most institutions are using all available modalities to establish a diagnosis, which is time consuming, uncomfortable, and not cost effective.
Specific points should be taken in consideration as a part of history taking; risk factors for liver cirrhosis like hepatitis and alcohol consumption, exposure to substances known to cause liver lesions, use of the oral contraceptive pill should be elucidated especially in childbearing aged women. Obesity and metabolic syndromes and diabetes are know pathognomic factors for non alcoholic fatty liver disease which is know to increase hepatocellular cancer[3]. Patients with a previous cancer should raise the suspicion of a liver metastatic lesion. A family history of malignancy should also be clarified[4]. A history of fever and travel should raise the suspicion of infective process.
During physical examination of the patient-jaundice, cachexia, palpable masses, palpable lymph nodes and stigmata of liver disease - should be looked for (Table 1)[4].
| General | Compensated | Decompensated |
| Jaundice | Xanthelasmas | Disorientation |
| Fever | Parotid enlargement | Drowsiness |
| Loss of body hair | Spider naevi | Coma |
| Gyanecomastia | Hepatic flap | |
| Large or small liver | Fetor hepaticus | |
| Splenomegaly | Ascites | |
| Clubbing | Dilated veins on abdominalwall | |
| Liver palms | Oedema | |
| Dupuytren’s contracture | ||
| Xanthoma | ||
| Scratch marks | ||
| Testicular atrophy | ||
| Purpura | ||
| Pigmented ulcers |
The differential diagnosis of a liver lesion is wide, and can be benign requiring no treatment or an advanced malignant condition beyond cure. The list can be minimized with a careful clinical, chemical and radiological assessment (Table 2).
| Benign lesions | Malignant lesions |
| Cystic lesion (5%-14%) | Metastasis (14.4) |
| Simple, infectious, pre malignant | Cystic lesions (8%) |
| Hemangioma (2%-20%) | Hepatocellular carcinoma (2%-6%) |
| Hepatic adenoma (3%) | Cholangiocarcinoma (2%) |
| Biliary hamartoma (1.5%) | Lymphoma |
| Regenerative nodule (11%) | Sarcoma |
When requesting blood investigation for patients with FLL the results should answer three essential points.
The general condition of the patient; using a full blood count, renal profile, liver function test and albumin level.
The assessment of liver status using the above with the addition of a coagulation profile. These will help obtain a Childs-Pugh score and can be determinant in planning proper management plan.
Tumor markers such as carcinembryonic antigen (CEA), alpha-feto protein (AFP) and cancer antigen 19-9 (CA19-9) should be requested. A high-level of CEA should raise the possibilities of metastatic colorectal cancer. HCC and cholangiocarcinoma could have raised level of AFP and CA19-9 respectively. An elevated AFP (over 400 ng/mL) may confirm the diagnosis if combined with the addition of two confirmatory imaging techniques[5].
The limitation of any type of ultrasonography (USS) (B-mode or contrast enhanced) is the visualization of the whole liver. When the whole liver can be seen USS is a very useful screening test but in certain patients views of parts of the liver can be very limited which limits the usefulness of the investigation.
B-US is one of the most commonly used modalities to investigate the liver and can help to diagnose different pathology. In patients presenting with liver disease, abdominal pain and jaundice a B-US is usually requested. In the Focused Assessment with Sonography for Trauma examination, liver lesions are found in approximately 12 of every 1000 patients examined[6]. B-US is also recommended in the surveillance for patients at a high risk of developing HCC[7,8].
The role of B-US in the diagnosing FLLs in a healthy patient is limited to a few diagnoses, of which hemangioma is the most common. Haematomas, hydatid cysts, and abscesses can be conveniently identified using B-US alone. The diagnosis of other FLLs with B-US alone is more challenging and rarely possible.
The use of pulsed and color Doppler USS is limited to focal nodular hyperplasia (FNH) in which the central artery with radial distribution is a characteristic element present in approximately 80% of cases[9].
There are two main types of contrast used with ultrasound, micro-bubbles (MBs) and Sonazoid. MBs can be defined within different vascular phases: Arterial, portal and the delayed venous phase and are very useful in the detection of malignancies. Sonazoid is approved only in Japan and has an extra post-vascular phase (also called the Kupffer phase), MBs become phagocytosed by Kupffer cells and hence there is no post vascular phase when MBs are used.
Malignancies are characterized by hypo enhancement in the portal and venous phases as well as in the post-vascular phase, making their detection with C-US possible. C-US has been shown to be a reliable imaging technique for follow-up of metastatic liver disease with an accuracy of 91% compared to CT scan and MR imaging[10].
In imaging of HCCs C-US is more complicated. Well-differentiated HCC lesions are iso enhancing in late phases in 51% of cases only, meaning that other imaging modalities are required[11].
The use of USS contrast agents has radically changed the approach to the characterization of FLLs. C-US allows the classification of the majority of FLLs with a high diagnostic accuracy. The typical pattern of FLLs has been well described in the European Federation of Societies for Ultrasound in Medicine and Biology guidelines for C-US, originally published in 2004, updated in 2008, and soon to be updated again[12,13].
Excluding simple cysts (without enhancement in all phases), benign FLLs are generally characterized by an iso echoic pattern in the portal and late phases; because of the persistence of USS contrast agents in the sinusoidal space. In contrast, the washout of these agents in late phases is characteristic of malignant lesions.
Hervé Trillaud confirmed the superior results of real-time C-US for FLLs characterization, compared to that of unenhanced ultrasound. Furthermore, it was demonstrated that the diagnostic accuracy of SonoVue®-enhanced ultrasound was better in comparison to C- CT and C-MR[14].
Hohmann et al[15] using MBs agents in C-US with a long-lasting late phase, showed no significant difference in lesion detection compared with C-MR imaging.
Real-time (RT) elastography is a technique that can estimate the strain modules from radiofrequency signals in response to external compression and provide an estimation of tissue elasticity. This technique has been studied for the characterization of nodules in superficial structures such as the breast, thyroid, and prostate. Few studies are available concerning its application to the liver, particularly for the evaluation of liver fibrosis. Apart from its use for characterization, RT elastography has been studied for the detection of liver nodules in animal models and during surgery[16,17]. In the latter setting, it has been demonstrated to have a higher diagnostic accuracy than B-mode intraoperative USS in detecting lesions surrounded by a heterogeneous background or with an iso echoic pattern (96% vs 89%). Nevertheless, its role in the detection of FLLs is yet to be definitively assessed.
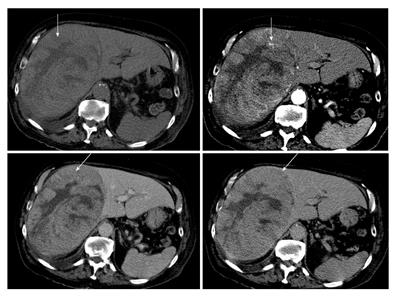
C-CT scan is one of the essential imaging studies of FLL. The protocol and ability to acquire a multiphasic study is paramount in characterizing liver lesions. Triphasic images are the method of choice, which give a significant improvement in the result compared to single-phase studies[18]. The ability for three-dimensional reconstruction helps in assessing the vascular anatomy, the liver and tumor volumes. It also provides a good screening tool to the rest of the abdomen as well as to stage a malignant pathology. Differentiation between benign and malignant conditions is based on the degree of uptake of the contrast agent at different phases of the study. For example, hepatocellular cancer has an early uptake of contrast in the arterial phase with an early washout in the portal and delayed phases (Figure 1)[8]. One of the limitations of C-CT is the large dose of radiation given to the patient and the nephrotoxic effect of the iodine contrast that limit its use in patients with renal impairment.
C-MR is the best modality for FLLs assessment, in both primary and metastatic malignancy. C-MR represents the current technique of choice in this setting since it is free of ionizing radiation as well as demonstrating a high contrast resolution using several sequences and different types of contrast media. The commonly used contrast media are gadolinium-chelates, which have an extra-cellular hepatic distribution which help in differentiating liver lesions and obtaining angiography. Other types of contrast agent have an intra-cellular distribution such as ferrumoxides and hence help to detect liver parenchymal lesions[19-21]. There is general agreement about the superiority of C-MR with extra-cellular contrast medium compared to the baseline study without contrast or with other types of contrast[22-26].
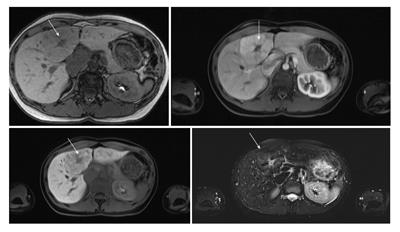
Primovist (Gd-EOB-DTPA) is a biphasic hepatobiliary magnetic resonance contrast agent. Dynamic C-MR imaging can be performed with the Gd-based extracellular contrast agents where the hemodynamic characteristics of the lesion can be studied. Following that, the hepatobiliary phase can be obtained when the contrast agents are excreted in both renal and biliary systems. Obtaining hepatobiliary phase can provide histological as well as functional information about lesions which might improve the diagnostic accuracy of FLLs[27]. Gd-EOB-DTPA-enhanced MR can provide useful information to help characterizing benign and malignant focal lesions and not only to detect them (Figure 2)[28].
Soussan et al[29] reported that using gadolinium-based C-MR gives a diagnostic accuracy of 52%-66% for incidentally found solid liver lesions compared to 52%-53% with C-US.
Chung et al[30] demonstrated that Gd-EOB-DTPA-enhanced MR is more accurate to differentiate between benign and malignant lesions and more specific to diagnosis FNH and focal eosinophilic infiltration. Both dynamic C-CT and Gd-EOB-DTPA-enhanced MR had similar high diagnostic accuracy for hemangiomas and HCCs, whereas other relatively uncommon lesions such as inflammatory myofibroblastic tumor, embryonal sarcoma or schwannoma are rarely diagnosed accurately on both modalities[30].
An advantage of C-MR is lack of ionizing radiation and the ability to use in renal impairment patients. It also provides a better characterization of liver lesions compared to other modalities. A drawback is the high cost and the longer procedure duration[30].
Radiological imaging, tumor markers and other information gathered through the assessment process are often diagnostic, and therefore biopsy is rarely needed. Biopsy increases risks of bleeding and needle-track seeding. Biopsy of hepatic adenomas, FNH, and hemangioma has an increased risk of bleeding[31]. It has been reported that biopsy of HCCs are associated with a significant risk of needle-track seeding (1.6%-5%)[4,32,33].
A group of investigators studied 160 patients with FLLs. Preoperative fine needle biopsy was not performed. After surgery, 98% of preoperative diagnosis was confirmed histologically[34].
In rare cases imaging might not be conclusive, and hence, a surgical resection for definitive diagnosis might be needed. Resection will confirm the diagnosis, prevent progression of premalignant conditions and will reduce the risk of bleeding or seeding if biopsy were done. Other indication for surgery is resectable lesion, which has been characterized on imaging, and a diagnosis has been made.
Fine-needle liver biopsy of FLLs is generally reserved for patients who are not surgical candidates and can be done at the same time of non-surgical treatments such as radiofrequency ablation or trans arterial chemoembolization.
Incidentally found FLLs should be thoroughly assessed using history and physical examination in association with blood tests as the starting point to formulate a differential diagnosis. Imaging modalities should be used wisely to save cost but to get the highest sensitivity possible. Ultrasound is fast, feasible, safe, cost effective and if combined with contrast, has an increased sensitivity in reaching the diagnosis but C-CT has a greater accuracy in diagnosis, is more widely applicable (less influenced by body morphology) and is helpful to study liver anatomy. C-MR is the modality of choice with the highest sensitivity. Biopsy should be reserved for questionable lesions where surgery is not an option.
P- Reviewer: Abu-Zidan FM, Tarantino G, Wang JS S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Boutros C, Katz SC, Espat NJ. Management of an incidental liver mass. Surg Clin North Am. 2010;90:699-718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Karhunen PJ. Benign hepatic tumours and tumour like conditions in men. J Clin Pathol. 1986;39:183-188. [PubMed] [Cited in This Article: ] |
| 3. | Scalera A, Tarantino G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J Gastroenterol. 2014;20:9217-9228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 37] [Reference Citation Analysis (1)] |
| 4. | Huang GT, Sheu JC, Yang PM, Lee HS, Wang TH, Chen DS. Ultrasound-guided cutting biopsy for the diagnosis of hepatocellular carcinoma--a study based on 420 patients. J Hepatol. 1996;25:334-338. [PubMed] [Cited in This Article: ] |
| 5. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] [Cited in This Article: ] |
| 6. | Sgourakis G, Lanitis S, Korontzi M, Kontovounisios C, Zacharioudakis C, Armoutidis V, Karaliotas C, Dedemadi G, Lepida N, Karaliotas C. Incidental findings in focused assessment with sonography for trauma in hemodynamically stable blunt trauma patients: speaking about cost to benefit. J Trauma. 2011;71:E123-E127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4059] [Cited by in F6Publishing: 4399] [Article Influence: 366.6] [Reference Citation Analysis (2)] |
| 8. | Tan CH, Low SC, Thng CH. APASL and AASLD Consensus Guidelines on Imaging Diagnosis of Hepatocellular Carcinoma: A Review. Int J Hepatol. 2011;2011:519783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Lim KJ, Kim KW, Jeong WK, Kim SY, Jang YJ, Yang S, Lee JJ. Colour Doppler sonography of hepatic haemangiomas with arterioportal shunts. Br J Radiol. 2012;85:142-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Dietrich CF, Kratzer W, Strobe D, Danse E, Fessl R, Bunk A, Vossas U, Hauenstein K, Koch W, Blank W. Assessment of metastatic liver disease in patients with primary extrahepatic tumors by contrast-enhanced sonography versus CT and MRI. World J Gastroenterol. 2006;12:1699-1705. [PubMed] [Cited in This Article: ] |
| 11. | Nicolau C, Catalá V, Vilana R, Gilabert R, Bianchi L, Solé M, Pagés M, Brú C. Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: correlation with cellular differentiation. Eur Radiol. 2004;14:1092-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 138] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 12. | Albrecht T, Blomley M, Bolondi L, Claudon M, Correas JM, Cosgrove D, Greiner L, Jäger K, Jong ND, Leen E. Guidelines for the use of contrast agents in ultrasound. January 2004. Ultraschall Med. 2004;25:249-256. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 247] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 13. | Claudon M, Cosgrove D, Albrecht T, Bolondi L, Bosio M, Calliada F, Correas JM, Darge K, Dietrich C, D’Onofrio M. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med. 2008;29:28-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 526] [Cited by in F6Publishing: 485] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 14. | Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of focal liver lesions using contrast-enhanced sonography with a low mechanical index mode and a sulfur hexafluoride-filled microbubble contrast agent. J Clin Ultrasound. 2006;34:261-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Hohmann J, Müller A, Skrok J, Wolf KJ, Martegani A, Dietrich CF, Albrecht T. Detection of hepatocellular carcinoma and liver metastases with BR14: a multicenter phase IIA study. Ultrasound Med Biol. 2012;38:377-382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Melodelima D, Chenot J, Souchon R, Rivoire M, Chapelon JY. Visualisation of liver tumours using hand-held real-time strain imaging: results of animal experiments. Br J Radiol. 2012;85:e556-e565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Inoue Y, Takahashi M, Arita J, Aoki T, Hasegawa K, Beck Y, Makuuchi M, Kokudo N. Intra-operative freehand real-time elastography for small focal liver lesions: “visual palpation” for non-palpable tumors. Surgery. 2010;148:1000-1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Chi Y, Zhou J, Venkatesh SK, Tian Q, Liu J. Content-based image retrieval of multiphase CT images for focal liver lesion characterization. Med Phys. 2013;40:103502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Semelka RC, Helmberger TK. Contrast agents for MR imaging of the liver. Radiology. 2001;218:27-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 260] [Cited by in F6Publishing: 268] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 20. | Harisinghani MG, Jhaveri KS, Weissleder R, Schima W, Saini S, Hahn PF, Mueller PR. MRI contrast agents for evaluating focal hepatic lesions. Clin Radiol. 2001;56:714-725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Hammerstingl R, Huppertz A, Breuer J, Balzer T, Blakeborough A, Carter R, Fusté LC, Heinz-Peer G, Judmaier W, Laniado M. Diagnostic efficacy of gadoxetic acid (Primovist)-enhanced MRI and spiral CT for a therapeutic strategy: comparison with intraoperative and histopathologic findings in focal liver lesions. Eur Radiol. 2008;18:457-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 22. | Mueller GC, Hussain HK, Carlos RC, Nghiem HV, Francis IR. Effectiveness of MR imaging in characterizing small hepatic lesions: routine versus expert interpretation. AJR Am J Roentgenol. 2003;180:673-680. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Mainenti PP, Mancini M, Mainolfi C, Camera L, Maurea S, Manchia A, Tanga M, Persico F, Addeo P, D’Antonio D. Detection of colo-rectal liver metastases: prospective comparison of contrast enhanced US, multidetector CT, PET/CT, and 1.5 Tesla MR with extracellular and reticulo-endothelial cell specific contrast agents. Abdom Imaging. 2010;35:511-521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Ward J, Guthrie JA, Scott DJ, Atchley J, Wilson D, Davies MH, Wyatt JI, Robinson PJ. Hepatocellular carcinoma in the cirrhotic liver: double-contrast MR imaging for diagnosis. Radiology. 2000;216:154-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 152] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Kim YK, Kim CS, Han YM. Detection of small hepatocellular carcinoma: comparison of conventional gadolinium-enhanced MRI with gadolinium-enhanced MRI after the administration of ferucarbotran. Br J Radiol. 2009;82:468-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Matsuo M, Kanematsu M, Itoh K, Ito K, Maetani Y, Kondo H, Kako N, Matsunaga N, Hoshi H, Shiraishi J. Detection of malignant hepatic tumors: comparison of gadolinium-and ferumoxide-enhanced MR imaging. AJR Am J Roentgenol. 2001;177:637-643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Kim MJ. Current limitations and potential breakthroughs for the early diagnosis of hepatocellular carcinoma. Gut Liver. 2011;5:15-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Purysko AS, Remer EM, Veniero JC. Focal liver lesion detection and characterization with GD-EOB-DTPA. Clin Radiol. 2011;66:673-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Soussan M, Aubé C, Bahrami S, Boursier J, Valla DC, Vilgrain V. Incidental focal solid liver lesions: diagnostic performance of contrast-enhanced ultrasound and MR imaging. Eur Radiol. 2010;20:1715-1725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Chung YE, Kim MJ, Kim YE, Park MS, Choi JY, Kim KW. Characterization of incidental liver lesions: comparison of multidetector CT versus Gd-EOB-DTPA-enhanced MR imaging. PLoS One. 2013;8:e66141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 31. | Reddy KR, Schiff ER. Approach to a liver mass. Semin Liver Dis. 1993;13:423-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Durand F, Regimbeau JM, Belghiti J, Sauvanet A, Vilgrain V, Terris B, Moutardier V, Farges O, Valla D. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol. 2001;35:254-258. [PubMed] [Cited in This Article: ] |
| 33. | Takamori R, Wong LL, Dang C, Wong L. Needle-tract implantation from hepatocellular cancer: is needle biopsy of the liver always necessary? Liver Transpl. 2000;6:67-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | Torzilli G, Minagawa M, Takayama T, Inoue K, Hui AM, Kubota K, Ohtomo K, Makuuchi M. Accurate preoperative evaluation of liver mass lesions without fine-needle biopsy. Hepatology. 1999;30:889-893. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 255] [Article Influence: 10.2] [Reference Citation Analysis (0)] |