Published online Mar 18, 2016. doi: 10.4254/wjh.v8.i8.411
Peer-review started: November 29, 2015
First decision: January 4, 2016
Revised: January 13, 2016
Accepted: March 7, 2016
Article in press: March 9, 2016
Published online: March 18, 2016
AIM: To evaluate the efficacy of technical modifications of total hepatic vascular exclusion (THVE) for hepatectomy involving inferior vena cava (IVC).
METHODS: Of 301 patients who underwent hepatectomy during the immediate previous 5-year period, 8 (2.7%) required THVE or modified methods of IVC cross-clamping for resection of liver tumors with massive involvement of the IVC. Seven of the patients had diagnosis of colorectal liver metastases and 1 had diagnosis of hepatocellular carcinoma. All tumors involved the IVC, and THVE was unavoidable for combined resection of the IVC in all 8 of the patients. Technical modifications of THVE were applied to minimize the extent and duration of vascular occlusion, thereby reducing the risk of damage.
RESULTS: Broad dissection of the space behind the IVC coupled with lifting up of the liver from the retrocaval space was effective for controlling bleeding around the IVC before and during THVE. The procedures facilitate modification of the positioning of the cranial IVC cross-clamp. Switching the cranial IVC cross-clamp from supra- to retrohepatic IVC or to the confluence of hepatic vein decreased duration of the THVE while restoring hepatic blood flow or systemic circulation via the IVC. Oblique cranial IVC cross-clamping avoided ischemia of the remnant hemi-liver. With these technical modifications, the mean duration of THVE was 13.4 ± 8.4 min, which was extremely shorter than that previously reported in the literature. Recovery of liver function was smooth and uneventful for all 8 patients. There was no case of mortality, re-operation, or severe complication (i.e., Clavien-Dindo grade of III or more).
CONCLUSION: The retrocaval liver lifting maneuver and modifications of cranial cross-clamping were useful for minimizing duration of THVE.
Core tip: Total hepatic vascular exclusion (THVE) is needed for resection of liver tumors involving inferior vena cava (IVC). Because THVE has a high risk of morbidity, compared to inflow occlusion alone, its duration should be shortened. The technical modifications reported here minimized the risk of damage of THVE. Specifically, the procedures include the retrocaval liver lifting maneuver, switching of the cranial IVC cross-clamp, and oblique IVC cross-clamping. For the 8 patients retrospectively assessed, the duration of THVE was 13.4 ± 8.4 min, which was remarkably shorter than that reported previously. Postoperative recovery was smooth for all patients, without severe complications.
- Citation: Ko S, Kirihataya Y, Matsumoto Y, Takagi T, Matsusaka M, Mukogawa T, Ishikawa H, Watanabe A. Retrocaval liver lifting maneuver and modifications of total hepatic vascular exclusion for liver tumor resection. World J Hepatol 2016; 8(8): 411-420
- URL: https://www.wjgnet.com/1948-5182/full/v8/i8/411.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i8.411
Despite recent advances in liver surgery, resection for tumors involving inferior vena cava (IVC) is still challenging[1-4]. This situation requires total hepatic vascular exclusion (THVE) for combined resection of the IVC. The THVE method was developed to control bleeding during hepatic parenchymal transection[5,6]; however, it is technically complicated and may cause profound liver damage and circulatory instability, and as a result inflow occlusion via Pringle’s maneuver is more frequently used to control bleeding during usual hepatectomy, rather than THVE[7]. While THVE can effectively limit blood loss during hepatic parenchymal transection, especially that from hepatic veins[8,9], its related rates of morbidity and mortality after hepatectomy for tumors involving IVC have been quite high[10,11]. Novel strategies to reduce the risk of damage and complications of THVE will benefit clinical practice and patient outcome.
Long occlusion time is one of the most significant hazards of operative morbidity and mortality after hepatectomy with THVE[2]. There is no doubt about the importance of minimizing duration of THVE to reduce ischemic damage of the liver. Different from the procedure of portal triad clamping applied alone, addition of THVE necessitates taking into account both hepatic ischemia and instability of systemic circulation, including renal congestion. Selective hepatic vascular exclusion with hepatic venous occlusion preserving IVC flow is an alternative of THVE in usual hepatectomy[12,13], but the method cannot be used for tumors involving IVC. Reports describing technical modifications aimed at minimizing hazards of THVE are available in the publicly available literature, but they are small in number[8,14]. Herein, we describe some technical modifications of THVE that were applied to patients in our hospital and which were found by retrospective analysis to have successfully minimized the duration and the extent of vascular occlusion. The purpose of reporting these results is to share these methods with clinicians and researchers in the field of hepatology so as to help reduce the rates of disastrous events during hepatectomy requiring THVE.
From January 2010 to April 2015, 301 patients underwent hepatectomy at our hospital. Patients who underwent hepatectomy with THVE or modified THVE with IVC cross-clamping were selected for retrospective analysis, and those patients who had required only a small portion of the IVC to be resected without cross-clamping of the IVC were excluded from the study. A total of 8 patients (2.7% of the 301 hepatectomized patients) fit the criteria for inclusion, namely tumors involving IVC and treatment by combined resection of the IVC.
For all 8 patients, tumor status had been evaluated using three-phase contrast-enhanced computed tomography (CT). Contrast-enhanced magnetic resonance imaging (MRI) and/or (18F)-fluoro-D-glucose positron emission tomography had been performed in addition as necessary. The indication of THVE during hepatectomy was massive tumor involvement of IVC or tumor involvement of major hepatic vein extending to its confluence on IVC. Tumors that had been deemed as necessitating resection by side clamping of the IVC were excluded from the indication of THVE. THVE was not used merely for controlling bleeding from the parenchymal transection plane.
By dissecting the coronary ligament around the suprahepatic IVC just below the diaphragm, the outer wall of the IVC was made visible. Ligation and division of infradiaphragmatic veins had been made as necessary. For all 8 patients, the attachment between the liver and diaphragm was dissected, and the dorsal space of the suprahepatic IVC was dissected. Subsequently, the suprahepatic IVC was encircled gently and taped in preparation for cranial cross-clamping of the IVC. The infrahepatic IVC was also dissected circumferentially and encircled in preparation for caudal cross-clamping. Principally, THVE was not applied before completion of hepatic parenchymal transection. The final step of resection involved clamping of the caudal and cranial IVC prior to the resection and reconstruction of the involved IVC via THVE.
The abdomen was opened via J-shape incision that included upper median and right oblique incisions. Tumor status and the relation between the tumors and major intrahepatic vasculature were confirmed by intraoperative ultrasonography. The liver was mobilized to the extent necessary for the planned surgery. The hepatic parenchyma was transected using the clamp-crushing method. Thin vessel branches were burned by electrocautery or soft coagulation devices. Thicker branches were ligated and divided. Intermittent Pringle’s maneuver was applied routinely during the hepatic parenchymal transection using a 15-min/5-min period of clamping and release.
The recorded postoperative complications were classified according to severity by using the Clavien-Dindo criteria (grades I-V). Hepatic failure was defined as serum level of total bilirubin > 5.0 mg/dL (equal to 85.5 μmol/L) at postoperative day 5 or later. Operative mortality was defied as all in-hospital deaths and death within 90 d after surgery.
Follow-up after discharge involved CT or MRI evaluation every 4-6 mo and blood testing and physical examination every 1-6 mo for a total period of 5 years after the last intervention.
All numerical values were calculated as mean and SD or median and range.
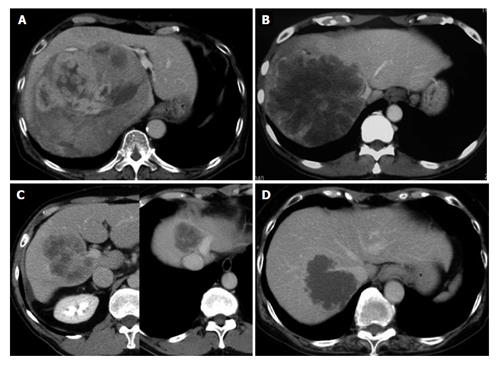
The 8 patients included in this analysis consisted of 6 men and 2 women, with median age of 72-years-old (range: 36-78 years). Seven of the patients had a diagnosis of colorectal liver metastases and 1 had a diagnosis of hepatocellular carcinoma (Table 1). The mean diameter of the total resected tumors was 10.8 ± 7.3 cm. All patients had tumors involving the IVC, with or without confluence of the hepatic veins as shown in Figure 1.
| Case No. | Diagnosis | No. of tumor | Size of the largest tumor, cm | THVE and modifications | THVE duration, min | Hepatectomy | Vascular reconstruction procedures | Outcome |
| 1 | HCC | 1 | 20.0 | Standard with switching1 | 5 | Extended right hepatectomy | Direct suture of IVC | Alive without disease, 60 mo postoperative |
| 2 | Liver metastasis | 1 | 20.0 | Standard with switching1 | 9 | Extended right hepatectomy | IMV patch for HV, Direct suture of IVC | Alive with disease, 17 mo postoperative |
| 3 | Liver metastasis | 2 | 9.0 | Standard with switching1 | 23 | Extended right hepatectomy | IMV patch for HV and IVC | Alive with disease, 10 mo postoperative |
| 4 | Liver metastasis | 1 | 6.0 | Oblique2 (right) | 26 | Right hepatectomy | IMV patch graft for IVC | Died of disease, 27 mo postoperative |
| 5 | Liver metastasis | 4 | 2.5 | Oblique2 (right) | 20 | Partial resection | IMV patch graft for IVC and HV | Alive without disease, 15 mo postoperative |
| 6 | Liver metastasis | 10 | 18.0 | Oblique2 (right) | 7 | Right hepatectomy, partial resection | Direct suture of IVC | Died of disease, 8 mo postoperative |
| 7 | Liver metastasis | 15 | 5.5 | Oblique2 (right) | 7 | Extended anterior sectionectomy, partial resection | Direct suture of IVC | Died of disease, 9 mo postoperative |
| 8 | Liver metastasis | 9 | 5.5 | Oblique2 (left) | 10 | Segmentectomy, partial resection | Direct suture of IVC and HVs | Alive without disease, 13 mo postoperative |
The surgical procedures used for all 8 patients are listed in Table 1. For all, the initial surgical step was broad dissection of the space behind the IVC, after which the liver was lifted up by the surgeon’s left hand to move it from the retrocaval space in order to control bleeding before and during THVE (retrocaval liver lifting maneuver). Standard THVE was applied to 3 patients (cases 1-3), for which the cranial cross-clamp for THVE was switched from the suprahepatic to retrohepatic levels or to the confluence of the major hepatic vein to shorten the duration of THVE (switching the cross-clamp). For the remaining 5 patients (cases 4-8), total ischemia of the whole liver was avoided by applying the cranial clamp obliquely to maintain the hepatic venous outflow of the liver remnant (oblique cross-clamping). Detailed descriptions of these techniques are provided below.
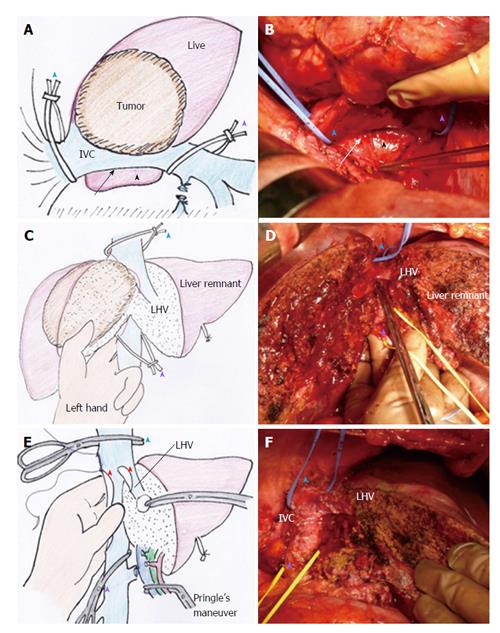
Retrocaval liver lifting maneuver: The liver cannot be detached from the IVC when the tumor involves the IVC. Instead, the retrocaval space behind the hepatic IVC should be dissected broadly to facilitate performance of standard or modified THVE (Figure 2A and B). In all 8 patients of this study, the IVC was compressed ventrally and the liver was lifted up from the retrocaval space by the surgeon’s left hand (Figure 2C and D). This maneuver was quite useful for controlling bleeding during transection of the hepatic parenchyma near the IVC. With the help of this maneuver parenchymal transection can be completed safely before applying THVE, as shown in Figure 2D. The THVE procedure was applied at the last step of the combined resection and reconstruction of IVC, with the involved part of the IVC excised en-bloc with the liver specimen (Figure 2E). Theoretically, no bleeding should come from the IVC under THVE, but a significant amount of backflow bleeding often comes from the cut orifice of IVC during THVE. For all 8 patients in this study, compressing the IVC upward from the retrocaval space, by means of the surgeon’s left hand, was effective for controlling backflow bleeding from the cut orifice of the IVC under THVE (Figure 2E).
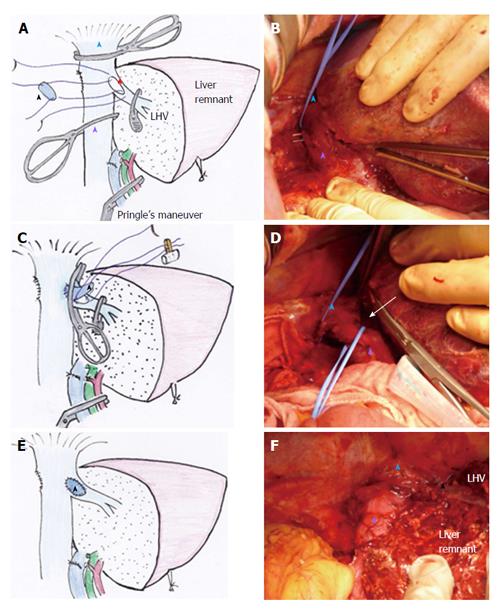
Switching the cross-clamp: Standard THVE cannot be avoided for combined resection of IVC involving the major hepatic vein of the liver remnant, as was performed in case 3 of this study (Figure 3A). After removal of the specimen and completion of the repair of the IVC under standard THVE, the position of the cranial IVC cross-clamp was switched from the suprahepatic IVC to the IVC confluence of the resected hepatic vein to be reconstructed (Figure 3C). Then, the IVC blood flow was restored to stabilize systemic hemodynamics.
Another surgical situation in this study involved switching of the cross-clamp of the suprahepatic IVC to the retrohepatic level, as performed in case 1. The tumor status is shown in Figure 1A. At the first step of this intervention, standard THVE was applied for resection of large hepatocellular carcinoma that involved retrohepatic IVC massively. Both the tumor and the involved wall of the IVC were resected under standard THVE. After repairing the IVC defect around the confluence of the hepatic vein, the suprahepatic IVC cross-clamp was switched to the level just below the hepatic vein confluence. Then, the portal triad occlusion was released to restore the blood flow of the liver remnant, after which the retrohepatic IVC was repaired without prolonging the ischemic time of the liver.
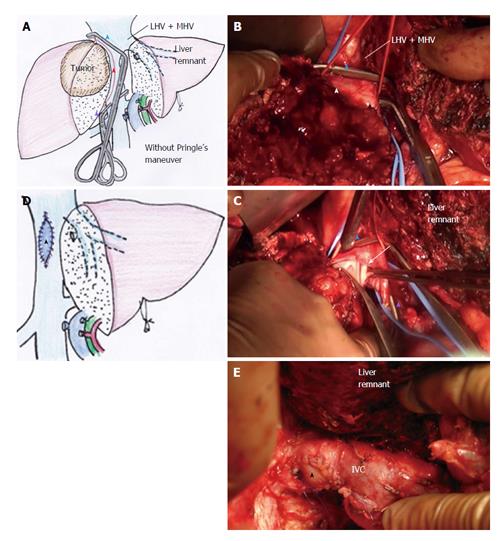
Oblique cranial cross-clamping: When the space between the right and middle hepatic veins is free from involvement, venous drainage of the residual liver can be preserved during vascular exclusion by applying the cranial cross-clamping obliquely (Figure 4A). For this purpose, the retrocaval space must be dissected sufficiently in advance, same as shown in Figure 2A. Prior to this step, hepatic parenchymal transection had been completed to facilitate visualization of the anterior aspect of the hepatic IVC (Figure 4A and B). In the next step, the vascular clamp was inserted behind the IVC obliquely, preserving the outflow orifice of the hepatic vein of the liver remnant (Figure 4A and B). By applying this modification of the THVE procedure, ischemic damage was avoided, as was intestinal congestion. In patients of this study for whom the right liver tumor involved the IVC massively around the orifice of right hepatic vein (RHV) (as in cases 4-7), the cranial IVC cross-clamp was applied from the right cranial side to the left caudal side of the IVC to allow venous drainage of the trunk of middle and left hepatic veins (MHV + LHV) (Figure 4A). In patients of this study for whom the left liver tumor involved the trunk of MHV + LHV (as in case 8), the clamp was applied from the left cranial line to the right caudal line to preserve the outflow of the RHV. In the 5 patients who represented these two special situations, the THVE duration presented in Table 1 equates to the occlusion time of oblique cross-clamping of the IVC.
The mean values of the operative parameters for the total 8 patients in this study are presented in Table 2. In no case did the THVE time exceed 30 min. Results from biochemical liver function tests and prothrombin time indicated the smooth recovery of liver function during the first postoperative week. In no case did the maximum serum total bilirubin level exceed 5 mg/mL, and no patient developed liver failure. For all of the patients, the serum total bilirubin levels gradually decreased during the first postoperative week to below 2 mg/mL (equal to 34.2 μmol/L) by day 7. No patient showed a prothrombin time less than 50% at any time during the postoperative period. In addition, no patient experienced a complication of Clavien-Dindo grade III or higher and there was no case of operative mortality. The postoperative complications that occurred included leg edema, pleural effusion, refractory ascites, and wound infection (n = 1 each). No patient required reoperation, subsequent surgical or radiological interventions, or management in the intensive care unit, and all patients were discharged within a month after the surgery. All of the reconstructed vessels remained patent through the end of follow-up.
| Parameters | Values or number of patients |
| Operation time | 482 ± 108 min |
| Blood loss | 1778 ± 1233 mL |
| THVE duration | 13.4 ± 8.4 min |
| Postoperative liver function | |
| TB (mg/dL) | |
| Maximum | 2.01 (0.98-4.4) |
| POD7 | 1.08 (0.75-1.86) |
| AST (IU/L) | |
| Maximum | 513 (238-1058) |
| POD7 | 30 (20-41) |
| ALT (IU/L) | |
| Maximum | 319 (179-824) |
| POD7 | 64 (36-115) |
| PT (%) | |
| Minimum | 64.4 (55.3-88.8) |
| POD7 | 88.1 (61.6-107.8) |
| Complications | |
| Clavien-Dindo classification | |
| I | 2 |
| II | 1 |
| ≥ III | 0 |
| Operative mortality | 0 |
| Hospital stay (d) | 15 (12-24) |
All patients returned to usual active life after discharge. The 1-year survival rate was 71%. Individual outcomes for each patient are presented in Table 1. At the date of this report, case 1 was alive without disease (5 years after surgery) and cases 6 and 7 presented with early multiple tumor recurrence in the liver remnant and died of rapid progression at 8 and 9 mo respectively.
Liver tumors involving IVC or its junction of major hepatic veins represent situations in which THVE is needed for curative resection. Such surgery is fraught with challenges, including unstable systemic circulation, ischemic damage of the liver and high risk of morbidity. Minimizing the duration and extent of exclusion is key to increasing the safety of THVE[2]. To this end, the present study highlights the feasibility of three technical modifications of THVE. The techniques include: (1) retrocaval liver lifting maneuver; (2) switching the cross-clamp; and (3) oblique cranial cross-clamping, which minimized duration of THVE and ischemic damage of the liver. It is noteworthy that application of these modified techniques minimized occlusion time; in addition, recovery of liver function was smooth, no severe complication developed, and operative mortality was not experienced. These operative results are quite favorable, particularly when compared to those reported by other studies[1-3,8,10,11].
To date, only a limited number of studies of THVE for resection of liver tumors involving IVC are present in the publicly available literature, probably because the number of experiences in individual institutes has been small. The most distinctive features of the present study are the extremely short duration of THVE and favorable recovery after surgery. While the mean durations of THVE were 29 min to 78 min in the reports from very experienced institutes[1,2,7,8], the mean THVE duration in the present study was only 13.4 ± 8.4 min. The mortality rates of hepatectomy with IVC resection were 4.5% to 25% in the previous reports[1-4,8,10,11,15]. In particular, the morbidity and mortality rates were quite high when standard THVE was applied frequently, even when hypothermic perfusion was used to attenuate the ischemic liver damage. In the previous reports, the major causes of mortality were liver failure and sepsis[1,3,4,8,11], both of which are likely to be relevant to ischemic injury of the liver and intestinal congestion since they may facilitate bacterial translocation. Of course, simple comparison to the present case series is not possible due to the differences in severity of tumor status and underlying conditions. Nevertheless, minimized duration of THVE might have contributed to the favorable postoperative course in the present study.
Hand manipulation of the IVC from the broadly dissected retrocaval space is a unique method to decrease bleeding around the IVC. This procedure is also essential as a preparation for modifying the THVE procedure to improve its safety. When the tumor involves the IVC, the liver cannot be freed from the IVC and Belghiti’s liver hanging maneuver is not possible (and is rather a contraindication)[16]. In such a situation, wide dissection of the retrocaval space makes subsequent procedures safer. Lifting-up the liver by hand from the retrocaval space proved quite useful to control bleeding from the hepatic parenchyma near the IVC and backflow bleeding from the cut orifice of the IVC during THVE. Although no bleeding is supposed to come from the IVC under THVE theoretically, significant amount of backflow bleeding, which occurs frequently, serves to disrupt and complicate the vascular reconstruction procedure of IVC. Sources of such backflow bleeding are lumbar veins, short hepatic veins, or small venous branches that extend into the major hepatic veins. Even with portal triad occlusion by Pringle’s maneuver, blood flow into the liver from the diaphragm or lesser omentum can exist. Compressing the IVC by hand, from the retrocaval space, was shown in the present study to be quite useful and the only way to control backflow bleeding during THVE.
The damage associated with THVE includes both systemic circulatory instability (due to absence of venous return via the IVC) and total cessation of hepatic blood flow. These conditions cause congestion of the kidney and intestine, which may explain why the damage and morbidity after THVE is much higher than that experienced after inflow occlusion alone[7]. In the current study, when the cranial IVC cross-clamp was switched from the supra- to the retrohepatic IVC (as performed in case 1), blood flow of the liver remnant was restored, thereby shortening the ischemic time of the liver remnant and intestine. When the clamp was switched to the confluence of hepatic vein of the liver remnant (as performed in case 3), IVC blood flow was restored, thereby resolving the systemic circulatory instability and renal congestion.
Oblique cranial IVC clamping is an option of standard THVE to avoid ischemic damage of the liver remnant as well as intestinal congestion; yet, this technique has not been precisely described in the literature. Even in the series of patients in this study with tumors with massive IVC involvement, the hepatic vein of the liver remnant side was free from involvement in many of the cases, and this is reported in other studies as well[1,8]. Such a situation is good indication for oblique cross-clamping. Sufficient dissection of the retrocaval space and completion of hepatic parenchymal transection in advance are essential for application of this method.
The timing of applying THVE may be one of the most important issues underlying its efficacy and safety. In most of the studies reported in the literature, THVE was applied during both parenchymal transection and combined resection/reconstruction of IVC[1,7,8]. This might be one of the reasons for the characteristic long occlusion time in the studies previous to ours. The primary policy of our hospital, however, is to make every effort to minimize the duration of THVE. Because the retrocaval liver lifting maneuver makes it possible to complete the hepatic parenchymal transection without much bleeding, THVE was applied at the final step only for IVC resection and reconstruction. We believe that its success depends primarily on the procedures that had been applied prior to the application of THVE, including sufficient dissection of the retrocaval space and smooth completion of the hepatic parenchymal transection.
The small number of included patients may be a limitation of the present study. In general, the number of patients requiring THVE for hepatectomy with IVC reconstruction is rather small for a single institute, such as ours, as reflected in the previous studies[1-4,10,11,15]. The accumulated literature on this topic includes no reports that provide a definitive description of modified procedures of THVE, especially the retrocaval liver lifting maneuver and oblique cranial cross-clamping that are described here for the first time. These techniques can be strategies to decrease the risk of disastrous bleeding and ischemic liver damage. Sharing these data with other surgeons will serve to increase the safety and feasibility of surgery for liver tumors involving IVC.
In conclusion, the retrocaval liver lifting maneuver and modifications of IVC cross-clamping are useful to attenuate damage related to THVE. The knowledge and techniques that have arisen from the current analysis of our case series may help to improve the surgical techniques and outcomes for advanced liver tumors involving IVC.
Total hepatic vascular exclusion (THVE) is needed during hepatectomy for liver tumors involving inferior vena cava (IVC). However, THVE carries a much greater risk than inflow occlusion alone.
The authors performed technical modifications that shortened the duration of THVE, thereby reducing the risk of damage.
The technical modifications described in this study for THVE included the retrocaval liver lifting maneuver, switching of the cranial IVC cross-clamp, and oblique cranial IVC cross-clamping. With these techniques, the mean duration of THVE was shortened remarkably, compared to that reported in the past literature. Moreover, postoperative recovery of liver function was smooth without any severe complications.
The retrocaval liver lifting maneuver and modifications of cranial cross-clamping minimized the duration of THVE. Thus, these techniques might might help to decrease risk of liver damage and increase likelihood of favorable postoperative courses in patients who undergo hepatectomy requiring THVE.
THVE is a method to control bleeding during hepatectomy by occluding both inflow and outflow of the liver. THVE is required especially for resection of liver tumors involving IVC.
The authors suggest some technical modifications of the THVE. The study is clearly planned and correctly managed. The technical details of the three variations are clearly explained as well as the figures.
P- Reviewer: Aurello P S- Editor: Gong ZM L- Editor: A E- Editor: Liu SQ
| 1. | Azoulay D, Andreani P, Maggi U, Salloum C, Perdigao F, Sebagh M, Lemoine A, Adam R, Castaing D. Combined liver resection and reconstruction of the supra-renal vena cava: the Paul Brousse experience. Ann Surg. 2006;244:80-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Azoulay D, Pascal G, Salloum C, Adam R, Castaing D, Tranecol N. Vascular reconstruction combined with liver resection for malignant tumours. Br J Surg. 2013;100:1764-1775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Madariaga JR, Fung J, Gutierrez J, Bueno J, Iwatsuki S. Liver resection combined with excision of vena cava. J Am Coll Surg. 2000;191:244-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Hemming AW, Mekeel KL, Zendejas I, Kim RD, Sicklick JK, Reed AI. Resection of the liver and inferior vena cava for hepatic malignancy. J Am Coll Surg. 2013;217:115-124; discussion 124-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Heaney JP, Stanton WK, Halbert DS, Seidel J, Vice T. An improved technic for vascular isolation of the liver: experimental study and case reports. Ann Surg. 1966;163:237-241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 179] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Huguet C, Nordlinger B, Galopin JJ, Bloch P, Gallot D. Normothermic hepatic vascular exclusion for extensive hepatectomy. Surg Gynecol Obstet. 1978;147:689-693. [PubMed] [Cited in This Article: ] |
| 7. | Belghiti J, Noun R, Zante E, Ballet T, Sauvanet A. Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Ann Surg. 1996;224:155-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 263] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Emre S, Schwartz ME, Katz E, Miller CM. Liver resection under total vascular isolation. Variations on a theme. Ann Surg. 1993;217:15-19. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 78] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Smyrniotis VE, Kostopanagiotou GG, Gamaletsos EL, Vassiliou JG, Voros DC, Fotopoulos AC, Contis JC. Total versus selective hepatic vascular exclusion in major liver resections. Am J Surg. 2002;183:173-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Dubay D, Gallinger S, Hawryluck L, Swallow C, McCluskey S, McGilvray I. In situ hypothermic liver preservation during radical liver resection with major vascular reconstruction. Br J Surg. 2009;96:1429-1436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Malde DJ, Khan A, Prasad KR, Toogood GJ, Lodge JP. Inferior vena cava resection with hepatectomy: challenging but justified. HPB (Oxford). 2011;13:802-810. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Fu SY, Lai EC, Li AJ, Pan ZY, Yang Y, Sun YM, Lau WY, Wu MC, Zhou WP. Liver resection with selective hepatic vascular exclusion: a cohort study. Ann Surg. 2009;249:624-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Cherqui D, Malassagne B, Colau PI, Brunetti F, Rotman N, Fagniez PL. Hepatic vascular exclusion with preservation of the caval flow for liver resections. Ann Surg. 1999;230:24-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 89] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Azoulay D, Maggi U, Lim C, Malek A, Compagnon P, Salloum C, Laurent A. Liver resection using total vascular exclusion of the liver preserving the caval flow, in situ hypothermic portal perfusion and temporary porta-caval shunt: a new technique for central tumors. Hepatobiliary Surg Nutr. 2014;3:149-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 8] [Reference Citation Analysis (0)] |
| 15. | Lodge JP, Ammori BJ, Prasad KR, Bellamy MC. Ex vivo and in situ resection of inferior vena cava with hepatectomy for colorectal metastases. Ann Surg. 2000;231:471-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 126] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Belghiti J, Guevara OA, Noun R, Saldinger PF, Kianmanesh R. Liver hanging maneuver: a safe approach to right hepatectomy without liver mobilization. J Am Coll Surg. 2001;193:109-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 344] [Article Influence: 15.0] [Reference Citation Analysis (0)] |