Published online Oct 18, 2015. doi: 10.4254/wjh.v7.i23.2432
Peer-review started: April 28, 2015
First decision: June 2, 2015
Revised: June 22, 2015
Accepted: September 2, 2015
Article in press: September 9, 2015
Published online: October 18, 2015
Processing time: 175 Days and 17.6 Hours
Liver transplantation (LT) has become the standard of care for patients with end stage liver disease. The allocation of organs, which prioritizes the sickest patients, has made the management of liver transplant candidates more complex both as regards their comorbidities and their higher risk of perioperative complications. Patients undergoing LT frequently display considerable physiological changes during the procedures as a result of both the disease process and the surgery. Transoesophageal echocardiography (TEE), which visualizes dynamic cardiac function and overall contractility, has become essential for perioperative LT management and can optimize the anaesthetic management of these highly complex patients. Moreover, TEE can provide useful information on volume status and the adequacy of therapeutic interventions and can diagnose early intraoperative complications, such as the embolization of large vessels or development of pulmonary hypertension. In this review, directed at clinicians who manage TEE during LT, we show why the procedure merits a place in challenging anaesthetic environment and how it can provide essential information in the perioperative management of compromised patients undergoing this very complex surgical procedure.
Core tip: The allocation of organs to the sickest liver transplant candidates has made their management more complex and anaesthesia for perioperative liver transplantation (LT) more challenging. Transoesophageal echocardiography, which can visualize dynamic cardiac function and overall contractility and provide real-time feedback on the adequacy of therapeutic interventions, has gained an irreplaceable role in the perioperative management of LT. We believe that echocardiography can play a key role in the care of and decision making for compromised liver transplant candidates undergoing this complex surgical procedure.
- Citation: De Pietri L, Mocchegiani F, Leuzzi C, Montalti R, Vivarelli M, Agnoletti V. Transoesophageal echocardiography during liver transplantation. World J Hepatol 2015; 7(23): 2432-2448
- URL: https://www.wjgnet.com/1948-5182/full/v7/i23/2432.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i23.2432
Liver transplantation (LT) is a life-saving procedure for patients with end-stage liver disease not responsive to other medical treatment. Unfortunately, many transplant candidates will die on the waiting list because of the marked shortage of donor organs[1]. In an effort to reduce waiting list mortality, organ allocation is based on the Model of End-Stage Liver Disease (MELD). This score prioritizes allocation to the sickest patients, and for this reason, patients undergoing LT today have more severe end stage liver disease (ESLD), are older (> 60 years), and are more complex regard both their comorbidity burden and their risk for perioperative complications[2]. The patient’s first examination will confirm his status as a LT candidate, and intraoperative transoesophageal echocardiography (TEE) will help optimize the anaesthetic management of these highly complex patients.
Patients with cirrhosis requiring LT have an increased cardiac output and a decreased peripheral vascular resistance and arterial pressure but a compromised ventricular response to stressors, such as haemorrhage, vasoactive drugs, vascular clamping, volume overload, and reperfusion. This condition is defined as cirrhotic cardiomyopathy[3,4] and is associated with increased left ventricular wall thickness and cardiac chamber enlargement. Assessing the optimal volaemia and excluding left or right ventricular dysfunction in the perioperative course of LT is a challenge for the anaesthesiologist. TEE, by providing a rapid visualization of dynamic cardiac function, volume status, overall contractility, regional wall motion, embolization of large vessels, and pericardial effusion, can provide irreplaceable help[5].
Liver transplant candidates usually have cardiac changes associated with their advanced age and the presence of several comorbidities that increase the potential for cardiovascular complications, particularly during the haemodynamic stresses that characterize the perioperative period. The preoperative cardiac evaluation usually includes a trans thoracic echocardiography which is essential to assess cardiac function and to look for the effects on the heart of the two main pulmonary syndromes caused by the end-stage liver disease: Hepatopulmonary syndrome and portopulmonary hypertension (POPH).
Echocardiography with agitated saline contrast is commonly used to detect intrapulmonary arteriovenous shunts, which are common in patients with ESLD. Microbubbles that appear late (after a time delay of 4 to 8 cardiac cycles) in the left side of the heart after agitated saline injection into the venous system are consistent with the diagnosis of hepatopulmonary syndrome. Immediate or early shunting (1-2 cardiac cycles) is more consistent with an atrial septal defect or patent foramen ovale[6].
If diagnostic questions remain after a transthoracic study, a TEE can provide increased sensitivity and can directly visualize bubbles entering the left atrium from the pulmonary veins rather than crossing the interatrial septum[7]. TEE is believed to be the test of choice for the diagnosis of patent foramen ovale or interatrial shunts[8].
For a preoperative patent foramen ovale (PFO) diagnosis, it is important to assess the severity of the problem and decide whether preoperative correction is needed to avoid significant amounts of venous air entering the systemic circulation at the time of reperfusion.
PFO, usually a benign and silent lesion (present in approximately 25% of adults in the general population), can cause hypoxemia and paradoxical embolic phenomena under circumstances when right atrial pressure exceeds left atrial pressure[9]. These circumstances may occur perioperatively as a result of mechanical ventilation, changes in intra-abdominal pressure, hypotension, and/or severe reperfusion syndromes. Numerous case reports implicate PFO as a cause of perioperative hypoxemia and systemic thromboembolism in LT[10]. Air from the right atrium can embolize the coronary arteries, particularly the right coronary artery, resulting in acute myocardial ischaemia, ventricular fibrillation, and severe right ventricular hypokinesis. Even in the absence of systematic study, screening for PFO before surgery has been suggested for high-risk patients to reduce the risk of paradoxical embolization[10,11].
Other authors, in contrast, state that LT can be performed safely in patients with a PFO and other types of intra-cardiac shunts because the overall incidence of this complication appears to be quite low (isolated case reports)[12]. They argue that although TEE offers the best sensitivity and specificity for PFO diagnosis, it is semi-invasive and expensive and that most patients with portal hypertension and oesophageal varices are at higher risk of oesophageal bleeding provoked by the TEE probe. Therefore, these authors argue that a TEE should be reserved for LT patients with specific indications[13]. Although a PFO is not a contraindication to LT, extra care should be taken to prevent thrombus formation and air entry into the venous system during surgery. Further studies are needed to determine impact of a PFO on LT morbidity and the potential role, if any, for percutaneous PFO closure in liver transplant candidates.
The value of a TEE must of course be balanced against the risk of performing the procedure. The insertion and manipulation of a transoesophageal echocardiographic probe may, even if infrequently, cause arrhythmias, respiratory distress, hemodynamic effects, provoke dental injuries, pharyngeal and/or laryngeal, esophageal and/or gastric trauma, and of course bleeding, that can be more severe and dangerous in cirrhotic patients with gastric varices or coagulopathy. Gastro-oesophageal varices are very common in patients listed for LT, and their presence is indicated by worse laboratory parameters and MELD scores. Varices are present in 73% of patients with ESLD awaiting LT[14,15], and 5% will develop new varices; moreover, up to 28% of patients will have oesophageal varices in the three years following a diagnosis of cirrhosis[16]. Preoperative endoscopy to evaluate the grade of the varices, oropharyngeal examination, limited probe manipulation and exam performance by an experienced operator is, recommended for patients with ESLD prior to TEE[17,18].
Patients with oesophageal stricture, cancer, diverticulum, and recent oesophageal surgery are generally considered to have near absolute contraindications for TEE.
Gastro-oesophageal varices are considered a relative contraindication to TEE, and the transgastric view is sometimes avoided to prevent damaging gastro-oesophageal varices in the distal oesophagus[19]; however, concerns over damage to these varices have been shown to be largely unfounded[20]. On the other hand, the right message should be that TEE is not completely safe in patients with oesophageal varices because their presence still remains a relative contraindication for TEE.
There is a paucity of data related to the manipulation of the TEE probe in patients with gastro-oesophageal varices, but a recent retrospective analysis by Spier et al[17] specifically analysed this cohort and found no major bleeding complications, even in higher-risk patients. No bleeding episodes were reported in a small study of 23 patients with oesophageal varices undergoing intraoperative TEE during LT[21].
An editorial by Spencer[22] accompanying Spier’s study provides some recommendations to aid decision-making regarding the use of TEE in patients with gastro-oesophageal varices and concludes by saying that “if a patient has an important indication for TEE, which cannot be answered first with TTE or any other noninvasive technique, then TEE should not be contraindicated by the presence of oesophageal varices”.
Physicians who perform TEE need to make individual decisions on the risk/benefit ratio of performing TEE in patients with known or suspected varices. Burger-Klepp et al[14] agreed, suggesting that TEE is a relatively safe method for monitoring cardiac performance of LT patients with a moderate MELD score and documented gastro-oesophageal varices but a low risk of major haemorrhagic complications. Markin et al[23], based on their retrospective study, state that intraoperative TEE is a relatively safe method for monitoring cardiac performance in liver transplant patients, with a major complication rate of 0.86%. Although not without risk, TEE provided valuable information that would not otherwise be detected, such as pulmonary thromboembolism occurring at the time of graft reperfusion, unidentified PFO and the presence of ventricular dysfunction after graft reperfusion[23].
Intraoperative TEE use during LT has been increasing because of its unique ability to rapidly visualize the dimensions and function of the heart chambers; to detect intra-cardiac air or thrombus, myocardial ischaemia, or pulmonary thromboembolism[24]; and for its invaluable role in intraoperative haemodynamic management[25]. It is an invasive medical procedure that is focused on intraoperative monitoring rather than specific diagnosis. It carries rare but potentially life threatening complications and therefore must be performed by only qualified physicians.
Although TEE is reported to be routine in 40%-72% of high-volume liver transplant centres[13,26], formal TEE certification is the exception. A thorough understanding of anatomy, physiology, and the surgical procedure is critical to its proper use. The intraoperative use of TEE is limited by the need for advanced training and the lack of credentialed anaesthesiologists. Most anaesthesiologists learn to use TEE in LT in an informal manner after completing training[26]. A significantly smaller proportion of anaesthesiologists who work in low volume liver transplant centres do not use perioperative TEE because they are unfamiliar with the procedure and the data it provides[27]. The American Society of Anesthesiology, in cooperation with the National Board of Echocardiography (NBE), defined the components of basic perioperative TEE training as including independent clinical experience, supervision, and continuing education requirements. The NBE’s Basic perioperative TEE training pathways require an extensive training, at least 150 basic intraoperative procedures, and a written exam to obtain certification[28,29].
The American Society of Echocardiography (ASE) and the Society of Cardiovascular Anesthesiologists (SCA) state that because of the risks, technical complexity, and potential impact of TEE on perioperative management, the basic TEE echocardiographer must be a licensed physician[28].
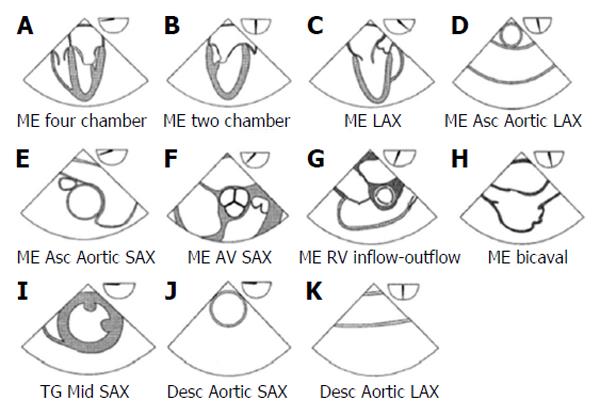
Although a basic perioperative TEE echocardiographer should be familiar with the 20 classical views necessary to obtain a comprehensive intraoperative transoesophageal echocardiogram[30], recent ASE and SCA guidelines state that it is more realistic to expect an anaesthesiologist to be familiar with the 11 most relevant TEE views (Figure 1), which can provide him with the necessary information for an aetiologic diagnosis of haemodynamic instability during surgery[28].
The basic perioperative TEE examination should be performed using three primary positions of the probe within the gastrointestinal tract: The mid-oesophageal level, the transgastric level, and the upper oesophageal level.
The haemodynamic instability typical of LT can result from heart failure, real hypovolaemia, or reduced peripheral vascular resistance[31].
Hemodynamic instability and rapid changes in volume status, mainly due to acute blood loss or vascular clamping, are the most serious complications and challenges that the anaesthetist has to manage during LT.
TEE allows the intraoperative causes of hypotension to be identified and can help optimize volaemia and avoid impaired organ perfusion and ischaemia. Identifying the cause of hypotension is the key to treatment. It is the inability of the cirrhotic patient to respond to cardiac stress together with their hypovolaemia and the massive fluid shifts that accompany clamping of the inferior vena cava and subsequent reperfusion of the liver graft that make intraoperative fluid management so difficult[32,33]. Unique haemodynamic changes occur in the transition from the anhepatic to the reperfusion phases of LT, and rapid fluid assessments and adjustments are needed to optimize the outcome. TEE analysis based on the close approximation of the papillary muscles in the transgastric mid papillary view (TG Mid SAX) allows for a rapid qualitative assessment of ventricular filling so that fluids can be adjusted for the desired preload.
Cirrhotic patients have an increased risk for right ventricular failure, so aggressive fluid repletion or blood transfusion or the increased blood flow to the right heart at reperfusion can cause volume overload and precipitate pulmonary oedema due to occult cardiac disease[3]. In these circumstances, TEE allows the right heart chambers to be visualized, allowing for a real-time diagnosis of right ventricle failure (due to pulmonary embolism or overload), pulmonary hypertension, or even a reduced preload. Right and left ventricular dysfunction can be exacerbated during the transplant by the clamping and unclamping of major vessels, such as the inferior vena cava or portal vein, and the sudden fluid shifts associated with such manoeuvres can be assessed during surgery by TEE.
TEE can be useful not only in assessing the patient’s volume status and intraoperative fluid management but also when evaluating any left ventricular hypertrophy or hyperdynamic systolic function typical of ESLD, which may result in haemodynamically significant left ventricular outflow tract obstruction (LVOTO) during LT. TEE makes the diagnosis of an haemodynamically significant LVOTO possible intraoperatively together with the recognition and management of refractory hypotension through inotropic agents and careful volume administration[34].
Monitoring cardiac output (CO) during LT is particularly important because it is considered, despite its limitations, one of the main determinants of oxygen transport and wrongly considered to be a surrogate for left ventricular function. TEE seems to be a valid alternative to standard methods for measuring CO, providing both a numerical value for CO and separate qualitative determinations of right and left ventricular function and ejection fraction.
Because a normal CO value does not always imply an adequate peripheral perfusion, it may be more useful to monitor CO variations over time, especially under conditions of haemodynamic instability or after therapeutic interventions rather than considering a single numerical value.
The thermodilution method is the most common technique used at the bedside to monitor CO during LT; this method uses a pulmonary artery catheter (PAC), which is considered a gold standard due to its extensive past use[35]. PAC measurements are based on changes in the temperature of the blood surrounding the catheter, but the large core body temperature shifts that are frequently witnessed before and after revascularization of the new graft can affect the reliability of both continuous and bolus determination of CO with PAC[36,37]. Massive peripheral or central venous infusion of fluids or unheated blood from a veno-venous by-pass (VVB) may affect the accuracy of thermodilution by increasing thermal noise and lead to erroneous measures of CO as well[38]. Using right-heart catheterization for volumetric left ventricular preload assessment highlights some limitations. Right ventricular function differs considerably from left ventricular one. The major determinant of left ventricular function is myocardial wall tension, whereas for the right it is ventricular afterload. Therefore, the relationship between right ventricular preload assessment and cardiac output readings may be weak[39]. Another underlined PAC limitation comes from a possible delayed reactivity to rapid changes in cardiac output and intravascular volume detection. CO monitored continuously by the thermodilution technique yields values averaged over a period of time (3-6 min or longer)[36], so changes in the left ventricular stroke volume (SV) or CO cannot be assessed with a high time resolution[36]. TEE cardiac output monitoring instead seems to detect changes in output during LT, as can occur during acute haemodynamic changes, more rapidly than thermodilution[40]. Other authors argue instead that a sudden change in filling pressures or SvO2, as indirect indicator of cardiac output, is an extremely valuable information provided by PAC, that allows the proper detection and identification of certain intraoperative events that TEE cannot detect[41]. TEE application does not guarantee a continuous monitoring and it’s not good at trending information (especially preload): No quantitative online evaluation of right ventricular function is available, and only sporadic right ventricular ejection fraction values can be obtained[42]. On the other hand TEE, unlike PAC, is not affected by blood temperature changes and provides a calculated numerical value for left ventricular volume and cardiac output (e.g., by Simpson’s rule) as well as a qualitative determination of right and left ventricular filling and ejection fraction.
In experienced hands, the correlation between echocardiographic and thermodilution measurements of cardiac output is generally acceptable[43,44] even if there is no general agreement on this subject. Other authors have declared that CO measurements by TEE are not interchangeable with PAC thermodilution because of limited agreement and a large percentage of errors[45]. Unlike PAC, TEE provides volumetric rather than pressure data, which can be misleading in the setting of pulmonary hypertension, valvular dysfunction or ventricular failure. The unique shape and function of the right ventricle may delay changes in PAC readings until the right ventricle is significantly dilated as central venous pressure readings do not necessarily correlate with right ventricular preload or ejection[21].
Despite PAC limits, transoesophageal echocardiography for monitoring left ventricular preload has some limitations which should be emphasized as well.
Determination of the left ventricular end diastolic area (LVEDA) index provides a measure of left ventricular filling that has been shown to correlate with changes in SV during volume therapy[46] only if, the compliance and contractility of the left ventricle remain unchanged[47]. Quantitative assessment of LVEDA may be altered by dislocation of the probe from the midpapillary level as well[47]. The technical complexity of TEE performance can be increased by the difficulty in obtaining short-axis visualization of the left ventricle which is limited due to the common posterior retraction of the stomach during LT[48]. The apparent reduced invasiveness of TEE compared to PAC, is however offset by a greater complexity of the technique. TEE users should be qualified in displaying standardized cross-sections, and skilled to interpret findings in order to avoid potential serious misinterpretation of the images[49]. Beside this technical difficulty this method is either not practicable in a perioperative setting or cannot be routinely performed for logistic and economic reasons.
In summary PAC allows for the nearly continuous measurement of CO, right ventricle ejection fraction, and right ventricle end diastolic volume showing some great advantages like its continuous nature and the relative lack of user input. TEE, besides aiding in the estimation of preload, is very valuable in the overall assessment of cardiac function, detection of air embolism or intracardiac clot formation, diagnosis of hepatopulmonary syndrome, and management of pulmonary hypertension[50]. Both methods have several limitations which make the actual available literature inconclusive about how to best monitor the hemodynamics during LT and whether a single monitoring device is absolutely superior to the others in terms of accuracy, validity and reproducibility of data. Nowadays a complete, accurate, non-invasive device, suitable for instantaneously detecting hemodynamic alterations typical of LT is still unavailable, and the integration of various data from different monitoring systems probably remains the only way to properly manage haemodynamic instability.
In the clinical setting, SV is an important parameter of cardiac performance. Assessment of CO is an important measure of responses to medical and surgical therapies, such as administration of inotropic agents to treat right and left heart failure[51]. SV and CO are most reliably and easily measured at the left ventricular outflow tract (LVOT) or at the level of the aortic valve. SV and CO can also be measured at the level of the mitral valve or the pulmonary artery, but this is less commonly done because, unlike the mitral or pulmonary valves, the cross sectional area of the LVOT and ascending aorta (because they are circular structures) change very little throughout the cardiac cycle.
SV and CO measurement at LVOT: The TEE-derived CO can be calculated as the product of SV and heart rate, where left ventricular SV is calculated by multiplying the time-velocity integral at the left ventricular outflow tract by the LVOT area. It is important to remember that area and flow measurements must be made at the same anatomical site. This calculation assumes that flow is laminar (i.e., not turbulent) and that the conduit being measured is an unchanging circular orifice such that it has the area of πr2.
Stroke volume measured at LVOT level is simplified by the following equations:
SV = VTI × CSALVOT
CSA: Cross-sectional area; VTI: Velocity-time integral.
The CSA (LVOT) is calculated from the LVOT diameter as follows:
CSALVOT = 0.785 × diameter2
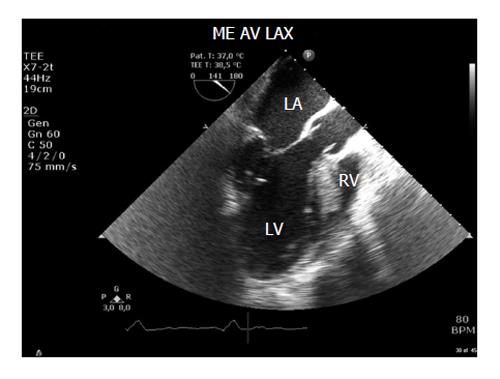
The CSA of the LVOT is usually obtained from the mid-oesophageal long axis (ME LAX) view at 110°-140° (Figure 2). Errors in diameter measurements are quadrupled because the formula requires squaring the diameter. Therefore, very small errors in measurement make a dramatic difference to the calculation.
The diameter should be measured multiple (usually three) times at mid-systole in the mid oesophageal aortic valve long axis (ME AV LAX) view, using the inner edge to inner edge technique, and then averaged. This measurement assumes that the annular size does not vary much throughout the cardiac cycle, so the timing of this measurement is not crucial.
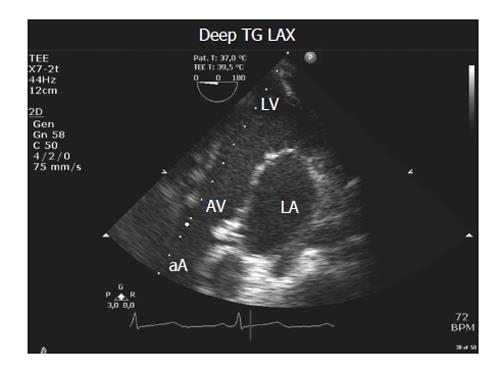
VTI measured at the level of the LVOT using pulse wave Doppler requires the sample volume to be positioned in the LVOT just proximal to the aortic valve. Because the blood flow is nearly parallel to the ultrasound beam, the best transoesophageal views for this measurement are the transgastric long axis (TG LAX) and the deep transgastric long axis (deep TG LAX) views with PW Doppler sample volume placed in the LVOT (Figure 3)[45,52].
When aortic stenosis is present, the CW Doppler signal shows a characteristic flow image with two densities[53]. The most intense part of the time velocity integral is the SV, whereas the outer contour shows the speed of the peak that allows the pressure gradient at the level of aortic valve to be calculated using the modified Bernoulli equation. This technique cannot be used when there is a significant aortic regurgitation.
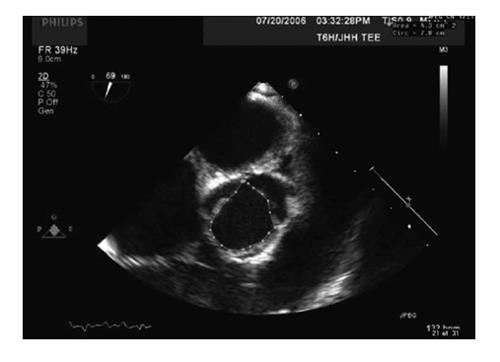
SV and CO measurement at aortic valve: If measuring at the level of the aortic valve, the CSA of the valve can be measured using planimetry of a short axis view of the aortic valve in mid systole (Figure 4). VTI is measured using continuous wave Doppler with the Doppler beam directed through the valve orifice; the TG LAX view or the deep TG LAX view are the most helpful views for this purpose.
The stroke volume measured at aortic valve is simplified by the following equation:
SV = VTI × aortic valve area
It is important to remember that if significant aortic stenosis is present, flow distal to the valve is not laminar and SV measurements will therefore be inaccurate.
Volume status assessments are a challenge in perioperative haemodynamic management of LT and the most common haemodynamic changes are secondary to changes in volume status and cardiac function.
The filling pressures (i.e., central venous pressure and pulmonary artery occlusion pressure), as indirect indicators of filling volumes, have been the “standard” methods for decades but, following significant criticism, volumetric measurements are now preferred[5,54]. The most commonly used parameters for left ventricular preload assessment are the left ventricular end-diastolic diameter (LVEDD) and the LVEDA, both obtained in the TG Mid SAX view[28]. It is important to remember that retractor placement during LT may, unfortunately, obstruct the transgastric view required to obtain these parameters[20,55]. Additionally, some authors recommend avoiding this view so as not to disrupt gastro-oesophageal varices in the distal oesophagus[19].
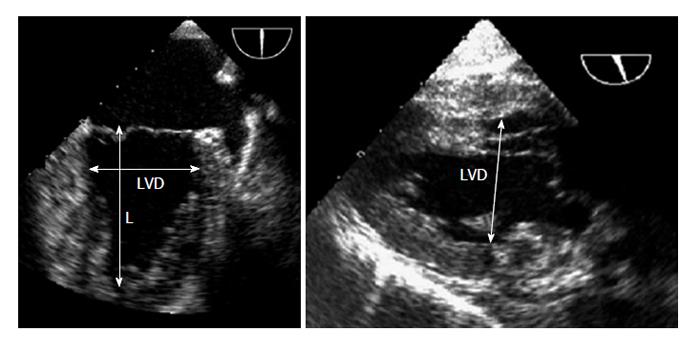
Left ventricular internal end diastolic diameter: Volume status or trends can be rapidly estimated by measuring LVEDD. A small left ventricular (LV) internal diameter at end-diastole can be indicative of hypovolemia, whereas the LV internal diameter at end systole (LVESD) is a less specific indicator of hypovolemia because a low value may be caused by a decreased systemic vascular resistance, an increased inotropic state, or by decreased ventricular filling. Both LVEDD and LVESD are decreased in hypovolemia, whereas LVEDD is normal and LVESD is decreased when systemic vascular resistance is decreased[52]. Serial measurements of both diameters can be useful to monitor a patient’s response to administered fluids. The LV diameters can be measured by the TEE mid oesophageal two chamber (ME 2C) view at the mitral valve leaflet tips and by the TG Mid SAX view using M-mode imaging at the mid-papillary level[52]; however, the TG LAX view has been recommended because it is easier to align. LV diameters are measured from the endocardium of the anterior wall to the endocardium of the inferior wall in a line perpendicular to the long-axis of the ventricle at the junction of the basal and middle thirds of the long-axis (Figure 5).
Reference ranges for un-indexed LVEDD are 3.9 to 5.3 cm in women and 4.2 to 5.9 cm in men[56].
LVEDA: Compared with baseline imaging, measurements of LVEDA can be used as an indirect measurement of LV preload[57] and can be used to monitor the response to fluid therapy[58].
Variations of LVEDA, measured by definition at end-diastole at the mid-papillary muscle level, closely reflect changes in left ventricular end diastolic volume (LVEDV)[59]. The LVEDA index provides a measure of LV filling, which correlates with changes in the stroke volume index during volume replacement[60].
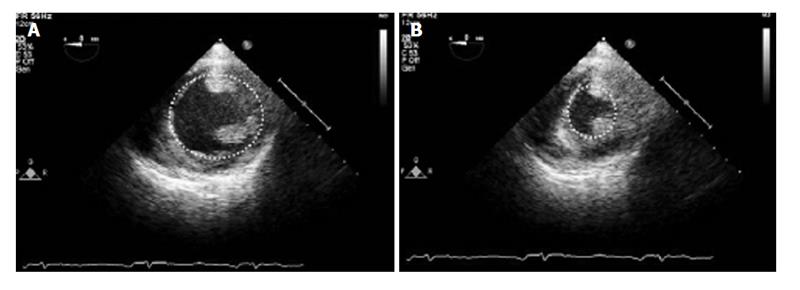
LVEDA can be calculated most easily in the TG Mid SAX view. The endocardium should be traced at the mid-papillary muscle level in end-diastole where the LV area is maximal (Figure 6)[61]. By convention, the papillary muscles are excluded from the tracing. Once traced, ultrasound software can calculate the LVEDA, which normally ranges from 8 to 14 cm2[56].
TEE provides a better index of LV preload in patients with normal LV function than filling pressure values obtained by the more invasive PA catheterization approach[62].
Intraoperative TEE monitoring offers an indirect assessment of preload, but the validity of LVEDA as a preload index is still under discussion because it only correlates with changes in stroke volume index during volume therapy if the compliance and contractility of the left ventricle remain unchanged[47]. An index LVEDA < 5.5 cm2/m2 is very suggestive of low filling of the left ventricle[63].
Although PAC study parameters provide an index of global myocardial function, they do not provide information on specific areas of myocardial performance. Assessments of left and right ventricular performance during surgery are advantages that TEE offers. TEE can detect areas of regional wall dysfunction that would otherwise remain undiagnosed. Clearly, time consuming methods requiring multiple equations or measurements are not helpful in an acute setting, such as with the hemodynamic instability of LT. So, despite many published quantitative measures of global left ventricular function[28,64], most basic echocardiographers rely on qualitative, visual estimations of systolic function. This approach is far from precise but allows a basic echocardiographer to differentiate those patients who might benefit from inotropic therapies from those who simply need more fluid volume.
Left ventricular function: The American Society of Echocardiography and by the Society of Cardiovascular Anaesthesiologists[28] has recommended that ventricular function is assessed by a regional wall motion analysis based on a 17-segment wall motion score, as described in the ASE guidelines[64]. This approach suggests that a physician trained in basic transoesophageal echocardiography obtains mid oesophageal four-chamber (ME 4C), ME 2C and ME LAX views for a more comprehensive evaluation and to monitor global and regional LV function. However, visualization of 6 mid-papillary segments from the TG Mid SAX view may suffice and is important for prognosis[65].
The TG Mid SAX view provides significant diagnostic information regarding regional and global ventricular function to allow for efficient patient care and to minimize any distraction under intraoperative conditions while the patient is haemodynamically unstable.
Quantitative measurements of left ventricular function can also be obtained by measuring the Fractional Shortening, fractional area change and Ejection Fraction.
Fractional shortening: Fractional shortening (FS) expresses the percentage change between the LVEDD and the LVESD according to the following formula:
FS (%) = (LVEDD - LVESD)/LVEDD × 100
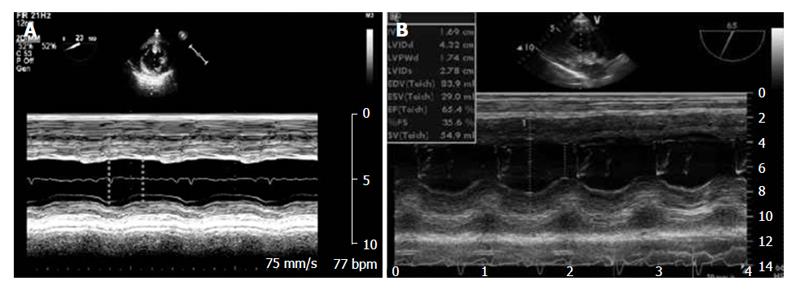
The LV internal diameters are measured at the ends of diastole and systole on an M-mode tracing of a TG Mid SAX view taken just above the papillary muscles and sometimes from an M-mode tracing of a TG LAX view from the inner edge to the inner edge of the endocardial borders (Figure 7).
Fractional shortening can be used to determine ventricular function, with normal values ranging from 25% to 45%[56].
Although FS gives a rapid and simple estimate of LV systolic function, it is not representative of global ventricular function if there are ventricular regional wall abnormalities or aneurysmal deformities[56]. It assesses only the selected cross section of the left ventricle, so there should be no alterations in regional LV contractility either at the apex or at the base if FS is to reflect global LV function accurately.
Fractional area change: Fractional area change (FAC) is a two-dimensional measurement that is easily obtained from the TG Mid SAX view. It expresses the percentage change between the LVEDA and the left ventricular end-systolic area (LVESA) according to the following formula:
FAC (%) = (LVEDA - LVESA)/LVEDA × 100
Normal values range from approximately 55% to 65%[56].
This approach requires both the LVEDA and LVESA measurements, tracing the left ventricular endocardium during the end of diastole and systole. The measurements are usually made in the TG Mid SAX view, but when this view is suboptimal, long axis views can also be used. The endocardium can be traced manually traced around the LV cavity, ignoring the papillary muscles, or the endocardial borders can be detected automatically (Figure 6)[61].
FAC can provide a reasonable global estimate of LV function but, like the FS, it has its limitations. It may represent global LV function poorly in cases of myocardial infarction or aneurysmal dilatation in areas of the ventricle other than the mid papillary level, where FAC is evaluated. Changes in loading condition may also influence the FAC.
Ejection fraction: This measure requires the application of algorithms that can approximate the left ventricle to a conventional solid. The ejection fraction (EF) is the most widely used index in clinical practice to describe left ventricular function, even if it measures ejection ability rather than the contractility of the left ventricle. The B-mode evaluation is most widely used to study systolic function because it is reliable even in the presence of geometric distortion or wall motion abnormalities and because it correlates well with radionuclide ventriculography and scintigraphy measurements[66]. In contrast to fractional shortening, which depends on a single cavity dimension in systole and diastole, the ejection fraction examines the entirety of myocardial contraction by expressing stroke volume as a percentage of LV end-diastolic volume:
LVEF% = (LVEDV - LVESV)/LVEDV × 100
Where LVEF is the left ventricle ejection fraction, LVEDV is the LV end-diastolic volume, and LV end-systolic volume (LVESV).
The LVEF represents a composite of cardiac performance involving preload, contractility, and afterload. It is widely regarded to be a predictor of outcome and survival. Normal EF values range from 55% to 70%.
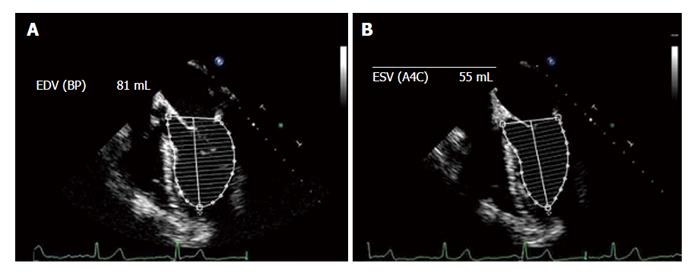
The area-length and the Simpson’s methods are among the commonest used to calculate the EF. The previously used Teichholz method of calculating LV ejection fraction from LV linear dimensions is not recommended for clinic practice because inaccuracies can arise from the geometric assumptions required to convert a linear measurement to a 3-D volume[67].
The area-length method is an alternative method to calculate LV volumes when the apical endocardial definition precludes an accurate tracing. This method assumes that the LV is bullet shaped[68], and the volumes are obtained by measuring LV areas and lengths at both end-diastole and end-systole. The measurements require a long-axis view of the chamber without foreshortening of the LV. This view may be difficult to obtain from the standard ME 4C view, and some retroflexion of the probe may be required to visualize the true apex and prevent any foreshortening of the LV.
The Simpson method is the most widespread EF calculation method and consists of 2-D measurements of volume with the biplane method of discs (modified Simpson’s rule). The EF is calculated from the summation of a series (20) of overlapping slices from apex to base, each of which is assumed to be an elliptical disc. This is the currently recommended method of choice of the American Society of Echocardiography and the European Association of Echocardiography[56]. Modern ultrasound is equipped with software to calculate the EF by using the area of the LV and its diameter measured from the apex to the mitral floor in the two phases of the cardiac cycle. The EF is calculated from projections of the ME 4C and 2C views (Figure 8).
A normal right ventricle (RV) has an oblong shape and a complex architecture and, in the ME 4C and TG Mid SAX views, it is approximately two-thirds the size of the LV. In the ME 4C view, the RV extends more than half way to but does not normally share the LV apex.
The RV cavity on the short axis has a half-moon shape with the concave side of the interventricular septum towards the VS. A first echocardiographic sight provides information regarding RV function because, at the onset of right ventricular dysfunction, the ventricular chamber enlarges and the usually convex septal wall of the RV, facing the crescent shaped RV cavity, loses its classical shape and anatomical relationships with VS. An increase in RV pressure or volume overload can cause flattening or leftward deviation of the septal wall, producing an elliptical or circular short-axis shape of the RV cavity. The normal right ventricle is accustomed to a low pulmonary resistance and hence low afterload; thus, normal RV pressure is low and right ventricular compliance is high. Elevations in RV afterload result acutely in RV dilatation, whereas chronic elevations cause RV hypertrophy. Right ventricular size is best estimated by TEE from a right ventricle focused ME 4C view, with the multiplane angle adjusted to maximize the tricuspid annulus diameter, usually between 10° and 20°[56]. Care should be taken to obtain the image of the maximal right ventricular diameter without foreshortening[69].
Many techniques have been described to obtain quantitative measurements of the overall function of the right ventricle, but in most cases, the physician relies on qualitative measures, and on visual estimates of systolic right ventricular function.
Assessments of right ventricular function are required whenever a patient presents with unexplained or refractory hypotension. Patients undergoing LT, for example, can present with hypotension secondary to right ventricular failure[70]. If their RV dysfunction is related to acute changes in pulmonary pressures associated with lung volume shifts or acid-base changes during the transplant, they can also be at risk for pulmonary hypertension[21].
TEE has the significant advantage that right ventricular failure can be identified by dynamic rather than the pressure changes, which can be easily missed in PAC measurements. Because right ventricular failure is an important complication during the reperfusion phase of LT, TEE monitoring confers very significant advantages at this stage.
When the evaluation is based on a quantitative assessment, a number of echocardiographic techniques may be used to assess RV function.
Tricuspidal annular plane systolic excursion: In systole, the tricuspid annulus will normally descend towards the apex 1.6-2.0 cm. A tricuspidal annular plane systolic excursion (TAPSE) of less than 1.6 cm has been associated with a poor prognosis in a variety of cardiovascular diseases; it is highly specific for RV dysfunction and can be used to monitor RV systolic function serially. It is important to place the cursor at the annulus side and to apply the M-Mode. Once the image is frozen, the TAPSE is the difference between the lowest and highest excursion points of the tricuspidal annulus. It has the advantage of easy reproducibility, speed of measurement and less dependence on optimal-quality images. There are some disadvantages: The measurement is angle dependent, and the displacement in the ME 4C view is representative of the function of the entire right ventricle only if there are no regional RV wall motion abnormalities. The TAPSE may also be load-dependent, even if less preload-dependent than other markers of RV function[69].
Right ventricular fractional area change: The FAC provides an estimate of the systolic function of the right ventricle. Usually measured in the ME 4C view, it is a simple method to assess RV function that correlates with RV ejection fractions measured by magnetic resonance imaging and has been related to outcome in a number of diseases[56,71]. It is important to verify that the entire right ventricle is in the view, including the apex and the lateral wall, in both systole and diastole. Care must be taken to exclude trabeculations while tracing the RV area. The normal range is 35%-60% and a two-dimensional FAC < 35% indicates RV systolic dysfunction[69].
In addition to all the pathophysiological processes that characterize ESLD, the three major stages of LT (pre-anhepatic, anhepatic and reperfusion phases) pose particular challenges in terms of anaesthetic management. TEE permits accurate monitoring of these clinical phases with rapid anaesthetic management during complicated procedures, maximizing the opportunities for a successful outcome.
The pre-anhepatic phase begins with surgical incision and concludes with cross clamping of the vascular inflow to the liver. The conventional technique involves clamping of the portal vein, the suprahepatic inferior vena cava, the infrahepatic inferior vena cava, and the hepatic artery. The piggyback technique requires temporary clamping of portal flow only and tangential clamping of the retrohepatic inferior vena cava, which allows venous return to the heart.
Surgical bleeding, when present, could be the main issue during the pre-anhepatic phase. Dissection may be complicated by steady and sometimes rapid haemorrhage from varices in the abdominal wall or adhesions within the abdominal cavity. Fluid shifts and third space fluid losses may result from the drainage of ascitic fluid and from venous congestion. Bleeding during this phase of surgery is related to the degree of pre-existing coagulopathy, the presence and severity of portal hypertension, and the duration and complexity of the surgical procedure[72,73].
Vascular clamping and manipulation of the liver, together with an inadequate volume resuscitation, result in decreased venous return and reduced cardiac output, resulting in critical organ hypoperfusion[74].
Sudden haemodynamic variations and a reduced preload are the most serious complications and challenges that the anaesthetist has to manage during LT.
TEE allows circulatory volumes to be optimized, avoiding impaired organ perfusion, ischaemia and, at the same time, volume overload. Identifying the cause of any hypotension is critical for successful treatment and aggressive fluid repletion leading to overload must be avoided to prevent pulmonary oedema or right ventricular failure due to unrecognized preoperative cardiac disease[3,75].
Close approximation of the papillary muscles and decreases in LVEDA and LVEDD in the TG Mid SAX view signal a reduced preload due to haemorrhage or vascular clamping: After a fluid challenge; TEE allows the anaesthetist to assess the adequacy the interventions rapidly. Signs of RV and right atrial dilation together with RV hypokinesis[76] or atypical regional wall motion abnormalities of the RV free wall[77] can draw attention to right ventricular failure.
If the conventional surgical technique with inferior vena cava and portal vein clamping is poorly tolerated, the VVB might be warranted to guarantee venous return to right atrium and to decompress the portal venous system, reducing bleeding, vascular congestion to the intestines, and injury to the bowel capillary bed[78].
In this case, TEE can assist in the placement of transcutaneous VVB lines, confirm the correct location of guide wires in the venous system, and detect the tip of the cannula in the superior vena cava[79]. Air embolism, thromboembolism, and inadvertent decannulation are among the reported and feared complications of VVB and can increase its morbidity[80].
The anhepatic phase starts after complete occlusion of vascular inflow to the liver; includes the removal of the native liver and the completion of the vascular anastomoses; and ends with the reperfusion of the newly grafted liver. This phase is mainly characterized by hemodynamic changes induced by the cross-clamping of the inferior vena cava and portal vein, which reduce venous return, cardiac output and renal perfusion pressure while increasing the splanchnic and lower caval pressures[25]. VVB is used routinely in some centres to facilitate return of blood from the portal system and lower body to the heart via a centrifugal pump to the axillary vein[78]. The TG Mid SAX view or ME 4C view can be ideal for monitoring ventricular function and volume status continuously during this phase. If preload is diminished or systemic vascular resistance is reduced, the left ventricular papillary muscles will approximate each other during systole and LVEDA will be reduced.
LT represents a special case of acute right ventricular stress. Cardiac output increases acutely at the time of reperfusion (up to 3-fold in 15 min) and this increased blood flow to the right heart can result in volume overload and pulmonary oedema due to occult cardiac disease[3].
This phase extends from the period immediately after the reperfusion of the graft to the end of surgery and includes the arterial anastomosis and biliary tract reconstruction. Cardiovascular instability is greatest during this phase of the operation, and it is as accompanied by a decrease in mean arterial pressure of 30% or more from baseline for at least 1 min’s duration, and occurring within 5 min of reperfusion[81,82].
The reperfusion syndrome is typically characterized by severe hypotension, decreased heart rate, a significant reduction in systemic vascular resistance, and increases in pulmonary arterial pressure and wedge pressure. All these changes are thought to result from the sudden release of cold, acidotic and hyperkalaemic preservation fluid into the circulation while myocardial dysfunction, often observed after reperfusion, is caused by several vasoactive mediators released into the circulation by the re-perfused graft[83].
The characteristic echocardiographic features of the reperfusion phase may include acute right ventricular systolic dysfunction, left ventricular systolic dysfunction or both; new global or focal wall motion abnormalities; decreased FAC; and because of rapid cardiac influx after vascular unclamping, increases in ventricular end-diastolic volume and LVEDA are common. Selecting and maintaining TEE, typically either in the ME 4C or TG Mid SAX views, allows for real time monitoring of all the effects of reperfusion on the heart. During transition from the anhepatic to the reperfusion phase, TEE views can also be useful for detecting intracardiac air, thrombosis, mitral or tricuspid valve regurgitation; severe diastolic dysfunction; or a previously undiagnosed outflow obstruction that can occur during reperfusion.
Following reperfusion TEE may be useful to detect the temporary opening of a foramen ovale[84] or elevation of pulmonary arterial pressures, facilitating prompt management of these complications.
Unique haemodynamic changes occur from the anhepatic to the reperfusion phases, and a real time TEE allows for a rapid assessment and adjustment of fluid shifts and an optimal outcome.
Although patients with liver disease were long assumed to have a natural bleeding tendency and to be protected from thrombosis, the real coagulation state of the cirrhotic patients combines changes in both pro- and anti-haemostatic pathways in a new haemostatic balance[85]. However, the occurrence of both bleeding and thrombotic complications in a significant proportion of patients shows that this haemostatic balance is relatively unstable[86]. Although LT is associated with increased bleeding and altered coagulation, a prothrombotic state may also occur. Intravascular thrombus formation and subsequent embolization is a potentially fatal complication that most often occurs after reperfusion. Both surgery (vascular clamping) and trauma pose an increased risk for pulmonary embolism (PE) and an incidental cardiac thrombosis, in particular, may lead to serious complications in LT, including intraoperative death[87]. Thus, anaesthesiologists may be responsible for both PE diagnosis and treatment, even if the diagnostic sensitivity of TEE for PE by direct visualization of a thrombus in the pulmonary artery is actually quite low[88]. Although TEE is not the gold standard for PE diagnosis, it can compare with the sensitivity of a TC scan when the PE is acute, central and characterized by severe haemodynamic instability[89,90]. Notably, only 30% obstruction is needed for RV dysfunction to be recognized on TEE[91] but the echocardiographic diagnosis of a PE using direct evidence often requires advanced TEE skills.
The Consensus Statement of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists recommends that “a physician trained in basic perioperative TEE at least should be able to use ME 4C, ME AV SAX, and ME RV inflow-outflow views to identify indirect echocardiographic findings consistent with a PE”[28]. Before initiating any specific treatment, the presence of thrombus and/or signs of RV dysfunction, typically due to elevated right-sided pressures, should be identified. The direct visualization of intracardiac thrombus or emboli in the main pulmonary artery or its right or left branches by TEE allows for diagnosis, though it is important to remember that the left pulmonary artery is not completely visualized with TEE due to the interposition of the left bronchus.
Echocardiographic findings consistent with acute PE include: Signs of RV dilation, RV hypokinesis[76], atypical regional wall motion abnormalities in the RV free wall[77], and decreased TAPSE.
Another typical sign of elevated right-sided pressures is the leftward shift of the interatrial septum or interventricular septum, which is easily seen in the ME 4C view. Although the RV is dysfunctional, the LV may appear hyperkinetic and underfilled, due to a leftward shift of the interventricular septum[92].
Cirrhosis is associated with increased left ventricular wall thickness, cardiac chamber enlargement, and a significantly impaired systolic and diastolic response to stress, especially in the setting of volume overload. The hypertrophic cardiomyopathy, which characterizes the cirrhotic patient, makes these patients particularly susceptible to LVOTO especially when hypovolemia, tachycardia, and increased ventricular contraction can cause apposition of the mitral valve anterior leaflet and the septal wall during systole[93]. The pre-anhepatic and anhepatic phases of LT are usually associated with decreases in the left ventricular preload secondary to intraoperative surgical or medical bleeding and to vascular clamping, whereas the post-reperfusion phase is usually associated with a marked decrease in systemic vascular resistance. Therefore, the patients undergoing LT often have several risk factors for dynamic LVOTO[94]. TEE, by virtue of its unique features, guarantees a continuous monitoring of cardiac function and structures, especially during the anhepatic and reperfusion phases[34] when the occurrence of dynamic LVOTO is more common, and can facilitate appropriate management and therapeutic interventions[95].
Dynamic LVOTO can be assessed readily in the ME LAX view at an approximately 120° angle. The characteristic features to look for are turbulence through the left ventricular outflow tract, hypercontractility of the left ventricle, systolic anterior leaflet motion of the mitral valve, and some degree of late mitral regurgitation[96]. A severe mitral regurgitation due to the systolic anterior leaflet motion represents a rapid qualitative method to quantify the degree of LVOTO. The degree of mitral regurgitation is a qualitative method to determine the severity of LVOTO because it usually correlates with the degree of outflow tract obstruction. Another rapid qualitative measure of LVOTO can be obtained by Colour-Doppler, where a mosaic pattern indicates turbulence associated with an elevated LVOT gradient. A quantitative assessment of the obstruction is otherwise obtained by a Doppler quantification of blood flow velocities through the LVOT, using the TG LAX view or the deep TG LAX view. Blood flow velocity in the LVOT is measured by positioning the continuous wave Doppler sample volume in the centre of the LVOT just proximal to the AV. Normal LVOT and AV flow velocities are less than 1.5 m/s[97]. Colour flow Doppler imaging of the LVOT and AV is useful in directing the Doppler beam through the area of maximum flow when these velocity measurements are made.
Liver disease and portal hypertension can be associated with pulmonary vascular complications, such as POPH, which is characterized by the presence of portal hypertension, a mean pulmonary artery pressure > 25 mmHg at rest, a mean pulmonary capillary wedge pressure < 15 mmHg, and a pulmonary vascular resistance > 240 dynxsxcm-5[98]. The prevalence of POPH in liver transplant candidates is reported to be 6.3%[99] and 8.5%[100]
Severe pulmonary hypertension and elevated right ventricular systolic pressure predicted a high risk of morbidity and mortality from fulminant right ventricular failure among patients undergoing LT[101]. Patients presenting for liver transplantation with pulmonary hypertension have an additional risk for RV dysfunction secondary to acute changes in pulmonary pressures associated with the volume shifts and acid base disturbances that characterize LT.
For this reason, most transplant centres consider severe POPH an absolute contraindication to transplantation[102]. On the other hand, a number of reports have confirmed that LT can be performed safely if the patient haemodynamic state is suitably controlled[102].
The most important test to screen for POPH is the two dimensional TTE, which is a routine part of an LT evaluation[103].
Unfortunately, the TTE cannot fully discriminate between increased PVR due to true vaso-occlusive arteriopathy, a hyperdynamic state, or fluid overload, with normal/low PVR. Therefore, right heart catheterization is the gold standard for the diagnosis of POPH[104].
For this reason, POPH may be missed by pre-operative echocardiography and may be recognized only during right heart catheterization in the intraoperative phase.
The diagnosis of unexpected POPH on the operating table may still be best handled through TEE. Continuous intraoperative transoesophageal echocardiography has been recommended for following right heart function, and in the event of a pulmonary hypertensive crisis, the anaesthetist has to be ready to address acute pulmonary hypertension with effective agents such as inhaled or intravenous vasodilators[102,105].
The echocardiographic intraoperative findings of severe pulmonary hypertension include right ventricular hypertrophy, dilatation and dysfunction, as well as right atrial enlargement. A paradoxical septal movement can be seen as well.
The best views to visualize the right heart chambers are ME 4C and ME RV inflow-outflow.
TEE has evolved as an important diagnostic tool outside the field of cardiac anaesthesia and it has gained increasing importance as a monitoring tool in liver anaesthesia. One of the most advantageous features of the TEE over PAC is the direct visualization of the heart in real time, which allows for instantaneous assessment of the state of the cardiovascular system, changes in global and regional contractility, and the rapid diagnosis of ventricular dilatation and failure. TEE can overcome the limitations of PAC measurements arising from the large core body temperature shifts typical of massive fluid infusion or revascularization of the new graft during LT.
TEE allows the intraoperative causes of hypotensive episodes during LT to be identified and can help rapidly optimize volaemia, avoiding organ perfusion impairment and ischaemia. TEE also allows for the intraoperative diagnosis and management of specific cardiovascular conditions that like portopulmonary hypertension, air embolism and thromboembolism, can complicate LT and the management of patients with hypertrophic cardiomyopathy, who may experience LVOTO.
Notwithstanding the published guidelines that define the basic and advanced competency requirements for TEE users, transoesophageal echocardiography is being used to monitor haemodynamics and for direct therapy in liver transplant patients, and clinically useful interpretations are possible even during the skill-acquisition phase of TEE training.
The value of TEE must of course be balanced against the risk of performing the procedure. Gastro-oesophageal varices are very common in patients listed for LT; thus, patients with ESLD should have a preoperative endoscopic surveillance and oropharyngeal examination, and probe manipulation should be limited to experienced operators.
Despite these limitations, the intraoperative TEE is a relatively safe method for monitoring cardiac performance in liver transplant patients and should not be contraindicated by the presence of oesophageal varices if the indications for the exam are important.
Although the interpretation of TEE remains mostly subjective, TEE has been helpful for assessing haemodynamic alterations, guiding fluid replacement and inotropic therapy, and identifying potential complications during LT, allowing for better management of patients.
P- Reviewer: Niu ZS S- Editor: Ma YJ L- Editor: A E- Editor: Liu SQ
| 1. | Wertheim JA, Petrowsky H, Saab S, Kupiec-Weglinski JW, Busuttil RW. Major challenges limiting liver transplantation in the United States. Am J Transplant. 2011;11:1773-1784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Petrowsky H, Rana A, Kaldas FM, Sharma A, Hong JC, Agopian VG, Durazo F, Honda H, Gornbein J, Wu V. Liver transplantation in highest acuity recipients: identifying factors to avoid futility. Ann Surg. 2014;259:1186-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 3. | Gaskari SA, Honar H, Lee SS. Therapy insight: Cirrhotic cardiomyopathy. Nat Clin Pract Gastroenterol Hepatol. 2006;3:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Alqahtani SA, Fouad TR, Lee SS. Cirrhotic cardiomyopathy. Semin Liver Dis. 2008;28:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | De Wolf A. Transesophageal echocardiography and orthotopic liver transplantation: general concepts. Liver Transpl Surg. 1999;5:339-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Gudavalli A, Kalaria VG, Chen X, Schwarz KQ. Intrapulmonary arteriovenous shunt: diagnosis by saline contrast bubbles in the pulmonary veins. J Am Soc Echocardiogr. 2002;15:1012-1014. [PubMed] |
| 7. | Vedrinne JM, Duperret S, Bizollon T, Magnin C, Motin J, Trepo C, Ducerf C. Comparison of transesophageal and transthoracic contrast echocardiography for detection of an intrapulmonary shunt in liver disease. Chest. 1997;111:1236-1240. [PubMed] |
| 8. | Mojadidi MK, Winoker JS, Roberts SC, Msaouel P, Gevorgyan R, Zolty R. Two-dimensional echocardiography using second harmonic imaging for the diagnosis of intracardiac right-to-left shunt: a meta-analysis of prospective studies. Int J Cardiovasc Imaging. 2014;30:911-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Meissner I, Whisnant JP, Khandheria BK, Spittell PC, O’Fallon WM, Pascoe RD, Enriquez-Sarano M, Seward JB, Covalt JL, Sicks JD. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Stroke Prevention: Assessment of Risk in a Community. Mayo Clin Proc. 1999;74:862-869. [PubMed] |
| 10. | Yerlioglu E, Krishnamoorthy V, Jeon H, Gustin A, Nicolau-Raducu R. Patent foramen ovale and intracardiac thrombus identified by transesophageal echocardiography during liver transplantation. J Cardiothorac Vasc Anesth. 2012;26:1069-1073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Garg A, Armstrong WF. Echocardiography in liver transplant candidates. JACC Cardiovasc Imaging. 2013;6:105-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Alba AC, Verocai Flaman F, Granton J, Delgado DH. Patent foramen ovale does not have a negative impact on early outcomes in patients undergoing liver transplantation. Clin Transplant. 2011;25:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Schumann R, Mandell MS, Mercaldo N, Michaels D, Robertson A, Banerjee A, Pai R, Klinck J, Pandharipande P, Walia A. Anesthesia for liver transplantation in United States academic centers: intraoperative practice. J Clin Anesth. 2013;25:542-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Burger-Klepp U, Karatosic R, Thum M, Schwarzer R, Fuhrmann V, Hetz H, Bacher A, Berlakovich G, Krenn CG, Faybik P. Transesophageal echocardiography during orthotopic liver transplantation in patients with esophagoastric varices. Transplantation. 2012;94:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 15. | Cárdenas A, Ginès P. Management of patients with cirrhosis awaiting liver transplantation. Gut. 2011;60:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Merli M, Nicolini G, Angeloni S, Rinaldi V, De Santis A, Merkel C, Attili AF, Riggio O. Incidence and natural history of small esophageal varices in cirrhotic patients. J Hepatol. 2003;38:266-272. [PubMed] |
| 17. | Spier BJ, Larue SJ, Teelin TC, Leff JA, Swize LR, Borkan SH, Satyapriya A, Rahko PS, Pfau PR. Review of complications in a series of patients with known gastro-esophageal varices undergoing transesophageal echocardiography. J Am Soc Echocardiogr. 2009;22:396-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086-2102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 19. | Hilberath JN, Oakes DA, Shernan SK, Bulwer BE, D’Ambra MN, Eltzschig HK. Safety of transesophageal echocardiography. J Am Soc Echocardiogr. 2010;23:1115-1127; quiz 1220-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 333] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 20. | Krenn CG, De Wolf AM. Current approach to intraoperative monitoring in liver transplantation. Curr Opin Organ Transplant. 2008;13:285-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Suriani RJ, Cutrone A, Feierman D, Konstadt S. Intraoperative transesophageal echocardiography during liver transplantation. J Cardiothorac Vasc Anesth. 1996;10:699-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Spencer KT. Transesophageal echocardiography in patients with esophageal varices. J Am Soc Echocardiogr. 2009;22:401-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Markin NW, Sharma A, Grant W, Shillcutt SK. The safety of transesophageal echocardiography in patients undergoing orthotopic liver transplantation. J Cardiothorac Vasc Anesth. 2015;29:588-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Robertson AC, Eagle SS. Transesophageal echocardiography during orthotopic liver transplantation: maximizing information without the distraction. J Cardiothorac Vasc Anesth. 2014;28:141-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Ozier Y, Klinck JR. Anesthetic management of hepatic transplantation. Curr Opin Anaesthesiol. 2008;21:391-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (1)] |
| 26. | Wax DB, Torres A, Scher C, Leibowitz AB. Transesophageal echocardiography utilization in high-volume liver transplantation centers in the United States. J Cardiothorac Vasc Anesth. 2008;22:811-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 27. | Schumann R. Intraoperative resource utilization in anesthesia for liver transplantation in the United States: a survey. Anesth Analg. 2003;97:21-28, table of contents. [PubMed] |
| 28. | Reeves ST, Finley AC, Skubas NJ, Swaminathan M, Whitley WS, Glas KE, Hahn RT, Shanewise JS, Adams MS, Shernan SK. Basic perioperative transesophageal echocardiography examination: a consensus statement of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2013;26:443-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Practice Guidelines for Perioperative Transesophageal Echocardiography. An Updated Report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology. 2010;112:1084-1096. |
| 30. | Shanewise JS, Cheung AT, Aronson S, Stewart WJ, Weiss RL, Mark JB, Savage RM, Sears-Rogan P, Mathew JP, Quiñones MA. ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. Anesth Analg. 1999;89:870-884. [PubMed] |
| 31. | Oxorn DC. Intraoperative echocardiography. Heart. 2008;94:1236-1243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Della Rocca G, Costa MG, Pompei L, Chiarandini P. The liver transplant recipient with cardiac disease. Transplant Proc. 2008;40:1172-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Feltracco P, Biancofiore G, Ori C, Saner FH, Della Rocca G. Limits and pitfalls of haemodynamic monitoring systems in liver transplantation surgery. Minerva Anestesiol. 2012;78:1372-1384. [PubMed] |
| 34. | Cywinski JB, Argalious M, Marks TN, Parker BM. Dynamic left ventricular outflow tract obstruction in an orthotopic liver transplant recipient. Liver Transpl. 2005;11:692-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Reuter DA, Huang C, Edrich T, Shernan SK, Eltzschig HK. Cardiac output monitoring using indicator-dilution techniques: basics, limits, and perspectives. Anesth Analg. 2010;110:799-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 36. | Bao FP, Wu J. Continuous versus bolus cardiac output monitoring during orthotopic liver transplantation. Hepatobiliary Pancreat Dis Int. 2008;7:138-144. [PubMed] |
| 37. | Böttiger BW, Sinner B, Motsch J, Bach A, Bauer H, Martin E. Continuous versus intermittent thermodilution cardiac output measurement during orthotopic liver transplantation. Anaesthesia. 1997;52:207-214. [PubMed] |
| 38. | Greim CA, Roewer N, Thiel H, Laux G, Schulte am Esch J. Continuous cardiac output monitoring during adult liver transplantation: thermal filament technique versus bolus thermo-dilution. Anesth Analg. 1997;85:483-488. [PubMed] |
| 39. | Hurford WE, Zapol WM. The right ventricle and critical illness: a review of anatomy, physiology, and clinical evaluation of its function. Intensive Care Med. 1988;14 Suppl 2:448-457. [PubMed] |
| 40. | Boucaud C, Bouffard Y, Dumortier J, Gaillac N, Sagnard P, Graber MC, Adham M, Boillot O. Transoesophageal echo-Doppler vs. thermodilution cardiac output measurement during hepatic vascular exclusion in liver transplantation. Eur J Anaesthesiol. 2008;25:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 41. | De Wolf AM. Pulmonary artery catheter: rest in peace? Not just quite yet. Liver Transpl. 2008;14:917-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Zink W, Nöll J, Rauch H, Bauer H, Desimone R, Martin E, Böttiger BW. Continuous assessment of right ventricular ejection fraction: new pulmonary artery catheter versus transoesophageal echocardiography. Anaesthesia. 2004;59:1126-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Savino JS, Troianos CA, Aukburg S, Weiss R, Reichek N. Measurement of pulmonary blood flow with transesophageal two-dimensional and Doppler echocardiography. Anesthesiology. 1991;75:445-451. [PubMed] |
| 44. | Muhiudeen IA, Kuecherer HF, Lee E, Cahalan MK, Schiller NB. Intraoperative estimation of cardiac output by transesophageal pulsed Doppler echocardiography. Anesthesiology. 1991;74:9-14. [PubMed] |
| 45. | Møller-Sørensen H, Graeser K, Hansen KL, Zemtsovski M, Sander EM, Nilsson JC. Measurements of cardiac output obtained with transesophageal echocardiography and pulmonary artery thermodilution are not interchangeable. Acta Anaesthesiol Scand. 2014;58:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Della Rocca G, Costa MG, Coccia C, Pompei L, Salandin V, Pierangelo DM, Pietropaoli P. Continuous right ventricular end-diastolic volume in comparison with left ventricular end-diastolic area. Eur J Anaesthesiol. 2009;26:272-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 47. | Cheung AT, Savino JS, Weiss SJ, Aukburg SJ, Berlin JA. Echocardiographic and hemodynamic indexes of left ventricular preload in patients with normal and abnormal ventricular function. Anesthesiology. 1994;81:376-387. [PubMed] |
| 48. | Della Rocca G, Brondani A, Costa MG. Intraoperative hemodynamic monitoring during organ transplantation: what is new? Curr Opin Organ Transplant. 2009;14:291-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Practice guidelines for perioperative transesophageal echocardiography. A report by the American Society of Anesthesiologists and the Society of Cardiovascular Anesthesiologists Task Force on Transesophageal Echocardiography. Anesthesiology. 1996;84:986-1006. [PubMed] |
| 50. | Vanatta JM, Dryn O, Berkley T, Nair S, Eason JD. Liver transplantation at the University of Tennessee Health Science Center in Memphis, Tennessee: the current era 2006-2012. Clin Transpl. 2012;103-109. [PubMed] |
| 51. | Lahm T, McCaslin CA, Wozniak TC, Ghumman W, Fadl YY, Obeidat OS, Schwab K, Meldrum DR. Medical and surgical treatment of acute right ventricular failure. J Am Coll Cardiol. 2010;56:1435-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 52. | Porter TR, Shillcutt SK, Adams MS, Desjardins G, Glas KE, Olson JJ, Troughton RW. Guidelines for the use of echocardiography as a monitor for therapeutic intervention in adults: a report from the American Society of Echocardiography. J Am Soc Echocardiogr. 2015;28:40-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 315] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 53. | Maslow AD, Mashikian J, Haering JM, Heindel S, Douglas P, Levine R. Transesophageal echocardiographic evaluation of native aortic valve area: utility of the double-envelope technique. J Cardiothorac Vasc Anesth. 2001;15:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 54. | De Wolf AM, Begliomini B, Gasior TA, Kang Y, Pinsky MR. Right ventricular function during orthotopic liver transplantation. Anesth Analg. 1993;76:562-568. [PubMed] |
| 55. | De Wolf AM, Aggarwal S. Monitoring preload during liver transplantation. Liver Transpl. 2008;14:268-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 56. | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2471] [Cited by in RCA: 2602] [Article Influence: 136.9] [Reference Citation Analysis (0)] |
| 57. | Schmidlin D, Jenni R, Schmid ER. Transesophageal echocardiographic area and Doppler flow velocity measurements: comparison with hemodynamic changes in coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 1999;13:143-149. [PubMed] |
| 58. | Swenson JD, Harkin C, Pace NL, Astle K, Bailey P. Transesophageal echocardiography: an objective tool in defining maximum ventricular response to intravenous fluid therapy. Anesth Analg. 1996;83:1149-1153. [PubMed] |
| 59. | Appleyard RF, Glantz SA. Two dimensions describe left ventricular volume change during hemodynamic transients. Am J Physiol. 1990;258:H277-H284. [PubMed] |
| 60. | Denault AY, Couture P, McKenty S, Boudreault D, Plante F, Perron R, Babin D, Buithieu J. Perioperative use of transesophageal echocardiography by anesthesiologists: impact in noncardiac surgery and in the intensive care unit. Can J Anaesth. 2002;49:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 61. | Guarracino F. Ecocardiografia transesofagea in area critica. 2008;213-250. |
| 62. | Thys DM, Hillel Z, Goldman ME, Mindich BP, Kaplan JA. A comparison of hemodynamic indices derived by invasive monitoring and two-dimensional echocardiography. Anesthesiology. 1987;67:630-634. [PubMed] |
| 63. | Skarvan K, Lambert A, Filipovic M, Seeberger M. Reference values for left ventricular function in subjects under general anaesthesia and controlled ventilation assessed by two-dimensional transoesophageal echocardiography. Eur J Anaesthesiol. 2001;18:713-722. [PubMed] |
| 64. | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8282] [Cited by in RCA: 8784] [Article Influence: 462.3] [Reference Citation Analysis (0)] |
| 65. | Reichert CL, Visser CA, van den Brink RB, Koolen JJ, van Wezel HB, Moulijn AC, Dunning AJ. Prognostic value of biventricular function in hypotensive patients after cardiac surgery as assessed by transesophageal echocardiography. J Cardiothorac Vasc Anesth. 1992;6:429-432. [PubMed] |
| 66. | Naik MM, Diamond GA, Pai T, Soffer A, Siegel RJ. Correspondence of left ventricular ejection fraction determinations from two-dimensional echocardiography, radionuclide angiography and contrast cineangiography. J Am Coll Cardiol. 1995;25:937-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 67. | Teichholz LE, Kreulen T, Herman MV, Gorlin R. Problems in echocardiographic volume determinations: echocardiographic-angiographic correlations in the presence of absence of asynergy. Am J Cardiol. 1976;37:7-11. [PubMed] |
| 68. | Hozumi T, Shakudo M, Shah PM. Quantitation of left ventricular volumes and ejection fraction by biplane transesophageal echocardiography. Am J Cardiol. 1993;72:356-359. [PubMed] |
| 69. | Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685-713; quiz 786-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4670] [Cited by in RCA: 5284] [Article Influence: 352.3] [Reference Citation Analysis (0)] |
| 70. | Ellis JE, Lichtor JL, Feinstein SB, Chung MR, Polk SL, Broelsch C, Emond J, Thistlethwaite JR, Roizen MF. Right heart dysfunction, pulmonary embolism, and paradoxical embolization during liver transplantation. A transesophageal two-dimensional echocardiographic study. Anesth Analg. 1989;68:777-782. [PubMed] |
| 71. | Maslow AD, Regan MM, Panzica P, Heindel S, Mashikian J, Comunale ME. Precardiopulmonary bypass right ventricular function is associated with poor outcome after coronary artery bypass grafting in patients with severe left ventricular systolic dysfunction. Anesth Analg. 2002;95:1507-1518, table of contents. [PubMed] |
| 72. | Hannaman MJ, Hevesi ZG. Anesthesia care for liver transplantation. Transplant Rev (Orlando). 2011;25:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Steadman RH. Anesthesia for liver transplant surgery. Anesthesiol Clin North America. 2004;22:687-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 74. | Møller S, Dümcke CW, Krag A. The heart and the liver. Expert Rev Gastroenterol Hepatol. 2009;3:51-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 75. | Burtenshaw AJ, Isaac JL. The role of trans-oesophageal echocardiography for perioperative cardiovascular monitoring during orthotopic liver transplantation. Liver Transpl. 2006;12:1577-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 76. | Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P, Bengel F, Brady AJ, Ferreira D, Janssens U. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29:2276-2315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1163] [Cited by in RCA: 1214] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 77. | McConnell MV, Solomon SD, Rayan ME, Come PC, Goldhaber SZ, Lee RT. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol. 1996;78:469-473. [PubMed] |
| 78. | Miranda LE, de Melo PS, Sabat BD, Tenório AL, Lima DL, Neto OC, Amorim AG, Fernandez JL, de Macedo FI, Lacerda CM. Orthotopic liver transplantation without venovenous bypass: 125 cases from a single center. Transplant Proc. 2012;44:2416-2422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 79. | Planinsic RM, Nicolau-Raducu R, Caldwell JC, Aggarwal S, Hilmi I. Transesophageal echocardiography-guided placement of internal jugular percutaneous venovenous bypass cannula in orthotopic liver transplantation. Anesth Analg. 2003;97:648-649. [PubMed] |
| 80. | Budd JM, Isaac JL, Bennett J, Freeman JW. Morbidity and mortality associated with large-bore percutaneous venovenous bypass cannulation for 312 orthotopic liver transplantations. Liver Transpl. 2001;7:359-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Aggarwal S, Kang Y, Freeman JA, Fortunato FL, Pinsky MR. Postreperfusion syndrome: cardiovascular collapse following hepatic reperfusion during liver transplantation. Transplant Proc. 1987;19:54-55. [PubMed] |
| 82. | Chung IS, Kim HY, Shin YH, Ko JS, Gwak MS, Sim WS, Kim GS, Lee SK. Incidence and predictors of post-reperfusion syndrome in living donor liver transplantation. Clin Transplant. 2012;26:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (2)] |
| 83. | Nanashima A, Pillay P, Crawford M, Nakasuji M, Verran DJ, Painter D. Analysis of postrevascularization syndrome after orthotopic liver transplantation: the experience of an Australian liver transplantation center. J Hepatobiliary Pancreat Surg. 2001;8:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 84. | Saada M, Liu N, Cherqui D, Beydon L, Maurel C, Catoire P, Bonnet F, Duvaldestin P. [Opening of a foramen ovale during liver transplantation. The value of transesophageal echocardiography]. Ann Fr Anesth Reanim. 1990;9:412-414. [PubMed] |
| 85. | Weeder PD, Porte RJ, Lisman T. Hemostasis in liver disease: implications of new concepts for perioperative management. Transfus Med Rev. 2014;28:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 86. | Lisman T, Porte RJ. Rebalanced hemostasis in patients with liver disease: evidence and clinical consequences. Blood. 2010;116:878-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 444] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 87. | Xia VW, Ho JK, Nourmand H, Wray C, Busuttil RW, Steadman RH. Incidental intracardiac thromboemboli during liver transplantation: incidence, risk factors, and management. Liver Transpl. 2010;16:1421-1427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 88. | Rosenberger P, Shernan SK, Body SC, Eltzschig HK. Utility of intraoperative transesophageal echocardiography for diagnosis of pulmonary embolism. Anesth Analg. 2004;99:12-16. [PubMed] |
| 89. | Pruszczyk P, Torbicki A, Kuch-Wocial A, Chlebus M, Miśkiewicz ZC, Jedrusik P. Transoesophageal echocardiography for definitive diagnosis of haemodynamically significant pulmonary embolism. Eur Heart J. 1995;16:534-538. [PubMed] |
| 90. | Krivec B, Voga G, Zuran I, Skale R, Pareznik R, Podbregar M, Noc M. Diagnosis and treatment of shock due to massive pulmonary embolism: approach with transesophageal echocardiography and intrapulmonary thrombolysis. Chest. 1997;112:1310-1316. [PubMed] |
| 91. | Wolfe MW, Lee RT, Feldstein ML, Parker JA, Come PC, Goldhaber SZ. Prognostic significance of right ventricular hypokinesis and perfusion lung scan defects in pulmonary embolism. Am Heart J. 1994;127:1371-1375. [PubMed] |
| 92. | Matthews JC, McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev. 2008;4:49-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 93. | Ramaraj R. Hypertrophic cardiomyopathy: etiology, diagnosis, and treatment. Cardiol Rev. 2008;16:172-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 94. | Lee AR, Kim YR, Ham JS, Lee SM, Kim GS. Dynamic left ventricular outflow tract obstruction in living donor liver transplantation recipients -A report of two cases-. Korean J Anesthesiol. 2010;59 Suppl:S128-S132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 95. | Roy D, Ralley FE. Anesthetic management of a patient with dynamic left ventricular outflow tract obstruction with systolic anterior movement of the mitral valve undergoing redo-orthotopic liver transplantation. J Cardiothorac Vasc Anesth. 2012;26:274-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 96. | Ashidagawa M, Ohara M, Koide Y. An intraoperative diagnosis of dynamic left ventricular outflow tract obstruction using transesophageal echocardiography leads to the treatment with intravenous disopyramide. Anesth Analg. 2002;94:310-312, table of contents. [PubMed] |
| 97. | Shanewise JS, Cheung AT, Aronson S, Stewart WJ, Weiss RL, Mark JB, Savage RM, Sears-Rogan P, Mathew JP, Quiñones MA. ASE/SCA guidelines for performing a comprehensive intraoperative multiplane transesophageal echocardiography examination: recommendations of the American Society of Echocardiography Council for Intraoperative Echocardiography and the Society of Cardiovascular Anesthesiologists Task Force for Certification in Perioperative Transesophageal Echocardiography. J Am Soc Echocardiogr. 1999;12:884-900. [PubMed] |
| 98. | Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43-S54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1574] [Cited by in RCA: 1440] [Article Influence: 90.0] [Reference Citation Analysis (0)] |
| 99. | Kawut SM, Krowka MJ, Trotter JF, Roberts KE, Benza RL, Badesch DB, Taichman DB, Horn EM, Zacks S, Kaplowitz N. Clinical risk factors for portopulmonary hypertension. Hepatology. 2008;48:196-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 177] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 100. | Ramsay MA, Simpson BR, Nguyen AT, Ramsay KJ, East C, Klintmalm GB. Severe pulmonary hypertension in liver transplant candidates. Liver Transpl Surg. 1997;3:494-500. [PubMed] |
| 101. | Porres-Aguilar M, Zuckerman MJ, Figueroa-Casas JB, Krowka MJ. Portopulmonary hypertension: state of the art. Ann Hepatol. 2008;7:321-330. [PubMed] |
| 102. | Ramsay M. Portopulmonary hypertension and right heart failure in patients with cirrhosis. Curr Opin Anaesthesiol. 2010;23:145-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 103. | Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, Beghetti M, Corris P, Gaine S, Gibbs JS. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30:2493-2537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2304] [Cited by in RCA: 2254] [Article Influence: 140.9] [Reference Citation Analysis (0)] |
| 104. | Duarte-Rojo A, Altamirano JT, Feld JJ. Noninvasive markers of fibrosis: key concepts for improving accuracy in daily clinical practice. Ann Hepatol. 2012;11:426-439. [PubMed] |
| 105. | Safdar Z, Bartolome S, Sussman N. Portopulmonary hypertension: an update. Liver Transpl. 2012;18:881-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |