Published online Aug 28, 2016. doi: 10.4254/wjh.v8.i24.1028
Peer-review started: March 24, 2016
First decision: May 23, 2016
Revised: June 4, 2016
Accepted: July 20, 2016
Article in press: July 22, 2016
Published online: August 28, 2016
Processing time: 154 Days and 21.2 Hours
To build a diagnostic non-invasive model for screening of large varices in cirrhotic hepatitis C virus (HCV) patients.
This study was conducted on 124 post-HCV cirrhotic patients presenting to the clinics of the Endemic Medicine Department at Mansoura University Hospital for evaluation before HCV antiviral therapy: 78 were Child A and 46 were Child B (score ≤ 8). Inclusion criteria for patients enrolled in this study was presence of cirrhotic HCV (diagnosed by either biopsy or fulfillment of clinical basis). Exclusion criteria consisted of patients with other etiologies of liver cirrhosis, e.g., hepatitis B virus and patients with high MELD score on transplant list. All patients were subjected to full medical record, full basic investigations, endoscopy, and computed tomography (CT), and then divided into groups with no varices, small varices, or large risky varices. In addition, values of Fibrosis-4 score (FIB-4), aminotransferase-to-platelet ratio index (APRI), and platelet count/splenic diameter ratio (PC/SD) were also calculated.
Detection of large varies is a multi-factorial process, affected by many variables. Choosing binary logistic regression, dependent factors were either large or small varices while independent factors included CT variables such coronary vein diameter, portal vein (PV) diameter, lieno-renal shunt and other laboratory non-invasive variables namely FIB-4, APRI, and platelet count/splenic diameter. Receiver operating characteristic (ROC) curve was plotted to determine the accuracy of non-invasive parameters for predicting the presence of large esophageal varices and the area under the ROC curve for each one of these parameters was obtained. A model was established and the best model for prediction of large risky esophageal varices used both PC/SD and PV diameter (75% accuracy), while the logistic model equation was shown to be (PV diameter × -0.256) plus (PC/SD × -0.006) plus (8.155). Values nearing 2 or more denote large varices.
This model equation has 86.9% sensitivity and 57.1% specificity, and would be of clinical applicability with 75% accuracy.
Core tip: Hepatitis C virus infection is a major global health problem, with over 14% of the Egyptian population currently infected. End-stage liver disease with cirrhosis is commonly complicated by potentially life-threatening esophageal varices, which require regular screening by endoscopy. However, this invasive procedure is burdened by patient non-compliance and possible complications, thus prompting the search for alternative non-invasive yet accurate means of diagnosis. This study group aimed to assess the use of computed tomography to evaluate and grade variceal size, and to compare its diagnostic value with other non-invasive predictors of portal hypertension, such as platelet count to splenic diameter ratio, aminotransferase-to-platelet ratio index, and Fibrosis-4 score.
- Citation: Elalfy H, Elsherbiny W, Abdel Rahman A, Elhammady D, Shaltout SW, Elsamanoudy AZ, El Deek B. Diagnostic non-invasive model of large risky esophageal varices in cirrhotic hepatitis C virus patients. World J Hepatol 2016; 8(24): 1028-1037
- URL: https://www.wjgnet.com/1948-5182/full/v8/i24/1028.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i24.1028
Hepatitis C virus (HCV) represents one of the major health problems affronting the medical community today, with chronic HCV infection affecting approximately 130-170 million people globally, or about 2%-3% of the world’s population[1]. The largest HCV epidemic is currently found in Egypt, with an estimated national prevalence reported to be 14.7%[2]. As with any chronic liver disease, the end stage of chronic HCV infection is cirrhosis, ultimately complicated by portal hypertension, an established contributing factor in the evolution of a variety of complications of cirrhosis including ascites, hepatic encephalopathy, and esophageal varices[3].
Portal hypertension generates development of porto-systemic collaterals, giving rise to esophageal varices (OV), most notably gastroesophageal varices because of their enhanced tendency for bleeding[4]. Esophageal varices can be found in 60%-80% of cirrhotic patients[5], with variceal hemorrhage presenting as the most devastating complication of cirrhosis. Because of this dramatic course of events, it is imperative to prevent variceal bleeding either with non-selective beta-blockers or endoscopic variceal ligation[6]. However, in spite of recent progress, mortality rate due to bleeding from ruptured esophageal varices remains between 10%-20%[7].
Current guidelines advocate screening for esophageal varices in all cirrhotic patients at the time of diagnosis. Lack of detection of esophageal varices at the first endoscopic evaluation warrants repeat endoscopy annually in patients with decompensated liver cirrhosis and every 2-3 years in patients with compensated cirrhosis[8]. Although upper endoscopy is regularly performed and conveys a diminished risk of adverse effects[9], repeated endoscopies are associated with several side effects including aspiration, perforation, and bacteremia[10]. Furthermore, these recommendations impose a huge burden on medical resources and branch from expert assumption rather than being evidence-based. In addition to the invasive nature of the procedure and lack of patient compliance restricting its use, there is also a cost-ineffectiveness of this policy in lack of actual detection of varices in many of the patients[11]. These considerations have spurred several attempts to identify non-invasive clinical, radiological, and biochemical parameters, used either separately or in conjunction, to determine the presence of portal hypertension and esophageal varices.
Perhaps the best predictor of esophageal varices developed to date is the platelet count to splenic diameter ratio, which proposes linking thrombocytopenia to spleen size by considering that diminished platelet count is probably the result of hypersplenism due to splenomegaly caused by portal hypertension[12]. Other parameters have attempted to determine the state of liver tissue with good accuracy by evaluating the extent of fibrosis and cirrhosis as a predictive indicator of progression of portal hypertension, these including aminotransferase-to-platelet ratio index (APRI), Fibroindex, and Fibrosis-4 score (FIB-4)[13].
Several radiological techniques have also been suggested for evaluation of esophageal varices. Doppler ultrasonography has been used for investigating portal and hepatic hemodynamics but its value in assessment of portal hypertension remains obscure. Although several indices for portal hypertension have been commonly used, inaccuracy remains due to fluctuating variations related to both observer and equipment[14].
Computed tomography (CT) has also been proposed as an evaluation tool for esophageal varices[15]. Examination of the correlation between CT findings and endoscopy from previous studies has shown better agreement between variceal size and radiological assessment than with endoscopic interpretation[16]. In addition, CT was found to be more desirable in initial screening of esophageal varices in comparison to endoscopy when considering patient preference and cost-effectiveness[17].
Therefore, considering these findings, we aimed to evaluate the use of CT in the diagnosis of esophageal varices, differentiating between small and large varices, and assessing its use in grading the size of varices. In addition, we aimed to compare the value of CT in diagnosis of esophageal varices with other non-invasive predictors of portal hypertension including laboratory indices such as platelet count to splenic diameter ratio, APRI, and FIB-4.
The objective of the study was to build a diagnostic non-invasive model for screening of large esophageal varices in cirrhotic HCV patients.
Informed consent was taken from each patient. The research protocol was approved by the Ethical Committee of Faculty of Medicine, Mansoura University.
This comparative cross sectional study included subjects presenting to the Endemic Medicine Department clinic at Mansoura University Hospital for evaluation before HCV antiviral therapy during the period between December 2014 and June 2015. Inclusion criteria for patients enrolled in this study was presence of cirrhotic HCV as diagnosed either by biopsy (F4) or on the basis of clinical evaluation combined with laboratory findings and ultrasonography. Exclusion criteria consisted of patients with other etiologies of liver cirrhosis or those ineligible for the HCV therapy program, e.g., HBV, Child C decompensated patients, and patients with high MELD score on transplant list. Patients with liver cirrhosis were then stratified according to endoscopic findings into groups with either no varices, small varices, or large varices.
The indication for CT imaging in the majority of cases was for evaluation of focal lesions for hepatocellular carcinoma while the entire laboratory assessment was done as a part of the HCV therapeutic evaluation program.
All subjects were HCV infected and thus subjected to complete laboratory assessment before antiviral therapy, including complete blood picture, PCR for HCV, alpha fetal protein (AFP), alanine and aspartate transaminases, albumin, bilirubin, INR, creatinine, TSH, as well as abdominal ultrasound and biopsy in selected cases.
Those with findings of F4 on biopsy or showed clinical, laboratory, or ultrasonographic features of cirrhosis were selected for this study, to be then classified into case and control groups.
Cases were patients with post-HCV liver cirrhosis with esophageal varices on endoscopy, divided into two groups with either small or large varices, while the control group was patients with post-HCV liver cirrhosis without varices.
Using slight sedation with IV midazolam administered just before the session, patients were stratified by risk of first variceal hemorrhage into either high-risk patients, i.e., those with medium/large varices, or low risk patients, i.e., those with small varices occurring in a Child A or B patient. Trials have shown that patients with medium/large varices can be treated with either non-selective β-blockers (propranolol, nadolol) or esophageal band ligation.
APRI = {[AST Level (IU/L)]/[AST (upper limit of normal) (IU/L)] × 100}/Platelet count (109/L)[18]
FIB4 = [age (years) × AST (IU/L)]/[PLT (× 109/L)] × [√ALT (IU/L)][19,20].
Platelet count (PC) to spleen diameter (SD) ratio = PC (N/μL)/the maximum bipolar diameter of the spleen (mm)[21].
These parameters were selected based on the criteria of being simple routine laboratory tests that are also inexpensive.
For all patients, multi-slice detector CT (MDCT) scan of the abdomen and pelvis was performed on a 16-MDCT scanner (Brilliance, Philips) using a tube collimation of 16 mm × 1.5 mm with overlapping reconstruction at 2 mm slice thickness and 0.8 mm increment.
Examination was carried out using a multiphasic liver protocol starting with non-contrast examination. Arterial phase examination was carried out using bolus tracking technique and post-threshold delay of 12 s. Low osmolar iodinated intravenous contrast [Omnipaque™ (iohexol) 350, GE Healthcare] was injected using a power injector [MEDRAD Vistron CT® Injector, Medrad] administered in a dose of 1.5 mL/kg at a flow rate of 4-5 mL/s. Portal phase examination was carried out 40 s after threshold and delayed phase examination after 5 min.
Images were reviewed on a dedicated workstation (Extended brilliance work space, Philips) in axial, coronal and sagittal planes. Images were evaluated for the following parameters: Maximum short axis diameter of the largest visible esophageal varix, diameter of coronary vein, diameter of the paraumbilical vein, maximum short axis diameter of the portal vein at the portahepatis, presence of ascites, and maximum height of the spleen. An esophageal varix was defined as an enhancing intramural nodular tubular structure (which may be bulging into the lumen of the esophagus or runs within the inner esophageal mucosa).
Data were statistically analyzed using the Statistical Package for Social Science (SPSS) version 20. The quantitative data were presented in the form of mean and standard deviation. One-way Anova was used to compare between the three groups. χ2 test was used to compare the qualitative data. Receiver operating curve (ROC) was done to determine a cut-off point predicting large varices. Logistic regression was done to construct a model for predicting the occurrence of large varices. Significance was considered at P value of 0.05.
A total of 124 patients with hepatic cirrhosis were included in this study. The mean age of the included patients were 56.52 ± 5.759 (range 37-66) years with 26 patients (52%) being males. The etiology of cirrhosis in all included patients was HCV. Most patients (59.7%) had esophageal varices and 50 patients (40.3%) had no varices. According to gastroscopy, among those who had esophageal varices, 28 patients (22.6%) were classified as having small varices and 46 patients (37.1%) had large varices. According to Child-Turcotte-Pugh Classification, 78 patients (62.9%) were classified as class A, proven by liver biopsy and 46 (37.1%) as class B. There were 34 patients with diuretic responsive ascites (27.5%) and 90 patients (72.5%) without ascites. Ten patients (8.06%) had hepatocellular carcinoma less than 3 cm (Table 1).
| Variables | All patients (n = 124) | No varices (n = 50) | Small varices (n = 28) | Large varices (n = 46) | P value |
| Age, mean ± SD | 56.52 ± 5.759 | 57.28 ± 4.513 | 58.57 ± 3.072 | 54.43 ± 7.423 | 0.005 |
| Gender | |||||
| Female | 52 (41.9) | 24 (48.0) | 12 (42.9) | 16 (34.8) | 0.421 |
| Male | 72 (58.1) | 26 (52) | 16 (57.1) | 30 (65.2) | |
| Laboratory data (mean ± SD) | |||||
| Serumalbumin (g/dL) | 3.329 ± 0.43 | 3.452 ± 0.404 | 3.271 ± 0.352 | 3.229 ± 0.474 | 0.028 |
| Serum bilirubin (mg/dL) | 1.312 ± 0.572 | 1.093 ± 0.534 | 1.414 ± 0.591 | 1.489 ± 0.532 | 0.001 |
| INR | 1.326 ± 0.238 | 1.249 ± 0.225 | 1.431 ± 0.24 | 1.347 ± 0.226 | 0.003 |
| Serum creatinine (mg/dL) | 0.999 ± 0.179 | 0.956 ± 0.169 | 0.994 ± 0.178 | 1.047 ± 0.180 | 0.044 |
| AST (IU/L) | 68.00 ± 40.086 | 65.84 ± 44.072 | 63.71 ± 22.993 | 72.96 ± 43.797 | 0.561 |
| ALT (IU/L) | 55.18 ± 23.607 | 51.84 ± 19.844 | 57.43 ± 24.848 | 57.43 ± 26.519 | 0.436 |
| Platelet count (cells/mm3) | 152.94 ± 22.012 | 145.96 ± 20.833 | 156.5 ± 27.941 | 1548.35 ± 17.071 | 0.001 |
| AFP (ng/mL) | 2.687 ± 2.687 | 3.646 ± 60.527 | 5.868 ± 1.854 | 3.274 ± 811.945 | 0.064 |
| Clinical data | |||||
| Spleen size (mm) | 152.94 ± 22.012 | 145.96 ± 20.833 | 156.5 ± 27.941 | 158.35 ± 17.071 | 0.013 |
| Presence of ascites | 0.017 | ||||
| Mild (respo-nsive) | 34 (27.5) | 6 (12) | 8 (28.5) | 20 (43.4) | |
| No | 90 (72.6) | 44 (88) | 20 (71.4) | 26 (56.5) | |
| MELD score (mean ± SD) | 1.103 ± 2.828 | 9.852 ± 2.480 | 1.2 ± 2.55 | 1.171 ± 2.942 | 0.001 |
| History of encepha-lopathy | |||||
| No | 124 (100) | 50 (100) | 28 (100) | 46 (100) | |
| Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Child Pugh Classification | |||||
| A | 78 (62.9) | 42 (84.3) | 18 (64.3) | 18 (39.1) | 0.001 |
| B | 46 (37.1) | 8 (16) | 10 (35.7) | 28 (60.9) |
The values of Fib4 and APRI were significantly higher in cirrhotic patients with large esophageal varices than those in cirrhotic patients without varices or with small esophageal varices (P = 0.001). Comparison of values of the PC/SD ratio between groups demonstrated a significant decrease in cirrhotic patients with large esophageal varices in comparison to cirrhotic patients without varices or with small esophageal varices (P = 0.001).
Regarding the values of CT parameters, there was a demonstrable difference between groups, as the cirrhotic patients with esophageal varices had higher values of portal vein diameter (PVD) and splenic vein diameter (SVD) than cirrhotic patients without varices (P = 0.012 vs 0.284, respectively). A coronary vein threshold ≥ 7 mm as measured by CT was present in 16 of these cirrhotic patients (12.7%), of which 4 patients were without varices, 4 patients had small varices, and 8 patients had large varices (P = 0.026).While the measurement of lieno-renal shunt by CT was ≥ 12 mm, there were 8 cirrhotic patients (6.45%) without esophageal varices (P = 0.006). In addition, CT significantly differentiated between presence and absence of OV. When CT reported that there were no variceal findings in 50 patients (40.3%), 46 of these were actually without varices and 4 patients had esophageal varices; CT indication of varices in 74 patients (59.7%) was confirmed in 70 of these patients who actually had OV (P = 0.001) (Table 2).
| Variables | All patients (n = 124) | No varices (n = 50) | Small varices (n = 28) | Large varices (n = 46) | P value |
| FIB-4 | 5.526 ± 3.239 | 4.432 ± 2.334 | 4.814 ± 2.457 | 7.149 ± 3.844 | 0.001 |
| APRI | 1.836 ± 1.256 | 1.408 ± 0.84 | 1.606 ± 1.02 | 2.442 ± 1.519 | 0.001 |
| PC/SD | 722.235 ± 316.5 | 891.133 ± 317.027 | 765.016 ± 326.324 | 512.611 ± 150.784 | 0.001 |
| PVD by CT | 14.116 ± 2.967 | 13.142 ± 2.959 | 14.929 ± 3.366 | 14.639 | 0.012 |
| SVD by CT | 10.903 ± 2.857 | 10.42 ± 2.989 | 11.071 ± 3.62 | 11.326 ± 2.063 | 0.284 |
| Coronary vein ≥ 7 mm by CT | 16 (12.7) | 4 (8.0) | 4 (14.29) | 8 (17.39) | 0.026 |
| Lienorenal shunt ≥ 12 mm by CT | 8 (6.45) | 8 (28.57) | 0 (0) | 0 (0) | 0.006 |
| Presence of varices in CT | 0.001 | ||||
| Yes | 74 (59.7) | 4 (8.0) | 28 (100) | 42 (91.3) | |
| No | 50 (40.3) | 46 (92.0) | 0 (0) | 4 (8.7) |
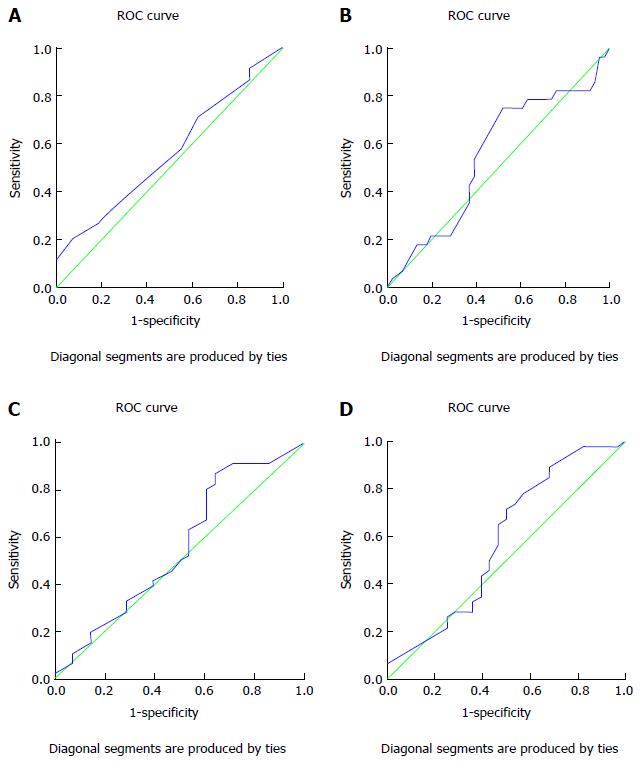
ROC curve was plotted to determine the accuracy of non-invasive parameters for predicting the presence of large esophageal varices rather than presence of varices and the area under the ROC curve for each one of these parameters was obtained. A FIB-4 ≥ 3.13 had a sensitivity of 71.7% and a specificity of 50% with an area under the ROC curve of 0.585 (95%CI: 0.442-0.728). The area under the ROC curve for APRI was 0.558 (95%CI: 0.417-0.699). An APRI value of ≥ 1.083 had a sensitivity of 63% and specificity of 46.4%. A PC/SD ratio of ≤ 806.93 had 75% sensitivity and 47.8% specificity, with the area under the ROC curve being 0.558 (95%CI: 0.417-0.699). In addition, the PVD as measured by CT had a sensitivity of 71.1% and specificity of 37% at cutoff ≥ 12.5 mm with an area under the ROC curve of 0.560 (95%CI: 0.425-0.630) (Table 3). ROC curves are demonstrated in Figure 1.
| Parameters | Sensitivity | Specificity | AUC | 95%CI | Cut-off | Significance |
| FIB-4 | 71.70% | 50% | 0.585 | 0.442-0.728 | 3.13 | 0.222 |
| APRI | 63% | 46.40% | 0.558 | 0.417-0.699 | 1.083 | 0.406 |
| PC/SD | 75% | 47.80% | 0.550 | 0.412-0.688 | 806.93 | 0.472 |
| PVD by CT | 71% | 37% | 0.560 | 0.425-0.695 | 12.5 | 0.396 |
The detection of large risky esophageal varices on the verge of rupture is a multi-factorial process affected by many variables. Statistically, the research team chose to use binary logistic regression. Dependent factors were either large or small varices while the independent factors measured by CT were coronary vein diameter, PVD and lieno-renal shunt in addition to various laboratory parameters including FIB-4, APRI, and PC/SD. The accuracy of this model was about 62.2%. After removal of insignificant predictors, i.e., APRI, FIB-4, coronary vein diameter, and lieno-renal shunt, the accuracy of the model becomes 75%. If only PC/SD was used, the accuracy was 73%, while use of both PC/SD and PVD raised the accuracy to 75.7% (Tables 4-6).
| Variables in the Equation | |||||||||
| B | SE | Wald | df | Sig. | Exp (B) | 95.0%CI for Exp (B) | |||
| Lower | Upper | ||||||||
| Step 11 | APRI | -0.444 | 0.813 | 0.298 | 1 | 0.585 | 0.641 | 0.130 | 3.155 |
| FIB-4 | 0.236 | 0.340 | 0.484 | 1 | 0.487 | 1.267 | 0.651 | 2.465 | |
| PVD by CT | -0.257 | 0.123 | 4.398 | 1 | 0.036 | 0.773 | 0.608 | 0.983 | |
| PC/SD | -0.006 | 0.002 | 8.327 | 1 | 0.004 | 0.994 | 0.990 | 0.998 | |
| Coronary vein diameter by CT | -0.853 | 0.687 | 1.544 | 1 | 0.214 | 0.426 | 0.111 | 1.637 | |
| Lieno-renal vein diameter by CT | -0.747 | 0.805 | 0.860 | 1 | 0.354 | 0.474 | 0.098 | 2.297 | |
| Constant | 10.125 | 3.838 | 6.959 | 1 | 0.008 | 2.497E4 | |||
| Variables in the equation | |||||||||
| B | SE | Wald | df | Sig. | Exp (B) | 95%CI for Exp (B) | |||
| Lower | Upper | ||||||||
| PC/SD1 | PC/SD | -0.005 | 0.001 | 10.721 | 1 | 0.001 | 0.995 | 0.992 | 0.998 |
| Constant | 3.457 | 0.926 | 13.935 | 1 | 0.000 | 31.707 | |||
| PVD by CT and PC/SD2 | PVD by CT | -0.256 | 0.116 | 4.879 | 1 | 0.027 | 0.774 | 0.617 | 0.972 |
| PC/SD | -0.006 | 0.002 | 13.057 | 1 | 0.000 | 0.994 | 0.990 | 0.997 | |
| Constant | 8.155 | 2.465 | 10.942 | 1 | 0.001 | 3.480E3 | |||
A major health problem facing the medical community today is chronic HCV infection, which affects about 2%-3% of the global population, or from 130-170 million people worldwide[1], with Egypt currently bearing the largest HCV epidemic which affects 14.7% of its national population[2]. Cirrhosis represents the end-stage of chronic liver disease, ultimately complicated by portal hypertension[3], the main inducing factor in the formation of esophageal varices[4], found in more than half (60%-80%) of cirrhotic patients[5]. These varices are ensuingly prone to consequent rupture and bleeding, with a devastatingly high mortality rate of 10%-20%[7].
Current guidelines advocate screening for esophageal varices using endoscopy in all cirrhotic patients at the time of diagnosis[8]. However, the invasive nature and subsequent complications associated with this maneuver have prompted the search for further accurate and non-invasive techniques to evaluate the presence of esophageal varices resulting from portal hypertension in these cirrhotic patients.
Founded on the basis that liver fibrosis is the primary factor enhancing hepatic resistance resulting in portal hypertension, use of non-invasive serum markers of liver fibrosis has shown favorable outcomes when predicting presence of esophageal varices[21]. Expected findings from previous studies have demonstrated that scores of FIB-4 and APRI were significantly higher in cirrhotic patients with or without portal hypertension when compared to healthy volunteers or patients with chronic liver disease[3]. In our study, significantly higher values of FIB-4 and APRI were also found in cirrhotic patients with large esophageal varices in comparison to those without varices or with small esophageal varices (P = 0.001).
Although several studies have previously demonstrated a strong relation between platelet count and splenic diameter with presence of esophageal varices[22,23], the decreased platelet count present in chronic liver disease may be the result of several factors other than portal hypertension, including diminished mean platelet life span, reduced production of thrombopoietin, or myelotoxic effects of hepatitis viruses[24]. An additional proposed underlying mechanism of “platelet exhaustion” states that hyperdynamic circulation causes platelet damage during intravascular activation with consequent hypofunction. However, the presence of splenomegaly in patients with cirrhosis is, in all likelihood, derived from vascular derangement mainly resulting from portal hypertension[25].
Consequently, Giannini et al[26] aimed to chart a new parameter bridging thrombocytopenia to splenomegaly so as to originate a variable that takes into account the diminished platelet count probably due to hypersplenism attributed to portal hypertension. A study performed by Giannini et al demonstrated that a PC/SD ratio cutoff < 909 had a positive predictive value of 96% and negative predictive value of 100%[26]. These data have been subsequently confirmed in a number of recent studies[27-30]; however, these studies focused mainly on presence of varices as a whole.
In the current study, our main target was detection of large risky varices subject to impending rupture. Comparison of values of PS/SD ratio between studied groups showed significant decrease in cirrhotic patients with large esophageal varices compared to those without varices or with small esophageal varices (P = 0.001). These findings are in concordance with several previous studies demonstrating a similar significant correlation between platelet count/splenic size ratio with stages according to Child-Turcotte-Pugh classification, extent of ascites, and size of esophageal varices[31,32].
Findings indicative of portal hypertension can also be commonly detected with use of CT imaging, these including, in addition to splenomegaly and ascites, the presence esophageal varices, augmentation of portal vein, and existence of collateral vessel enlargement[33]. Several previous studies have investigated the interconnection between findings from both CT and endoscopy, and have demonstrated an agreement between variceal size and radiologic interpretations rather than between variceal size and endoscopic valuation[16,34]. However, CT scanning cannot adequately differentiate between small and large varices nor can it detect red signs on small varices that are also subject to a higher risk of bleeding[35].
Comparison of CT parameters between groups in this study demonstrated evident differences, as cirrhotic patients with esophageal varices had higher values for PVD as well as SVD when compared with cirrhotic patients without varices (0.0012 and 0.284 respectively). In addition, CT significantly differentiated between presence and absence of esophageal varices. Interpretation of CT imaging showing no varices in 50 patients (40.2%) proved accurate in 46 of these patients who truly had no varices while only 4 patients indeed had esophageal varices as detected by endoscopy. Furthermore, demonstration of varices by CT in 74 patients (59.7%) was correct in 70 of these patients who had endoscopic evidence of esophageal varices. These results indicate that CT is almost as effective in detection of esophageal varices as endoscopy, hence possibly providing an acceptable substitute to endoscopy in detection of esophageal varices in cirrhotic patients.
To evaluate the efficacy of these non-invasive parameters in detecting presence of large esophageal varices, our study group plotted a ROC curve and the area under the curve was obtained for each individual parameter. FIB-4 score of ≥ 3.13 was shown to have a sensitivity of 71.7% and a specificity of 50% with an area under the ROC curve of 0.585 (95%CI: 0.442-0.728), which are higher than those for APRI which at a value of ≥ 1.083 had a sensitivity of 63% and a specificity of 46.4%, with the area under the ROC curve being 0.550 (95%CI: 0.442-0.728). In addition, a PC/SD ratio of ≤ 806.93 had a sensitivity of 75% and specificity of 47.8%, while PVD measurement by CT had 71.1% sensitivity and 37% specificity at a cutoff of ≥ 12.5 mm, with area under the ROC curve of 0.560 (95%CI: 0.425-0.630). These results indicate that use of CT in detection of large esophageal varices offers results comparable to those provided by both FIB-4 and APRI values, as well by evaluation of PC/SD ratio.
Based on these data, we proposed a non-invasive model for the prediction of large esophageal varices in patients with cirrhosis. Being a multi-factorial process, the detection of large varies is affected by many variables. In order to construct a model for the prediction of large esophageal varices, the research team chose binary logistic regression as a statistical means of evaluation. Dependent factors were either large or small varices, while independent factors were coronary vein diameter, PVD, lieno-renal shunt, FIB-4, APRI, and PC/SD. The accuracy of this model was shown to be about 62.2%. However, after removal of insignificant factors such as APRI, FIB-4, coronary vein diameter, and lieno-renal shunt, accuracy of the model becomes 75%. With use of PC/SD alone, the model accuracy was shown to be 73%, but combined use of both PC/SD and PVD offered an accuracy of 75.7% for prediction of large risky esophageal varices.
In conclusion, endoscopy continues to be the mainstay in diagnosis of esophageal varices, in spite of its invasive nature, unacceptability by a large number of patients, and diverse side effects and complications; however, there remains a need for further non-invasive, effective tools for detection of large esophageal varices which may be subject to imminent rupture and hemorrhage in patients with cirrhosis. Thus, CT scanning may afford an adequate alternative to endoscopy in diagnosis of esophageal varices in patients afflicted with cirrhosis, as it appears to offer similar diagnostic value for large esophageal varices as other non-invasive parameters, with the added benefit of detection of other pathology of the liver, such as various hepatic lesions or masses, most notably hepatocellular carcinoma. In addition, parameters easily detectable by CT, such as PC/SD and PVD, form the basis for the model proposed by this study group, which provides accuracy of 75% for detection of large risky esophageal varices threatening to rupture in cirrhotic patients.
Chronic hepatitis C virus (HCV) infection currently affects approximately 130-170 million people globally, or about 2%-3% of the world’s population, with the largest HCV epidemic currently found in Egypt, affecting about 14.7% of the Egyptian population. The end stage of chronic HCV infection is cirrhosis, often complicated by esophageal varices. While upper gastroscopy remains the gold standard for diagnosis of esophageal varices, several disadvantages of this invasive procedure have prompted the search for non-invasive parameters to determine the presence of esophageal varices.
Several parameters have emerged as predictors of esophageal varices including platelet count to splenic diameter ratio, aminotransferase-to-platelet ratio index (APRI), Fibroindex, and Fibrosis-4 score (FIB-4) as well as a number of radiological techniques including Doppler ultrasonography and computed tomography (CT). However, all of these elements are plagued by limitations. The research hotspot is to acknowledge these various parameters and their limitations to help other peers understand the background behind the search for an accurate.
The search for a non-invasive method to accurately diagnose the presence of esophageal varices has been advancing in recent years. The present study involved a significant number of cirrhotic patients who underwent upper gastroscopy followed by CT imaging in addition toa series of simple inexpensive investigations to determine values for APRI, FIB-4, and platelet count/splenic diameter ratio (PC/SD). Patients undergoing the latter procedures were much more willing to comply when compared to those consenting for endoscopy, giving further support that endoscopy, in spite of its established benefits, remains a costly, uncomfortable procedure for many patients who prefer to avoid this invasive maneuver in any way possible, particularly when other accurate diagnostic tools are readily available.
Data from this study suggest that CT scanning may afford an adequate alternative to endoscopy in diagnosis of esophageal varices in cirrhotic patients. In addition, parameters easily detectable by CT, such as PC/SD and portal vein diameter, form the basis for the model proposed by this study group.
Chronic HCV infection is a long-standing infection of the liver with HCV culminating into development of cirrhosis and its associated complications, including portal hypertension and esophageal varices. Esophageal varices are abnormally enlarged veins in the lower part of the esophagus, which may leak or even rupture, possibly causing life-threatening bleeding. Endoscopy continues to be the mainstay in diagnosis of esophageal varices.
The work is really valuable occult blood in stool is always a diagnostic challenge. Besides esophageal varices, acute mucosa lesions, venous bleeding caused by portal hypertension in any site of the gastrointestinal tract are equally causes.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Egypt
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Bordas JM, Caboclo JL, Yoshida H S- Editor: Gong ZM L- Editor: A E- Editor: Li D
| 1. | Cuadros DF, Branscum AJ, Miller FD, Abu-Raddad LJ. Spatial epidemiology of hepatitis C virus infection in Egypt: analyses and implications. Hepatology. 2014;60:1150-1159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 2. | Guerra J, Garenne M, Mohamed MK, Fontanet A. HCV burden of infection in Egypt: results from a nationwide survey. J Viral Hepat. 2012;19:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 3. | Zhang W, Wang L, Wang L, Li G, Huang A, Yin P, Yang Z, Ling C, Wang L. Liver stiffness measurement, better than APRI, Fibroindex, Fib-4, and NBI gastroscopy, predicts portal hypertension in patients with cirrhosis. Cell Biochem Biophys. 2015;71:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Peñaloza-Posada MA, Pérez-Torres E, Pérez-Hernández JL, Higuera-de la Tijera F. Non-invasive parameters as predictors of high risk of variceal bleeding in cirrhotic patients. Rev Med Hosp Gen Mex. 2014;77:179-184. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Jensen DM. Endoscopic screening for varices in cirrhosis: findings, implications, and outcomes. Gastroenterology. 2002;122:1620-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Bosch J, Abraldes JG, Berzigotti A, Garcia-Pagan JC. Portal hypertension and gastrointestinal bleeding. Semin Liver Dis. 2008;28:3-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | de Franchis R. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63:743-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2011] [Cited by in RCA: 2267] [Article Influence: 226.7] [Reference Citation Analysis (3)] |
| 8. | de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1021] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 9. | ASGE Standards of Practice Committee; , Ben-Menachem T, Decker GA, Early DS, Evans J, Fanelli RD, Fisher DA, Fisher L, Fukami N, Hwang JH, Ikenberry SO. Adverse events of upper GI endoscopy. Gastrointest Endosc. 2012;76:707-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 245] [Article Influence: 18.8] [Reference Citation Analysis (2)] |
| 10. | Spiegel BM, Targownik L, Dulai GS, Karsan HA, Gralnek IM. Endoscopic screening for esophageal varices in cirrhosis: Is it ever cost effective? Hepatology. 2003;37:366-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 99] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Talwalkar JA, Kamath PS. Screening for esophageal varices among patients with cirrhosis of the liver. Am J Gastroenterol. 2001;96:3039-3040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Giannini E, Botta F, Borro P, Risso D, Romagnoli P, Fasoli A, Mele MR, Testa E, Mansi C, Savarino V. Platelet count/spleen diameter ratio: proposal and validation of a non-invasive parameter to predict the presence of oesophageal varices in patients with liver cirrhosis. Gut. 2003;52:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 297] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 13. | Crisan D, Radu C, Lupsor M, Sparchez Z, Grigorescu MD, Grigorescu M. Two or more synchronous combination of noninvasive tests to increase accuracy of liver fibrosis assessement in chronic hepatitis C; results from a cohort of 446 patients. Hepat Mon. 2012;12:177-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Choi YJ, Baik SK, Park DH, Kim MY, Kim HS, Lee DK, Kwon SO, Kim YJ, Park JW. Comparison of Doppler ultrasonography and the hepatic venous pressure gradient in assessing portal hypertension in liver cirrhosis. J Gastroenterol Hepatol. 2003;18:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Kim H, Choi D, Gwak GY, Lee JH, Park MK, Lee HIe, Kim SH, Nam S, Yoo EY, Do YS. Evaluation of esophageal varices on liver computed tomography: receiver operating characteristic analyses of the performance of radiologists and endoscopists. J Gastroenterol Hepatol. 2009;24:1534-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Yu NC, Margolis D, Hsu M, Raman SS, Lu DS. Detection and grading of esophageal varices on liver CT: comparison of standard and thin-section multiplanar reconstructions in diagnostic accuracy. AJR Am J Roentgenol. 2011;197:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4491] [Article Influence: 224.6] [Reference Citation Analysis (0)] |
| 18. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3219] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 19. | Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2633] [Cited by in RCA: 3484] [Article Influence: 183.4] [Reference Citation Analysis (0)] |
| 20. | Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology. 2007;45:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 183] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Stefanescu H, Procopet B. Noninvasive assessment of portal hypertension in cirrhosis: liver stiffness and beyond. World J Gastroenterol. 2014;20:16811-16819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Thomopoulos KC, Labropoulou-Karatza C, Mimidis KP, Katsakoulis EC, Iconomou G, Nikolopoulou VN. Non-invasive predictors of the presence of large oesophageal varices in patients with cirrhosis. Dig Liver Dis. 2003;35:473-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | Madhotra R, Mulcahy HE, Willner I, Reuben A. Prediction of esophageal varices in patients with cirrhosis. J Clin Gastroenterol. 2002;34:81-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Peck-Radosavljevic M. Thrombocytopenia in liver disease. Can J Gastroenterol. 2000;14 Suppl D:60D-66D. [PubMed] |
| 25. | Witters P, Freson K, Verslype C, Peerlinck K, Hoylaerts M, Nevens F, Van Geet C, Cassiman D. Review article: blood platelet number and function in chronic liver disease and cirrhosis. Aliment Pharmacol Ther. 2008;27:1017-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Giannini EG, Botta F, Borro P, Dulbecco P, Testa E, Mansi C, Savarino V, Testa R. Application of the platelet count/spleen diameter ratio to rule out the presence of oesophageal varices in patients with cirrhosis: a validation study based on follow-up. Dig Liver Dis. 2005;37:779-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Giannini EG, Zaman A, Kreil A, Floreani A, Dulbecco P, Testa E, Sohaey R, Verhey P, Peck-Radosavljevic M, Mansi C. Platelet count/spleen diameter ratio for the noninvasive diagnosis of esophageal varices: results of a multicenter, prospective, validation study. Am J Gastroenterol. 2006;101:2511-2519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Sharma J, Yadav MK, Gupta A, Arya TS. A study of the role of platelet count/splenic diameter ratio as a predictor of esophageal varices in patients with chronic liver disease. Nat J Med Res. 2014;4:232-234. |
| 29. | Amin K, Muhammad D, Anjum A, Jamil K, Hassan A. Platelet count to splenic diameter ratio as a predictor of esophageal varices in patients of liver cirrhosis due to hepatitis C virus. JUMDC. 2012;3:6-11. |
| 30. | González-Ojeda A, Cervantes-Guevara G, Chávez-Sánchez M, Dávalos-Cobián C, Ornelas-Cázares S, Macías-Amezcua MD, Chávez-Tostado M, Ramírez-Campos KM, Ramírez-Arce Adel R, Fuentes-Orozco C. Platelet count/spleen diameter ratio to predict esophageal varices in Mexican patients with hepatic cirrhosis. World J Gastroenterol. 2014;20:2079-2084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Mahassadi AK, Bathaix FY, Assi C, Bangoura AD, Allah-Kouadio E, Kissi HY, Touré A, Doffou S, Konaté I, Attia AK. Usefulness of Noninvasive Predictors of Oesophageal Varices in Black African Cirrhotic Patients in Côte d’Ivoire (West Africa). Gastroenterol Res Pract. 2012;2012:216390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Ying L, Lin X, Xie ZL, Hu YP, Shi KQ. Performance of platelet count/spleen diameter ratio for diagnosis of esophageal varices in cirrhosis: a meta-analysis. Dig Dis Sci. 2012;57:1672-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Somsouk M, To’o K, Ali M, Vittinghoff E, Yeh BM, Yee J, Monto A, Inadomi JM, Aslam R. Esophageal varices on computed tomography and subsequent variceal hemorrhage. Abdom Imaging. 2014;39:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Shen M, Zhu KS, Meng XC, Zhang JS, Liu LY, Shan H. [Evaluation of esophageal varices and predicting the risk of esophageal varices bleeding with multi-detector CT in patients with portal hypertension]. Zhonghua Yi Xue Za Zhi. 2010;90:2911-2915. [PubMed] |
| 35. | Perri RE, Chiorean MV, Fidler JL, Fletcher JG, Talwalkar JA, Stadheim L, Shah ND, Kamath PS. A prospective evaluation of computerized tomographic (CT) scanning as a screening modality for esophageal varices. Hepatology. 2008;47:1587-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |