Published online Sep 18, 2015. doi: 10.4254/wjh.v7.i20.2245
Peer-review started: December 10, 2014
First decision: February 7, 2015
Revised: May 16, 2015
Accepted: August 30, 2015
Article in press: August 31, 2015
Published online: September 18, 2015
Processing time: 279 Days and 5.2 Hours
Many years after therapeutic wilderness, sorafenib finally showed a clinical benefit in patients with advanced hepatocellular carcinoma. After the primary general enthusiasm worldwide, some disappointments emerged particularly since no new treatment could exceed or at least match sorafenib in this setting. Without these new drugs, research focused on optimizing care of patients treated with sorafenib. One challenging research approach deals with identifying prognostic and predictive biomarkers of sorafenib in this population. The task still seems difficult; however appropriate investigations could resolve this dilemma, as observed for some malignancies where other drugs were used.
Core tip: The approval of sorafenib in advanced hepatocellular carcinoma is based on the positive results of two large randomized phase III clinical trials. The inter- and intra-individual variability regarding tumor response and clinical outcome highlighted the unmet need of effective biomarkers of response. These biomarkers could be useful for monitoring treatment activity, detecting early resistance to treatment and identifying patients who would more likely benefit from treatment. An overview of prognostic/predictive biomarkers of sorafenib in hepatocellular carcinoma is discussed in this review.
- Citation: Bouattour M, Payancé A, Wassermann J. Evaluation of antiangiogenic efficacy in advanced hepatocellular carcinoma: Biomarkers and functional imaging. World J Hepatol 2015; 7(20): 2245-2263
- URL: https://www.wjgnet.com/1948-5182/full/v7/i20/2245.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i20.2245
Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third leading cause of cancer-related deaths worldwide[1,2]. The incidence of HCC is steadily increasing with about 625000 new cases per year and the disease results in around 600000 deaths yearly over the world[1,2]. Less than 30% of patients diagnosed with HCC are eligible for curative treatment[3] and during the course of the natural evolution of HCC; a significant proportion of patients are candidates for systemic therapies. In recent years, considerable progress has been made in furthering the knowledge of molecular biology of HCC, including better understanding of the role of signaling pathways and angiogenesis[4-8]. These advances have led to the development of targeted therapies in HCC[9-11]. Nevertheless, only sorafenib, a multikinase inhibitor, remains till date the sole approved drug in advanced HCC, based on the clinical benefit observed in properly selected patients enrolled in clinical trials[12,13]. With only three months of survival gain compared to placebo, many practitioners and country health authorities consider the cost-efficacy ratio of sorafenib somewhat insufficient[14-16]. In some emerging countries, the drug is not even approved for patients with advanced HCC. Otherwise, published data and clinical practice highlight a great inter-individual and even intra-individual variation regarding clinical benefit and toxicity[17-22]. For clinicians, there is an unmet need to identify patients more likely to benefit from treatment. Thus, to dispose of predictive markers of response and to support the decision to continue treatment when better outcome has been detected early. Thus, to improve patient management, avoid side effects when sorafenib has proved ineffective, and control health expenses and clinical research. Numerous clinical, plasma and tumor-derived biomarkers have already been studied. Some of them have been proposed as predictive surrogate markers of activity of sorafenib and other antiangiogenic agents. Furthermore, Response Evaluation Criteria in Solid Tumors (RECIST) criteria[23,24] were proposed to evaluate tumor size changes during treatment in patients with cancer. Novel imaging techniques and radiological methods were suggested to strengthen the standard RECIST criteria in HCC to evaluate, directly in patients, the effects of drugs on tumor angiogenesis.
Herein, we review the current knowledge about prognostic/predictive and pharmacodynamics biomarkers for sorafenib and other antiangiogenic agents in advanced HCC and their potential integration into clinical practice. We also discuss the place of functional imaging to evaluate tumor response in advanced HCC. The Tables 1-3 give an overview of different studies of biomarkers in advanced HCC referred to in this review.
| Ref. | Markers | Patients (n) | Study design | Treatment | Level values | Clinical impact | Conclusion/comments |
| Schoenleber et al[85] | VEGF-A | 1018 | Systemic review and meta-analysis including only serum-based studies | Various (surgery, LRT and systemic therapies) | High serum VEGF level | Poorer OS | Serum VEGF method detection varied among studies |
| Poorer DFS | Serum VEGF levels seem more reliable than tissue VEGF for HCC prognosis | ||||||
| Poon et al[115] | bFGF | 88 | Prospective | Surgery | High serum level > 10.8 pg/mL | Larger tumor > 5 cm | High bFGF serum level before surgery was shown to be an independent factor of early recurrence. No further studies confirmed these findings |
| Venous invasion | |||||||
| Vejchapipat et al[105] | HGF | 55 | Retrospective | BSC | High level (≥ 1.0 ng/mL | Advanced pTNM stage Poorer prognosis Poorer OS | Although a control group was included, results of this small cohort study need confirmation in larger prospective analysis |
| Chau et al[104] | 40 | Retrospective | Resection | High portal and serum HGF level (> 699 pg/mL) | Multiple tumor | One limit of this study were the feasibility in routine of intraoperative puncture of the portal vein was difficult | |
| Poorer prognosis | |||||||
| Mizuguchi et al[106] | 100 | Retrospective | Resection | High serum level (≥ 0.35 ng/mL) | Postoperative complications | No correlation was observed between HGF level and RFS | |
| Poorer OS | |||||||
| Kaseb et al[87] | IGF-1 | 288 | Prospective | Various | Low plasma level (26 ng/mL) | High Child-Pugh score | The authors proposed that IGF-1 plasma level to be integrated into the BCLC staging system to predict OS for personal management in patients with HCC. This proposal was not yet adopted in clinical practice |
| High AST level | |||||||
| High tumor size | |||||||
| Multiple tumor | |||||||
| Vascular invasion | |||||||
| Poorer OS |
| Ref. | Marker | Patient (n) | Study type | Treatment | Levels values | Prognostic value | Conclusion/comments |
| Kaseb et al[86] | VEGF-A | 394 | Systemic review including only serum or plasma-based studies | Various (AA alone or combined with CT) | High serum or plasma level | Poorer outcome | Plasma VEGF seemed more relevant than serum VEGF as prognostic factor for HCC |
| Llovet et al[63] | 490 | Prospective phase III trial | Sorafenib vs placebo | High plasma level (> 101 pg/mL) | Poor OS | The VEGF level was a prognostic factor for all patient's cohort but surprisingly it did not affect prognosis in patients receiving sorafenib. Moreover, the VEGF level did not predict response | |
| Better clinical/ demographic parameters | |||||||
| Llovet et al[63] | HGF | 251 | Prospective phase III trial | Sorafenib vs placebo | High plasma level | Poorer OS | HGF was a prognostic factor for the entire cohort. However, it does not predict response to sorafenib (only a nonsignificant trend) |
| Miyahara et al[112] | Ang2 | 30 | Prospective? | Sorafenib | High serum level | Shorter PFS Progressive disease | The small cohort and the lack of control arm hamper conclusion on the role of Ang2 as predictive of response to sorafenib |
| Llovet et al[63] | 490 | Prospective phase III trial | Sorafenib vs placebo | High plasma level (> 6043.5 pg/mL) | Poorer OS Better clinical/demographic parameters | Ang2 was shown to be a prognostic factor in HCC but did not predict response to sorafenib | |
| Llovet et al[63] | c-KIT | 245 | Prospective phase III trial | Sorafenib vs placebo | High plasma level (> 11.3 ng/mL) | Trend to a better OS | Soluble c-KIT was shown to be a prognostic factor for HCC. However, it showed only a nonsignificant trend to predict response to sorafenib |
| Trend to better TTP | |||||||
| Better clinical/demographic parameters | |||||||
| Llovet et al[63] | IGF-2 | 254 | Prospective phase III trial | Sorafenib vs placebo | High plasma level (> 797.7 ng/mL) | Better OS | IGF-2 was shown to be prognostic factor in HCC but did not predict response to sorafenib |
| Better clinical/demographic parameters | |||||||
| Shao et al[126] | CEC/CECP | 40 | Prospective | Sorafenib + CT | High CECP level | Poorer PFS Poorer OS | The predictive value of CECP was not confirmed in further investigations |
| Ref. | Marker | Patient (n) | Study design | Treatment | Marker treatment-induced changes | Impact value | Comments |
| Llovet et al[63] | VEGF-A | 490 | Prospective phase III trial | Sorafenib vs placebo | Increase | No association with OS and ORR | The VEGF-A could serve as pharmacodynamic marker of exposure to sorafenib but did not have prognostic or predictive value |
| Harmon et al[93] | 37 | Prospective single arm phase II | Sunitinib | Reversible Increase | Better DCR | Inconsistent results were observed in these trials. The value of VEGF-A to predict response to sunitinib could be confirmed in larger trial | |
| Better PFS | |||||||
| Better OS | |||||||
| Zhu et al[91] | VEGF-C | 34 | Prospective single arm phase II | Sunitinib | Sustained increase | No predictive value | |
| Harmon et al[93] | 37 | Prospective single arm phase II | Sunitinib | Decrease | Better DC | The predictive value of VEGF-C was not shown for sorafenib probably because of its limited action against the VEGFR-3 | |
| Better ORR | |||||||
| Harmon et al[93] | sVEGFR-2/ sVEGFR-3 | 37 | Prospective single arm phase II | Sunitinib | Reversible decrease | Better OS (for sVEGFR-2) | The small cohort did not allow a definite conclusion |
| Zhu et al[91] | 34 | Prospective single arm phase II | Sunitinib | Decrease | No predictive value | ||
| Llovet et al[63] | Ang2 | 490 | Prospective phase III trial | Sorafenib vs placebo | No significant change (for sorafenib) Increase (for placebo) | Shorter TTP Shorter OS (for patients who experienced increase) | Ang2 was probably a prognostic biomarker than predictive of response to sorafenib |
| Llovet et al[63] | c-KIT | 245 | Prospective single arm phase II | Sorafenib vs placebo | Decrease (sorafenib) no change (placebo) | No predictive value | Tumor expression of KIT was considered as low in HCC, and the role of soluble KIT remains unclear |
| Zhu et al[91] | 34 | Prospective single arm phase II | Sunitinib | Decrease | Better TTP | ||
| Better OS | |||||||
| Harmon et al[93] | 37 | Prospective single arm phase II | Sunitinib | Decease | Better TTP | ||
| Boige et al[98] | CEC | 36 | Prospective single arm phase II | Bevacizumab | Early increase | Better OR | CEC level was not associated with prognosis in this study. However, it could predict response to bevacizumab. The rarity of CEC level and non-standardized measurement methods limited the use of CEC as a predictive marker of response to treatment in HCC |
| Better DCR | |||||||
| Zhu et al[91] | CECP | 34 | Prospective single arm phase II | Sunitinib | Decrease | Progression |
The national institute of health defined “biological marker (biomarker): a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention”[25]. Additionally, Ludwig et al[26] defined biomarkers as molecular, cellular or functional quantifiable or quantitative parameters indicative of particular genetic, epigenetic histological or cytological tumor abnormality. Initially, biomarkers were used for risk assessment and screening in cancers and later, to enhance cancer staging, to refine prognosis and to evaluate the response to biological therapy[27]. Biomarkers could then be clinical, biological, molecular or imaging parameters. Identifying prognostic and predictive biomarkers to antiangiogenic therapies is a crucial issue in HCC to be integrated into clinical care in the future. Previously, some predictive biomarkers of anticancer therapy response were identified in the field of oncology. Indeed, the efficacy of anti-epidermal growth factor receptors, such as cetuximab and panitumumab, in metastatic colorectal cancer is limited to proto-oncogene proteins p21(ras) (KRAS) wild-type cancer[28-30]. Other predictive biomarkers are used in clinical practice. For instance, the human epidermal growth factor receptor 2 expression in gastric and breast cancers to predict response to trastuzumab[31-33] and pertuzumab[34]. Moreover, gefitinib and erlotinib showed significant efficacy in patients with specific endothelial growth factor receptor (EGFR) mutations[35,36]. Recently, proto-oncogene proteins B-raf (BRAF) V600 E mutation in patients with metastatic melanoma was proved to be predictive of response to vemurafenib[37]. Regarding HCC, biomarkers should ideally meet at least the following criteria[26,38]: (1) to be easily measurable through minimally invasive procedures, ideally using blood tests; (2) to have a prognostic value in relation to the natural history and the outcome of HCC; (3) to have a predictive value wherein its presence correlates with the clinical response to sorafenib therapy; and (4) preferably not to be detectable in premalignant diseases (e.g., cirrhosis).
Positive impact of drug-related cutaneous adverse events on clinical outcome was initially reported in patients treated with epidermal growth factor receptor inhibitors for advanced colorectal cancers[29,39], non-small-cell lung cancers[40] and pancreatic cancers[41]. Some retrospective studies have shown in patients with advanced HCC treated with sorafenib a positive association with early skin drug-related toxicities and clinical benefit[42-44] and disease control[44,45] (Table 4). Recently, the Barcelonan group reported the results of a prospective single-arm, monocentric study that assessed the link between early sorafenib-related skin toxicities and outcome in patients with advanced HCC[46]. Added to baseline performance status and barcelona-clinic-liver-cancer staging system[47], early sorafenib-induced skin reactions were an independent predictor of overall survival (OS). Patients who experienced skin adverse events have a better outcome compared to patients without any cutaneous reactions. The time to progression (TTP) was significantly longer in the first group (8.1 mo, 95%CI: 1.6-14.5, vs 3.9 mo, 95%CI: 2.08-5.7; P = 0.016) as well as OS (18.2 mo, 95%CI: 11.9-24.4, vs 10.1 mo, 95%CI: 10.1-13.0; P = 0.009)[46]. Accordingly, early skin reactions during sorafenib treatment may indicate antitumor effect and clinical benefit in patients with advanced HCC. These findings support the need to maintain treatment provided that these side effects are well managed.
| Ref. | Side effect | Patients (n) | Study design | Impact on survival | Impact on other parameters | Predictive value |
| Otsuka et al[42] | Skin reaction | 94 | Retrospective | Better OS | No impact on ORR, DCR, and TTP | No |
| Vincenzi et al[45] | 65 | Retrospective | Trend to a better OS | Better DCR | Early skin toxicity could predict efficacy of sorafenib | |
| Better TTP | ||||||
| Di Costanzo et al[43] | 65 | Retrospective | Better OS | Not reported | Skin toxicity could predict survival | |
| Shomura et al[44] | 37 | Retrospective | Better OS | Better DCR | Skin toxicity could predict efficacy | |
| Reig et al[46] | 147 | Prospective | Better OS | Better TTP | Early skin reaction could predict efficacy of sorafenib and survival | |
| Otsuka et al[42] | Arterial hypertension | 94 | Retrospective | No impact | No impact | No |
| Estfan et al[55] | 41 | Retrospective | Better OS | Trend to better TTP |
Arterial hypertension is a frequent side effect observed in patients treated with antiangiogenic agents. The incidence of arterial hypertension in patients treated with sorafenib for advanced cancers was estimated at 23.1%[48]. Previous studies showed a positive link between arterial hypertension due to bevacizumab and outcome in patients with advanced colorectal cancer[49,50] and renal cell cancer[51] or related to axitinib in pancreatic cancer[52]. However, a recent systematic review of all placebo-controlled phase III trials with bevacizumab failed to demonstrate any positive impact of drug-related arterial hypertension and clinical benefit [progression-free survival (PFS) and OS] in patients with advanced cancers[53]. Sorafenib-induced arterial hypertension was reported to be predictive of clinical benefit in patients with metastatic renal cell cancer[54]. Estfan et al[55] found in a small cohort of patients with advanced HCC that arterial hypertension related to sorafenib correlated with better OS[55]. These results were not reproduced in other retrospective[42] and prospective[46] studies. Thus, no robust data is available to prove the link between an increase in blood pressure during sorafenib treatment and clinical benefit or antitumor activity for HCC (Table 4). In summary, no clinical biomarkers of response to sorafenib were validated in clinical practice. Based on the Barcelonan prospective study, cutaneous adverse events seem to be the best track to explore in patients treated with sorafenib for advanced HCC. These results should be interpreted with caution since no untreated control arm was evaluated in this study.
Alpha-fetoprotein: Serum alpha-fetoprotein (AFP) is the only biomarker that passed all five phases of biomarker development as defined by Pepe et al[56]. AFP remains a useful prognostic marker and probably a predictive marker of treatment response in HCC (Tables 5 and 6). In a large Chinese retrospective cohort, high serum AFP level correlated with larger HCC size, vascular invasion and low tumor differentiation[57]. Previous studies showed that AFP levels could be useful to predict recurrence after surgery[58,59], liver transplantation[60-62]. The value of AFP as a prognostic marker was reported in several studies evaluating sorafenib in advanced HCC. The SHARP trial[12] is a phase 3, placebo-controlled trial that studied the benefit of sorafenib vs placebo in 602 patients with advanced HCC. Llovet et al[63] showed in patients included in this study that high baseline AFP plasma levels (> 200 ng/mL) have a negative impact on OS[63]. These findings confirmed previous results reported with sorafenib a small cohort of patients with advanced HCC[64], in retrospective analysis[65]. High baseline serum AFP level (≥ 400 ng/mL) also seemed associated with shorter TTP[63]. Noticeably, in a recent analysis of six prospective phase II trials evaluating systemic therapies for patients with advanced HCC, no association between baseline AFP levels and prognosis was observed[66]. More interestingly, some authors evaluated the kinetics of AFP during treatment in HCC as a predictive marker of response or outcome. Previous studies showed a positive correlation between the decrease of AFP plasma levels and objective response and OS in patients with advanced HCC receiving systemic therapies[67,68]. Small series reported the value of baseline and changes in AFP plasma levels to predict response and outcome for patients with advanced HCC treated with sorafenib. Several studies showed consistent correlation between early (varying from 2 to 8 wk) decrease of AFP level more than 20% following sorafenib and objective response[69-73] and better outcome[69-71,73] in patients with advanced HCC. Personeni et al[71] showed that early responders, defined by a 20% decrease of AFP 8 wk after sorafenib treatment, had significantly better median OS and TTP compared to non-responders (13.8 mo vs 8.2 mo, P = 0.022 and 7.9 mo vs 2.4 mo, P = 0.004; respectively)[71]. In a recent study, Nakazawa et al[74] did not find a significant link between pretreatment AFP levels and tumor response in patients with advanced HCC treated with sorafenib. However, an early increase in AFP levels correlates with poorer outcome with shorter OS and PFS[74].
| Ref. | Patient (n) | Study design | Treatment | Level values | Impact value | Comments | |
| Liu et al[57] | AFP | 2034 | Retrospective | Resection (79.2%) NA (20.8) | High AFP levels (> 20 μg/L) | Large tumors (≥ 10 cm) | This large cohort study showed that High AFP level was associated with poor prognosis and poor clinicopathological features of HCC |
| Higher vascular invasion | |||||||
| Lower differentiated tumor | |||||||
| Wang et al[139] | 160 | Retrospective | Resection | High AFP level (> 4000 UI/L) | Shorter median TTR | In this study, the value of AFP levels to predict recurrence is limited since only a few numbers of patients (9%) have AFP level higher than the cutoff level | |
| Ma et al[58] | 108 | Retrospective | Resection | High AFP level (> 20 ng/mL) | Lower differentiated tumor | This study demonstrated the negative impact of high AFP levels on surgery benefit and the need to closely screen patients after resection for recurrence | |
| Higher vascular invasion | |||||||
| Higher postoperative 2-yr recurrence rate | |||||||
| Lower 24-mo survival rate | |||||||
| Ikai et al[59] | 12118 | Japanese nationwide | Resection | High AFP level (≥ 20 ng/mL) | Worsen OS after surgery | This large cohort study showed better outcome of patient resected for HCC in the last decade but the persistence of the negative impact of high AFP level on prognosis | |
| Analysis | |||||||
| Comparative study | |||||||
| Vibert et al[60] | 153 | Retrospective | LT | AFP level increase > 15 μg/L per month | Lower OS | This study showed the negative impact on the outcome of AFP levels increases in patients undergoing LT | |
| Lower RFS | |||||||
| Higher recurrence rate | |||||||
| Hakeem et al[61] | 12159 | Systemic review | LT | AFP > 1000 ng/mL (based on the majority of study included in the review) | Poorer OS | The authors stressed the poor quality of previous studies and the need for high-quality evidence on outcomes to use AFP levels as a prognostic indicator for patients undergoing LT | |
| Poorer DFS | |||||||
| Higher vascular invasion | |||||||
| Poorer differentiated tumor | |||||||
| Duvoux et al[62] | 972 | Prospective/retrospective | LT | High AFP level | Tumor recurrence | A new score model including AFP level was proposed to select patients for LT | |
| Vascular invasion | |||||||
| Poor differentiation |
| Ref. | Patients (n) | Study design | Treatment | Level values | Clinical impact | Comments | |
| Shim et al[160] | AFP | 57 | Retrospective | Sorafenib | High level ≥ 400 ng/mL | Shorter TTP | This study suffers from some limits: a retrospective study, a small cohort including only hepatitis B patients, short median follow-up duration, lack of correlation with OS or ORR |
| Shao et al[69] | 72 | Prospective | Various AA + CT | AFP response (> 20% decrease from baseline within the first four weeks) | Better DCR | The magnitude of AFP decline (20% or 50%) from baseline was not clearly defined. Similarly, the time point for evaluation of AFP level was not clear also (4 wk? 7 wk?). Limits: a small number of patients with heterogeneous treatment | |
| Better ORR | |||||||
| Better PFS | |||||||
| Better OS | |||||||
| Yau et al[70] | 94 | Retrospective | Sorafenib | AFP response (> 20% decrease from baseline within the first six weeks) | Clinical benefit rate | The cutoff value to define AFP response was inconsistent between various studies | |
| Better PFS | |||||||
| Marginal better OS | |||||||
| Personeni et al[71] | 85 | Retrospective | Sorafenib | AFP response (> 20% decrease from baseline within the first six weeks) | Better DCR | The authors used the landmark method to limit the potential favorable outcome due to tumor features than to AFP response | |
| Better TTP | |||||||
| Better OS | |||||||
| Køstner et al[72] | 76 | Retrospective | Sorafenib | AFP response (> 20% decrease from baseline within the first four weeks) | Better ORR | No correlation was observed between AFP response and OS probably because of the limited number of patients evaluated and the unusual poor OS seen in all cohort (5.4 mo) | |
| Kuzuya et al[73] | 48 | Retrospective | Sorafenib | AFP response (decrease from baseline within 2 and 4 wk) | Better DCR | Limits of the study: retrospective design and the small number of patients included | |
| Better TTP | |||||||
| Better OS | |||||||
| Nakazawa et al[74] | 59 | Retrospective | Sorafenib | AFP response (increase from baseline within four weeks) | Progressive disease | Limits of the study: a small number of patients was enrolled in this and retrospective study. No association between AFP level before treatment and tumor response was observed | |
| Shorter PFS | |||||||
| Shorter OS | |||||||
| Llovet et al[63] | 491 | Prospective Phase III trial | Sorafenib vs placebo | High plasma level > 200 ng/mL | Poorer OS | The impact of baseline AFP on survival was observed in both groups of patients treated with placebo or sorafenib | |
| Hsu et al[64] | 53 | Prospective single-arm Phase II trial | Sorafenib + mT/U | > 400 ng/mL | Poorer OS? | The prognostic value of baseline AFP level was shown only in univariate analysis and only score CLIP ≥ 3 was an independent prognostic factor of poor OS | |
| Baek et al[65] | 201 | Retrospective | Sorafenib | ≥ 400 ng/mL | Shorter FFS | Baseline AFP level, tumor size, PS, albumin and bilirubin levels were the independent factor associated with OS in this study | |
| Poorer OS | |||||||
| Lin et al[66] | 156 | Systemic review of the prospective phase II trials | Various systemic therapies | ≥ 400 ng/mL | No impact | Limits of the study: heterogeneous population | |
| Shao et al[119] | 45 | Pooled analysis of single-arm phase II trials | Sorafenib + mT/U and beva + C | > 400 ng/mL | No impact | This study especially focused on the impact of IGF factors on outcome and the small cohort analyzed limits the interpretation of the effect of AFP levels on survival |
Japanese groups proposed the lens culinaris agglutinin reactive AFP (AFP-L3), an isoform of AFP, as a good diagnostic and prognostic biomarker for HCC[75-77]. However, scant data is/are available regarding the value of AFP-L3 as predictive of response to antiangiogenic agents in HCC[78].
In summary, available data are not consistent enough to confirm the value of baseline AFP level as a predictive marker of response to antiangiogenic treatment for patients treated for advanced HCC[79].
Des-gamma-carboxy prothrombin: Des-gamma-carboxy prothrombin (DCP) is a prognostic factor for HCC as shown by Japanese research[80]. Changes in DCP plasma level were evaluated in patients treated with sorafenib[73,81,82]. Some studies reported that DCP could be an independent factor of survival in patients treated with sorafenib[81,82]. These results were not reproduced in other reports[73]. DCP is currently used mainly in Japan and should be investigated more in a western HCC population.
Vascular endothelial growth factors: The vascular endothelial growth factors (VEGF) is one of the potent pro-angiogenic factors implicated in cancer angiogenesis. The activation of the complex VEGF/VEGF receptor (VEGFR) stimulates endothelial cell growth, proliferation, invasion and survival[83]. Circulating VEGF level may be useful in evaluating VEGF expression in HCC tumor[84] and were found suitable for HCC prognosis[85]. The VEGF-A isoform promotes angiogenesis and the dual VEGF-C/VEGF-D isoforms stimulates the lymphangiogenesis through activation of the VEGFR-2 and VGEFR-3 respectively. Several studies showed that high baseline levels of VEGF-A impacts negatively on prognosis in patients with advanced HCC[63,85-87]. Ebos et al[88] demonstrated that monitoring of soluble VEGFR-2 (sVEGFR-2) in mouse tumor models could be suggestive of the overall circulating VEGF levels and therefore, a potential surrogate biomarker for VEGF-dependent tumor growth[88]. An inverse link between sVEGFR-2 plasma levels and tumor size was detected. Recently, sVEGFR-1 levels were shown to be associated with more advanced-stage HCC and tumor differentiation and sVEGFR-2 levels to be associated with poorly differentiated tumor[89]. Llovet et al[63] reported changes of plasma VEGF level in patients treated for HCC enrolled in the SHARP study. Compared to baseline level, a significant increase in plasma level of VEGF was observed in the sorafenib group (P = 0.010) and a significant decrease in plasma level of sVEGFR-2 and sVEGFR-3 was seen in the placebo group (P < 0.0001)[63]. The increase of VEGF plasma level found after sorafenib treatment was somewhat surprising since sorafenib showed OS improvement. However, similar findings were observed in patients treated with sorafenib for renal cell carcinoma[90], with sunitinib for advanced HCC[91-93] or renal cell carcinoma[94-96]. Increase of VEGF plasma level could be subsequent to hypoxia induced by the antiangiogenic agents[94]. Noticeably, a reversible increase in the VEGF level induced with sunitinib was also observed in non-tumor-bearing mice suggesting a systemic response that possibly masks tumor-specific changes or any difference in responding patients. Therefore, the increase in VEGF in response to treatment could also occur independently of tumor[97] and might explain the absence of correlation between this change and the outcome in HCC patients treated with antiangiogenic agents[63]. In the SHARP trial, the increase of VEGF-A plasma concentration during sorafenib treatment observed in patients with advanced HCC did not predict OS or tumor response[63]. Similarly, no association between VEGF-A plasma level changes and outcome was observed in patients treated with bevacizumab for advanced HCC[98]. Accordingly, the VEGF-A could serve as a pharmacodynamic marker of exposure to antiangiogenic agents but did not have prognostic or predictive value[85]. Sunitinib induced in patients with HCC, a reduction of VEGF-C (the ligand of VEGFR-3) plasma level that was associated with disease control and tumor response according to the RECIST criteria[23] and Choi criteria[99,100] respectively[93]. Likewise, sunitinib-induced decrease of sVEGFR-3 plasma levels in patients with renal cell cancer and breast cancer correlated with a better outcome[95,101]. Baseline level of VEGF-C may be regarded as a potential predictive biomarker of sunitinib efficacy in patients with advanced HCC[92,93]. However, as sorafenib has limited action against the VEGFR-3[102], the value of this biomarker to predict response in HCC patients could be anecdotal.
In summary, further robust studies are warranted to demonstrate the predictive value of circulating VEGF in patients treated with sorafenib or other antiangiogenic agents for advanced HCC. The plasma VEGF should be assessed more than serum VEGF because it was more reproducible and consistent in estimating the activity of VEGF[86].
Hepatocyte growth factor: The hepatocyte growth factor (HGF) is a strong promoter of hepatocarcinogenesis through the activation of the HGF axis and its receptor MET[103]. Previous studies showed that high serum levels of HGF in patients with HCC negatively associated with OS and outcome[104-106]. In the recent SHARP study biomarkers analysis, patients treated with sorafenib experienced a decrease in a mean plasma level of HGF although; patients treated with placebo have mean HGF concentration increase[63]. Added to circulating stem-cell factor receptor (c-KIT) and angiopoietin 2 (Ang2) concentrations, HGF level was shown to be an independent factor of survival in patients with advanced HCC[63]. Low baseline HGF plasma level trends toward better OS (12.4 mo vs 6.3 mo, P = 0.073) and TTP in patients treated with sorafenib for HCC[63]. Noticeably, in contrast to plasma levels, tissue HGF expression carries low prognostic information[107]. Further investigations are needed to identify the role of HGF as a predictor of response to sorafenib in patients with advanced HCC.
Ang2: Ang2, one of the families of angiopoietins, is an angiogenic factor implicated in tumor angiogenesis stimulation and progression in human HCC[108]. Tumor overexpression of Ang2 was associated with vascular invasion, tumor size microvessel density level, poorly prognosis HCC[108,109] and poor differentiated tumor[110]. Preoperative presence of Ang2 in the hepatic vein was also associated with portal invasion and poor outcome in HCC resected patients[111]. In a small uncontrolled cohort of patients treated with sorafenib for advanced HCC, the authors reported that Ang2 could predict the outcome[112]. High Ang2 serum baseline level was associated with PFS but not with OS in HCC patients treated with sorafenib[112]. Llovet et al[63] confirmed the negative impact on prognosis of baseline high plasma level of Ang2 in HCC. In patients treated with sorafenib or placebo, median OS was significantly shorter in those with high baseline Ang2 plasma levels compared to those with low baseline concentrations (6.3 mo vs 14.1 mo, HR = 2.407; 95%CI: 1.9-3.03; P < 0.001). In the group of patients treated with sorafenib, no significant changes in median Ang2 plasma levels were observed during the treatment. However, concentration increase was reported in the group of patients treated with placebo[63]. Both patient groups treated with sorafenib or placebo that experienced an increase of Ang2 plasma levels during follow-up had shorter OS and TTP[63]. Ang2 seems, therefore, a prognostic factor of HCC aggressiveness but not an adequate predictive factor of sorafenib efficacy. Llovet et al[63] suggested that dosing Ang2 plasma levels during treatment with sorafenib could be an attractive option to monitor patients with advanced HCC.
Basic fibroblast growth factor: The basic fibroblast growth factor (bFGF) is one of the identified angiogenic factors with a potent stimulus for HCC growth[113]. Tumor overexpression of bFGF seems mainly implicated in HCC invasiveness than tumor neovascularization[114]. Moreover, a significant correlation between high preoperative serum bFGF level and larger tumor, venous invasion, advanced tumor staging and early recurrence was reported in resected HCC[115]. In the SHARP study, no difference was observed concerning changes in mean bFGF plasma concentration between sorafenib and placebo in patients with advanced HCC[63].
Stem-cell factor receptor - KIT: The role of stem-cell factor receptor and its soluble forms has not been entirely elucidated in HCC. Soluble forms of KIT were fundamentally implicated in tumor-cell survival and proliferation[93]. Llovet et al[63] reported a trend to a positive impact of high baseline soluble c-KIT level on OS and TTP in patients treated with sorafenib. Sorafenib induced a significant decrease in mean plasma levels of soluble c-KIT, unlike the placebo that resulted in no changes in c-KIT concentration[63]. Likewise, following exposure to sunitinib, plasma levels of soluble c-KIT decreased significantly in patients with renal cell carcinoma[95], breast cancer[101] and HCC[91-93]. SHARP biomarker analysis showed a nonsignificant trend of soluble c-KIT in predicting sorafenib response in patients with advanced HCC. In the sorafenib cohort, patients with high baseline soluble c-KIT level showed better median OS and TTP compared to those with low soluble c-KIT level but without reaching significance (10.4 mo vs 9.4 mo, P = 0.081 and 6.7 mo vs 4.1 mo, P = 0.052; respectively)[63]. In a phase II study, Zhu et al[91] reported that soluble KIT plasma levels decrease following 14 d of sunitinib treatment in patients with advanced HCC and correlated with better PFS and OS. Similarly, improvement of TTP and trend towards better OS were reported when soluble KIT plasma level decreased from baseline following sunitinib in patients with HCC, metastatic breast cancer and neuroendocrine tumor[93,95,101]. Nowadays, the role of soluble c-KIT in HCC pathogenesis remains unclear since the expression of this protein kinase in HCC tissue appears to be anecdotal[116].
Insulin growth factors: The insulin growth factors (IGF) signaling pathway, including its ligand, IGF-1, and IGF-2, plays a crucial role in carcinogenesis of various tumors[117,118]. In patients with HCC, independently to the tumor stage, low baseline IGF-1 plasma level correlated with poorer OS[87]. In a small cohort of patients with advanced HCC receiving first-line antiangiogenic treatment associated with metronomic chemotherapy, serum levels of IGF-1 could predict treatment efficacy in this population. Indeed, high baseline IGF-1 serum levels before treatment correlate with better OS, PFS and disease control rate[87]. Moreover, high baseline IGF-2 plasma levels associated with a better OS in the placebo group enrolled in the SHARP trial[63]. In this large phase III controlled trial, the IGF-2 failed to predict response to sorafenib in patients with advanced HCC[63] confirming previous results observed with other antiangiogenic agents[119].
Circulating endothelial cells and circulating endothelial cell progenitors: In preclinical models, levels of circulating endothelial cells (CEC) and bone-marrow-derived CEC progenitors (CECP) were shown to be potential surrogate markers of angiogenesis[120,121]. High circulating level of CECP in patients with HCC correlates with advanced disease[122]. Previous studies reported levels of CEC and CECP decrease and return to normal values following antiangiogenic therapy in cases of complete remission[123]. Willett et al[124] showed that high doses of bevacizumab induce an increase of viable CEC and CECP percentage in a small cohort of patient with rectal cancer. Bevacizumab treatment induced in patients with advanced HCC, an early increase of viable CEC levels that correlated with objective response[98]. In patients with imatinib-resistant gastrointestinal stromal tumor, sunitinib induced early, but not subsequent increase of CEC blood levels that seemed to be correlating with clinical benefit[125]. Otherwise, sunitinib was shown to cause a decrease of CECP level in patients with advanced HCC[91]. Shao et al[126] showed that high baseline CECP level, but not CEC level, was associated with poor OS in patients treated with sorafenib combined with metronomic chemotherapy. The value of CEC and CECP levels as biomarkers of angiogenesis and antiangiogenic therapies in HCC needs further prospective analysis. In fact, methods and techniques of measurement were inconsistent, and unreliable results were reported depending on the type of study (clinical or preclinical studies), cancer types, and antiangiogenic agents[98,115,116,121].
In summary, none of the above biomarkers is validated to predict response to sorafenib in patient with advanced HCC. Except the SHARP biomarkers analysis study, the majority of available data was reported from no control arm retrospective studies. Validation through further large, controlled randomized trials are required to confirm the predictive value of such predictive biomarkers so to be integrated with clinical use. Moreover, techniques used to assess drug-induced variation in circulating factors should be standardized for reliable interpretation. An important issue should also be questioned of whether the presence or change in circulating biomarkers could discriminate between treatment benefit and tumor resistance or escape.
In addition to tissue prognosis markers obtained from tumor samples, some studies tried to identify predictive factors of response and outcome following anticancer agents. Table 7 summarizes studies evaluating tissue biomarkers used as prognostic and predictive of HCC. Abou-Alfa et al[127] evaluated the impact of tumor expression of phosphorylated extracellular signal-regulated kinase (pERK) and outcome in patients treated with sorafenib for advanced HCC. A high pretreatment tumor level of pERK correlated with TTP, but the survival impact was not analyzed. Tumor-cell expression and staining levels of pERK using immunohistochemistry analysis were performed in 33 patients. Patients with high pretreatment tumor-cell pERK expression had better TTP compared to those low staining intensity. The authors speculated that tissue expression of pERK could be predictive of response to sorafenib since tumors with higher levels of pERK were associated with more sensitive, or responsive, to sorafenib[127]. Our immunohistochemistry analysis did not confirm these findings[128]. Indeed, immunophenotypical markers (including pERK, VEGF, CD34, CK19, and STAT3) were evaluated in 21 patients treated with sorafenib for advanced HCC. None of these tissue markers was predictive of survival in our population[128]. These inconsistent results could be explained by the significant variability of detection of ERK expression by immunohistochemistry between samples obtained from biopsies compared to their subsequent resected HCC specimens[129] and the potential for rapid dephosphorylation and variable time of tissue fixation[130].
| Ref. | Marker | Patient (n) | Origin of specimen | Method assay | Quantification | Marker level | Clinical impact |
| Mitsuhashi et al[108] | Ang2 | 46 | Resected specimens | RT-PCR and IHC | Quantitative | High tumor Ang2/1 ratio | Tumor portal vein invasion |
| Large tumor | |||||||
| Increase MVD | |||||||
| Poor OS | |||||||
| Zhang et al[109] | 38 | Resected specimens | RT-PCR | No | High tumor Ang2/1 ratio | Large tumor | |
| Portal vein invasion | |||||||
| Metastasis | |||||||
| Torimura et al[110] | 59 | Resected specimens (19) and Biopsy (40) | RT-PCR and IHC | Semi-quantitative | High tumor Ang2 | Poor differentiated tumor | |
| Abou-Alfa et al[127] | pERK | 33 | Biopsy before sorafenib | IHC | Semi-quantitative | High tumor pERK | Better TTP |
| Ozenne et al[128] | 20 | Biopsy before sorafenib | IHC | Semi-quantitative | High tumor pERK | No impact | |
| Hagiwara et al[131] | JNK | 39 | Biopsy before sorafenib | IHC and Western Blot | Quantitative | High JNK tumor | Lower ORR |
| Poorer TTP | |||||||
| Poorer OS | |||||||
| Peng et al[134] | pVEGFR-2 | 35 | Resected specimen before sorafenib | RT-PCR and IHC | Semi-quantitative | Low tumor expression | Poorer OS |
| Poon et al[84] | VEGF | 60 | Resected specimen | IHC and ELISA | Semi-quantitative | High tumor expression | Advanced HCC stage |
Recently, a Japanese group found in patients treated with sorafenib for advanced HCC, a negative impact of tumor expression of phospho-c-Jun on outcome[131]. Tumor expression of phosphor-c-Jun was associated with low tumor response rate, shorter TTP and OS[131]. These data need further validation since limited samples were evaluated.
Otherwise, previous analysis showed that VEGF expression in HCC tumor was associated with aggressive disease and worse outcome[132,133]. Peng et al[134] showed that tumor expression of VEGFR (phosphorylated VEGFR-1 and VEGFR-2) could affect the outcome of patients treated with sorafenib for advanced HCC[134]. Using immunohistochemistry analyzes, low pVEGFR-1 and pVEGFR-2 expressions in previously resected HCC specimens; a subsequent treatment with sorafenib was associated with worse outcome and poorer OS. The authors postulated that high autocrine VEGF signaling activity in tumor tissue could be predictive of response and outcome in patients treated with sorafenib[134]. These results could be hampered somewhat by the retrospective feature of the analysis, the small number of patients included and the low feasibility in clinical practice.
Furthermore, overexpression of “stemness”-related proteins (including c-KIT, K19, and CD34) was shown to be associated with aggressive HCC and poor prognosis[135-138]. Recently, the stem-cell factor, a ligand of c-KIT, was shown to be an independent prognostic factor for HCC after resection[139]. In patients with low tumor expression of stem-cell factor, the median time to recurrence was 24 mo compared to 12 mo in patients with overexpression > 85% of the marker[139].
Microvessel density (MVD) was another tissue biomarker proposed to predict response to antiangiogenic agents. Willett et al[124] observed a decrease of tumor MVD following antiangiogenic therapies in rectal cancers and this parameter was suggested as predictive of clinical benefit. However, inconsistent results were reported in an exploratory analysis of a large pivotal trial evaluating the addition of bevacizumab to chemotherapy in patients with metastatic colorectal cancer[140]. The tumor MVD did not predict the survival benefit in this large trial[140]. Noticeably, measurement methods of MVD were not standardized explaining partially the inconsistent results[140]. MVD analysis of HCC tumor tissue was shown to have only prognostic value[141]. The feasibility of tumor MVD expression was very limited in clinical practice hampering its use in predicting response to antiangiogenic agents for HCC.
Some tissue markers of response were evaluated in HCC using other antiangiogenic agents. Tivantinib, a selective MET inhibitor, was evaluated in a second line setting through a randomized, placebo-controlled phase II trial in patients with advanced HCC[142]. In this study, tumor expression of MET influenced treatment benefit. Patients with tumor overexpression of MET clearly benefit from tivantinib treatment. High-MET tumor expression was associated with longer TTP on tivantinib compared to placebo (2.7 mo vs 1.4 mo, HR = 0.43, 95%CI: 0.19-0.97; P = 0.03) and OS (7.2 mo vs 3.8 mo, HR = 0.38, 95%CI: 0.18-0.81; P = 0.01). Interestingly, tivantinib did not show any benefit when tumor expression of MET was low[142].
Nowadays, no tissues biomarkers can identify patients who might respond to sorafenib. Tumor analysis data were/was unavailable in large clinical trials, probably because of lack of tumor samples biopsies since HCC diagnosis was frequently made according to imaging features[143,144].
The clinical benefit of sorafenib with OS gain in patients with advanced HCC contrasted largely with a low objective response rate noted in this population. The low response rates could be considered as a sign of lack of antitumor activity in early phases of clinical trials but were favorably balanced by sustained tumor stabilization and small numbers of tumor progression in the waterfall plot activity. Fortunately, the decision to proceed with phase III trials was not hampered by the apparent lack of tumor response.
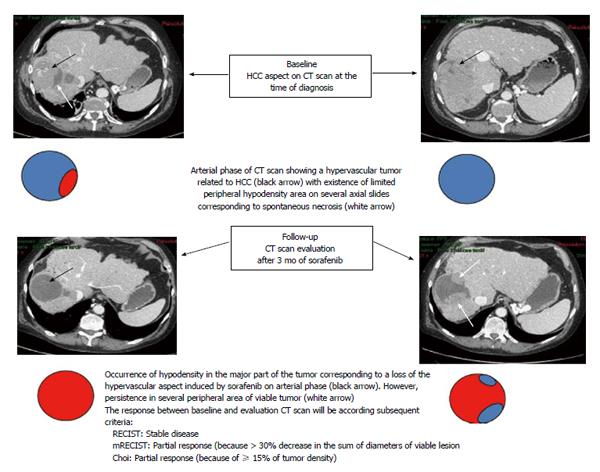
The conventional RECIST criteria[23,24] usually used for tumor response evaluation of conventional chemotherapy appear clearly inappropriate to evaluate the response to sorafenib in patients with advanced HCC. Major features were reported following antiangiogenic agents consisting of decreased tumor vascularization[145] and density[146] on computer tomography (CT) scans. The modified RECIST (mRECIST) criteria are a new assessment method proposed by Lencioni and Llovet[145] to overcome the limitations of RECIST criteria. They include vascularization and tumor arterial enhancement changes of the target lesion on CT. Other new criteria including European Association for the Study of the Liver (EASL) criteria and Choi criteria, that evaluated tumor density changes, were also proposed to evaluate tumor response to sorafenib in patients with HCC[100,146-148]. A representative case of discrepancies between these criteria is shown in Figure 1. Several studies used CT-scan evaluation to predict early response to sorafenib and to adjust treatment strategy according to the potential clinical benefit[100,147,149].
Edeline et al[147] showed in patients treated with sorafenib for advanced HCC that overall response rate was higher when mRECIST criteria were applied compared to RECIST criteria (22.7% vs 1.9%). Interestingly, tumor response assessment according to mRECIST criteria, reclassified 22.6% of patients as responders while they were initially categorized as having stable disease by RECIST criteria[147]. Our group found consistent results when alternative radiological criteria to RECIST were applied[100]. We evaluated early tumor response in 64 patients with advanced HCC treated with sorafenib using RECIST, mRECIST, Choi and EASL criteria[100]. These new criteria identified a higher objective response rate compared to the conventional RECIST criteria (varying from 51% for Choi to 28% and 28% for mRECIST and EASL respectively; compared to only 3% for RECIST criteria). Responder patients according to Choi criteria at the first tumor assessment had better OS compared to non-responders (22.4 mo vs 10.6 mo, 95%CI: 0.15-086; P = 0.097)[100].
Further evaluations of these new criteria in comparison to RECIST criteria are needed in prospective clinical trials evaluating sorafenib or other antiangiogenic agents for advanced HCC.
In summary, we believe that, combining early reduction of AFP levels following sorafenib initiation with new radiological criteria could be helpful in detecting patients who might benefit from antiangiogenic treatment and to propose better tailor-made strategy management.
Various functional imaging tools [including contrast-enhanced ultrasound, dynamic contrast-enhanced magnetic resonance imaging (MRI) and dynamic contrast-enhanced CT and positron emission tomography (PET)] were proposed to evaluate the antiangiogenic effects[150] (Table 8). Functional imaging approaches consist of infusion of intravenous contrast agent that enhances vascular and tumor structures and the acquisition of sequential images before, during, and after injection.
| Ref. | Imaging tools | Patients (n) | Study design | Treatment | Imaging findings and clinical impact | Conclusion/comments |
| Sugimoto et al[152] | DCE-US | 37 | Prospective | Sorafenib | Tumor vascularity decreases and blood volume within seven days trends towards better PFS and OS | These studies enrolled small cohort of patients hampering adequate interpretation. However, DCE-US remains a promising noninvasive imaging, but operator dependent, to predict response in patients with HCC treated with sorafenib and larger cohort of patients should be evaluated |
| Zocco et al[153] | 28 | Prospective | Sorafenib | An early decrease in AUC and increase of median transit time was associated with better PFS and OS | ||
| Zhu et al[91] | DCE-MRI | 34 | Prospective | Sunitinib | Decrease in vascular permeability was associated with better disease control | The decrease of vascular permeability induced by antiangiogenic agents seems to be a good predictive of tumor response and clinical benefit. These promising findings should be confirmed by largest cohort of patient |
| Hsu et al[156] | 31 | Prospective | Sorafenib + mT/U | A ≥ 40% decrease in vascular permeability with 14 d was associated with better PFS and OS | ||
| Lee et al[159] | FGD-PET | 29 | Retrospective | Sorafenib | SUV < 5.00 correlated with longer PFS and OS | Prospective studies are needed to evaluate the predictive value of the FDG-PET in HCC |
Some small cohort studies evaluated the useful of dynamic contrast-enhanced ultrasound (DCE-US) to predict early tumor response to sorafenib in patients with advanced HCC[151-153]. In a Japanese prospective monocentric study, a total of 37 patients with advanced HCC treated with sorafenib were evaluated using DCE-US, before treatment and on days 7, 14 and 28 of treatment[152]. Significant changes in different US perfusion parameters between responders and non-responders (according to RECIST and mRECIST criteria) were observed at the prescheduled time of the follow-up. Correlation between reduction in tumor blood volume 7 d after treatment initiation and better PFS and OS was found. The authors suggest that DCE-US performed earlier could be useful to identify patients with advanced HCC, who may benefit from sorafenib[152]. Consistent results were obtained in an Italian prospective study that enrolled 28 patients treated with sorafenib and monitored with DCE-US at baseline, days 7, 15 and 30 of treatment[154]. Early decrease of tumor vascularity occurring during treatment was predictive of tumor response, better PFS and OS.
Dynamic contrast-enhanced magnetic resonance imaging has already been proposed to assess vascular disruption of antiangiogenic compounds in early clinical trials. However, this technique remains considerably more complex than conventional imaging and needs real expertise[155]. Using DEC-MRI, changes in tumor blood flow following VEGFR tyrosine kinase inhibitors were observed in patients with advanced HCC[91,156]. Significant decrease in vascular permeability (Ktrans) and reverse reflux rate constant between the extracellular space and plasma (Kep) were reported in patients with advanced HCC treated with sunitinib[91]. These changes were associated with better prognosis since the extent of decrease in Ktrans was significantly greater in patients with partial response or stable disease compared to those with progressive disease or those who died early following sunitinib treatment[91]. DEC-MRI was also evaluated to predict response and benefit in 31 patients with advanced HCC treated with sorafenib plus metronomic tegafur/uracil[156]. In this study, Ktrans before treatment was significantly higher in patients with partial response or stable disease compared to patients with progressive disease. Following 14 d of treatment, significant change in median Ktrans was observed in responders compared to non-responder patients (-47.1% vs 9.6%; P < 0.001). The percentage of Ktrans change following treatment was an independent predictor of tumor response, PFS, and OS. Better PFS, and OS was seen when a vascular response, defined as ≥ 40 decrease in Ktrans at day 14 of treatment, was detected (29.1 wk vs 8.7 wk, P = 0.033 and 53.0 wk vs 14.9 wk, P = 0.016; respectively)[156].
Currently, the use of DEC-MRI is limited to clinical research and has not been extended to routine practice. Further studies combining cost-effectiveness are needed to define the place of this innovative tool as predictive of tumor response and clinical benefit with sorafenib in advanced HCC.
Few studies evaluated the prognostic value of 18F-fluorodeoxyglucose-PET (18-FDG-PET) in patients receiving antiangiogenic agents for advanced HCC[157,158]. In a small cohort study, Lee et al[159] found that the degree of FGD uptake correlates with outcome in Korean patients with advanced HCC treated with sorafenib. Patients who experienced pretreatment standardized uptake values (SUV) < 5.00 had better PFS and OS compared to those with SUV ≥ 5.00[159]. Undeniably, such findings should be verified by prospective evaluation in large cohort patients. Finally, no data are/is available regarding the prognostic or predictive value of 18F-fluorocholine, a PET tracer of lipid metabolism, that is supposed to be more sensitive than 18F-FDG for HCC detection[158], in patients receiving antiangiogenic drugs for HCC.
In summary, several studies with antiangiogenic agents have shown the need for additional criteria, beyond RECIST criteria, for early evaluation of antitumor activity and identification of patients who could benefit from these therapies. Furthermore, promising findings of the correlation between biomarkers and radiological response were shown in some studies, warranting further validation in larger clinical trials.
Measurement of tumor hypodensity, intratumor necrosis, and vascular parameters are the main criteria to be explored by dynamic functional imaging. These parameters are not already validated, but they represent prospective radiological investigations of primary interest for the assessment of antiangiogenic therapy effects beyond tumor size.
The sorafenib success story in advanced HCC raised new questions regarding the suitable approach to select patients who would likely benefit from treatment, ideally before its initiation. In routine practice, identifying predictive tools and biomarkers of response or early resistance seems to be an unmet need. Nowadays, no one of biomarkers the cited above biomarkers was validated in routine. AFP and some proangiogenic factors, such as VEGF and Ang2, seem to be promising prognostic and predictive biomarkers in HCC. However, there is probably no single ideal biomarker to predict response to antiangiogenic agents.
Controlled-arm prospective studies are required to improve the robustness of result interpretation. New endpoints are necessary for these biomarkers, such as monitoring angiogenesis, predicting early treatment response or even before starting therapy, defining optimum biological dose and identifying early resistance to antiangiogenic agents. Translational research using sequential tumor biopsy analysis while the patient is his own witness could probably be the most reliable method to identify robust biomarkers. Furthermore, advances in functional imaging techniques could allow evaluation of these molecules in real time, by assessing tumor density rather than tumor size. New tumor assessment criteria, particularly in cases of stable disease according to RECIST, should be identified and validated through large prospective cohort analysis. Finally, combining imaging response and efficient circulating biomarkers such AFP or proangiogenic factors (e.g., VEGF or Ang2) could be a practical option and may be helpful to detect patients more likely to benefit from antiangiogenic treatment and to propose better tailor-made strategy management.
The authors would like to thank Enago (http://www.enago.com) for the English language review.
P- Reviewer: Penkova-Radicheva MP, Vradelis S, Wang JY S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
| 1. | Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137-2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2591] [Cited by in RCA: 2640] [Article Influence: 138.9] [Reference Citation Analysis (0)] |
| 2. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11820] [Article Influence: 844.3] [Reference Citation Analysis (4)] |
| 3. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3276] [Article Influence: 148.9] [Reference Citation Analysis (0)] |
| 4. | Thomas MB, Abbruzzese JL. Opportunities for targeted therapies in hepatocellular carcinoma. J Clin Oncol. 2005;23:8093-8108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Pang RW, Poon RT. From molecular biology to targeted therapies for hepatocellular carcinoma: the future is now. Oncology. 2007;72 Suppl 1:30-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312-1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 823] [Cited by in RCA: 829] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 7. | Psyrri A, Arkadopoulos N, Vassilakopoulou M, Smyrniotis V, Dimitriadis G. Pathways and targets in hepatocellular carcinoma. Expert Rev Anticancer Ther. 2012;12:1347-1357. [PubMed] [DOI] [Full Text] |
| 8. | Kudo M. Signaling pathway/molecular targets and new targeted agents under development in hepatocellular carcinoma. World J Gastroenterol. 2012;18:6005-6017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Faivre S, Bouattour M, Raymond E. Novel molecular therapies in hepatocellular carcinoma. Liver Int. 2011;31 Suppl 1:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Shen YC, Lin ZZ, Hsu CH, Hsu C, Shao YY, Cheng AL. Clinical trials in hepatocellular carcinoma: an update. Liver Cancer. 2013;2:345-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Bouattour M, Wassermann J. Molecular targeted drugs under investigation in hepatocellular carcinoma. 4th ed. Frontiers in Anti-Cancer Drug Discovery. 2014;39-87 Available from: http://ebooks.benthamscience.com/book/9781608059225/chapter/124572/. |
| 12. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10194] [Article Influence: 599.6] [Reference Citation Analysis (2)] |
| 13. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4619] [Article Influence: 271.7] [Reference Citation Analysis (0)] |
| 14. | Connock M, Round J, Bayliss S, Tubeuf S, Greenheld W, Moore D. Sorafenib for the treatment of advanced hepatocellular carcinoma. Health Technol Assess. 2010;14 Suppl 1:17-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Cammà C, Cabibbo G, Petta S, Enea M, Iavarone M, Grieco A, Gasbarrini A, Villa E, Zavaglia C, Bruno R. Cost-effectiveness of sorafenib treatment in field practice for patients with hepatocellular carcinoma. Hepatology. 2013;57:1046-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Palmer DH, Hussain SA, Smith AJ, Hargreaves S, Ma YT, Hull D, Johnson PJ, Ross PJ. Sorafenib for advanced hepatocellular carcinoma (HCC): impact of rationing in the United Kingdom. Br J Cancer. 2013;109:888-890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, Cammà C, Colombo M; SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54:2055-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 291] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 18. | Tod M, Mir O, Bancelin N, Coriat R, Thomas-Schoemann A, Taieb F, Boudou-Rouquette P, Ropert S, Michels J, Abbas H. Functional and clinical evidence of the influence of sorafenib binding to albumin on sorafenib disposition in adult cancer patients. Pharm Res. 2011;28:3199-3207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Arrondeau J, Mir O, Boudou-Rouquette P, Coriat R, Ropert S, Dumas G, Rodrigues MJ, Rousseau B, Blanchet B, Goldwasser F. Sorafenib exposure decreases over time in patients with hepatocellular carcinoma. Invest New Drugs. 2012;30:2046-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Mir O, Coriat R, Blanchet B, Durand JP, Boudou-Rouquette P, Michels J, Ropert S, Vidal M, Pol S, Chaussade S. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PLoS One. 2012;7:e37563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 239] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 21. | Lee JH, Chung YH, Kim JA, Shim JH, Lee D, Lee HC, Shin ES, Yoon JH, Kim BI, Bae SH. Genetic predisposition of hand-foot skin reaction after sorafenib therapy in patients with hepatocellular carcinoma. Cancer. 2013;119:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Widmer N, Bardin C, Chatelut E, Paci A, Beijnen J, Levêque D, Veal G, Astier A. Review of therapeutic drug monitoring of anticancer drugs part two--targeted therapies. Eur J Cancer. 2014;50:2020-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 23. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. [PubMed] |
| 24. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21336] [Article Influence: 1333.5] [Reference Citation Analysis (1)] |
| 25. | Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther. 2001;69:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4510] [Cited by in RCA: 4101] [Article Influence: 170.9] [Reference Citation Analysis (0)] |
| 26. | Ludwig JA, Weinstein JN. Biomarkers in cancer staging, prognosis and treatment selection. Nat Rev Cancer. 2005;5:845-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1164] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 27. | Sessa C, Guibal A, Del Conte G, Rüegg C. Biomarkers of angiogenesis for the development of antiangiogenic therapies in oncology: tools or decorations? Nat Clin Pract Oncol. 2008;5:378-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Lièvre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Côté JF, Tomasic G, Penna C, Ducreux M. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992-3995. [PubMed] |
| 29. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pintér T, Lim R, Bodoky G. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2901] [Cited by in RCA: 3111] [Article Influence: 194.4] [Reference Citation Analysis (1)] |
| 30. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1296] [Cited by in RCA: 1388] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 31. | Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3742] [Cited by in RCA: 3674] [Article Influence: 183.7] [Reference Citation Analysis (0)] |
| 32. | Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673-1684. [PubMed] |
| 33. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5255] [Article Influence: 350.3] [Reference Citation Analysis (3)] |
| 34. | Baselga J, Cortés J, Im SA, Clark E, Ross G, Kiermaier A, Swain SM. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol. 2014;32:3753-3761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 269] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 35. | Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5906] [Cited by in RCA: 6450] [Article Influence: 403.1] [Reference Citation Analysis (0)] |
| 36. | Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2700] [Cited by in RCA: 3247] [Article Influence: 231.9] [Reference Citation Analysis (0)] |
| 37. | Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507-2516. [PubMed] |
| 38. | Brunetto AT, Kristeleit RS, de Bono JS. Early oncology clinical trial design in the era of molecular-targeted agents. Future Oncol. 2010;6:1339-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [PubMed] |
| 40. | Pérez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, Rigas J, Clark GM, Santabárbara P, Bonomi P. Determinants of tumor response and survival with erlotinib in patients with non--small-cell lung cancer. J Clin Oncol. 2004;22:3238-3247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 826] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 41. | Xiong HQ, Rosenberg A, LoBuglio A, Schmidt W, Wolff RA, Deutsch J, Needle M, Abbruzzese JL. Cetuximab, a monoclonal antibody targeting the epidermal growth factor receptor, in combination with gemcitabine for advanced pancreatic cancer: a multicenter phase II Trial. J Clin Oncol. 2004;22:2610-2616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 337] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 42. | Otsuka T, Eguchi Y, Kawazoe S, Yanagita K, Ario K, Kitahara K, Kawasoe H, Kato H, Mizuta T; Saga Liver Cancer Study Group. Skin toxicities and survival in advanced hepatocellular carcinoma patients treated with sorafenib. Hepatol Res. 2012;42:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 43. | Di Costanzo GG, Tortora R, Iodice L, Lanza AG, Lampasi F, Tartaglione MT, Picciotto FP, Mattera S, De Luca M. Safety and effectiveness of sorafenib in patients with hepatocellular carcinoma in clinical practice. Dig Liver Dis. 2012;44:788-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Shomura M, Kagawa T, Shiraishi K, Hirose S, Arase Y, Koizumi J, Mine T. Skin toxicity predicts efficacy to sorafenib in patients with advanced hepatocellular carcinoma. World J Hepatol. 2014;6:670-676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 45. | Vincenzi B, Santini D, Russo A, Addeo R, Giuliani F, Montella L, Rizzo S, Venditti O, Frezza AM, Caraglia M. Early skin toxicity as a predictive factor for tumor control in hepatocellular carcinoma patients treated with sorafenib. Oncologist. 2010;15:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 46. | Reig M, Torres F, Rodriguez-Lope C, Forner A, LLarch N, Rimola J, Darnell A, Ríos J, Ayuso C, Bruix J. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol. 2014;61:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 47. | Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2645] [Cited by in RCA: 2855] [Article Influence: 109.8] [Reference Citation Analysis (1)] |
| 48. | Funakoshi T, Latif A, Galsky MD. Risk of hypertension in cancer patients treated with sorafenib: an updated systematic review and meta-analysis. J Hum Hypertens. 2013;27:601-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Österlund P, Soveri LM, Isoniemi H, Poussa T, Alanko T, Bono P. Hypertension and overall survival in metastatic colorectal cancer patients treated with bevacizumab-containing chemotherapy. Br J Cancer. 2011;104:599-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 124] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 50. | Scartozzi M, Galizia E, Chiorrini S, Giampieri R, Berardi R, Pierantoni C, Cascinu S. Arterial hypertension correlates with clinical outcome in colorectal cancer patients treated with first-line bevacizumab. Ann Oncol. 2009;20:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 256] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 51. | Bono P, Elfving H, Utriainen T, Osterlund P, Saarto T, Alanko T, Joensuu H. Hypertension and clinical benefit of bevacizumab in the treatment of advanced renal cell carcinoma. Ann Oncol. 2009;20:393-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 52. | Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C, Létourneau R, Bajetta E, Pithavala Y, Bycott P. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet. 2008;371:2101-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 53. | Hurwitz HI, Douglas PS, Middleton JP, Sledge GW, Johnson DH, Reardon DA, Chen D, Rosen O. Analysis of early hypertension and clinical outcome with bevacizumab: results from seven phase III studies. Oncologist. 2013;18:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 54. | Ravaud A, Sire M. Arterial hypertension and clinical benefit of sunitinib, sorafenib and bevacizumab in first and second-line treatment of metastatic renal cell cancer. Ann Oncol. 2009;20:966-967; author reply 967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Estfan B, Byrne M, Kim R. Sorafenib in advanced hepatocellular carcinoma: hypertension as a potential surrogate marker for efficacy. Am J Clin Oncol. 2013;36:319-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 56. | Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, Winget M, Yasui Y. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054-1061. [PubMed] |
| 57. | Liu C, Xiao GQ, Yan LN, Li B, Jiang L, Wen TF, Wang WT, Xu MQ, Yang JY. Value of α-fetoprotein in association with clinicopathological features of hepatocellular carcinoma. World J Gastroenterol. 2013;19:1811-1819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 58. | Ma WJ, Wang HY, Teng LS. Correlation analysis of preoperative serum alpha-fetoprotein (AFP) level and prognosis of hepatocellular carcinoma (HCC) after hepatectomy. World J Surg Oncol. 2013;11:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 59. | Ikai I, Arii S, Kojiro M, Ichida T, Makuuchi M, Matsuyama Y, Nakanuma Y, Okita K, Omata M, Takayasu K. Reevaluation of prognostic factors for survival after liver resection in patients with hepatocellular carcinoma in a Japanese nationwide survey. Cancer. 2004;101:796-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 350] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 60. | Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, Lemoine A, Bismuth H, Castaing D, Adam R. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant. 2010;10:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 61. | Hakeem AR, Young RS, Marangoni G, Lodge JP, Prasad KR. Systematic review: the prognostic role of alpha-fetoprotein following liver transplantation for hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:987-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 62. | Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986-994.e3; quiz e14-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 717] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 63. | Llovet JM, Peña CE, Lathia CD, Shan M, Meinhardt G, Bruix J; SHARP Investigators Study Group. Plasma biomarkers as predictors of outcome in patients with advanced hepatocellular carcinoma. Clin Cancer Res. 2012;18:2290-2300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 453] [Article Influence: 34.8] [Reference Citation Analysis (2)] |
| 64. | Hsu CH, Shen YC, Lin ZZ, Chen PJ, Shao YY, Ding YH, Hsu C, Cheng AL. Phase II study of combining sorafenib with metronomic tegafur/uracil for advanced hepatocellular carcinoma. J Hepatol. 2010;53:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 65. | Baek KK, Kim JH, Uhm JE, Park SH, Lee J, Park JO, Park YS, Kang WK, Lim HY. Prognostic factors in patients with advanced hepatocellular carcinoma treated with sorafenib: a retrospective comparison with previously known prognostic models. Oncology. 2011;80:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Lin ZZ, Hsu C, Hu FC, Shao YY, Chang DY, Yang CH, Hong RL, Hsu CH, Cheng AL. Factors impacting prognosis prediction in BCLC stage C and Child-Pugh class A hepatocellular carcinoma patients in prospective clinical trials of systemic therapy. Oncologist. 2012;17:970-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB, Ho WM, Lam KC, Chan AT, Mok TS, Yeo W. New utility of an old marker: serial alpha-fetoprotein measurement in predicting radiologic response and survival of patients with hepatocellular carcinoma undergoing systemic chemotherapy. J Clin Oncol. 2009;27:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 213] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 68. | Vora SR, Zheng H, Stadler ZK, Fuchs CS, Zhu AX. Serum alpha-fetoprotein response as a surrogate for clinical outcome in patients receiving systemic therapy for advanced hepatocellular carcinoma. Oncologist. 2009;14:717-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 69. | Shao YY, Lin ZZ, Hsu C, Shen YC, Hsu CH, Cheng AL. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer. 2010;116:4590-4596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 70. | Yau T, Yao TJ, Chan P, Wong H, Pang R, Fan ST, Poon RT. The significance of early alpha-fetoprotein level changes in predicting clinical and survival benefits in advanced hepatocellular carcinoma patients receiving sorafenib. Oncologist. 2011;16:1270-1279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 71. | Personeni N, Bozzarelli S, Pressiani T, Rimassa L, Tronconi MC, Sclafani F, Carnaghi C, Pedicini V, Giordano L, Santoro A. Usefulness of alpha-fetoprotein response in patients treated with sorafenib for advanced hepatocellular carcinoma. J Hepatol. 2012;57:101-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 72. | Køstner AH, Sorensen M, Olesen RK, Grønbæk H, Lassen U, Ladekarl M. Sorafenib in advanced hepatocellular carcinoma: a nationwide retrospective study of efficacy and tolerability. ScientificWorldJournal. 2013;2013:931972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Kuzuya T, Asahina Y, Tsuchiya K, Tanaka K, Suzuki Y, Hoshioka T, Tamaki S, Kato T, Yasui Y, Hosokawa T. Early decrease in α-fetoprotein, but not des-γ-carboxy prothrombin, predicts sorafenib efficacy in patients with advanced hepatocellular carcinoma. Oncology. 2011;81:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 74. | Nakazawa T, Hidaka H, Takada J, Okuwaki Y, Tanaka Y, Watanabe M, Shibuya A, Minamino T, Kokubu S, Koizumi W. Early increase in α-fetoprotein for predicting unfavorable clinical outcomes in patients with advanced hepatocellular carcinoma treated with sorafenib. Eur J Gastroenterol Hepatol. 2013;25:683-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 75. | Tamura Y, Igarashi M, Kawai H, Suda T, Satomura S, Aoyagi Y. Clinical advantage of highly sensitive on-chip immunoassay for fucosylated fraction of alpha-fetoprotein in patients with hepatocellular carcinoma. Dig Dis Sci. 2010;55:3576-3583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 76. | Toyoda H, Kumada T, Tada T. Highly sensitive Lens culinaris agglutinin-reactive α-fetoprotein: a new tool for the management of hepatocellular carcinoma. Oncology. 2011;81 Suppl 1:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Nakao K, Ichikawa T. Recent topics on α-fetoprotein. Hepatol Res. 2013;43:820-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Kudo M, Ueshima K. Positioning of a molecular-targeted agent, sorafenib, in the treatment algorithm for hepatocellular carcinoma and implication of many complete remission cases in Japan. Oncology. 2010;78 Suppl 1:154-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 79. | Shao YY, Hsu CH, Cheng AL. Predictive biomarkers of antiangiogenic therapy for advanced hepatocellular carcinoma: where are we? Liver Cancer. 2013;2:93-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 80. | Nagaoka S, Yatsuhashi H, Hamada H, Yano K, Matsumoto T, Daikoku M, Arisawa K, Ishibashi H, Koga M, Sata M. The des-gamma-carboxy prothrombin index is a new prognostic indicator for hepatocellular carcinoma. Cancer. 2003;98:2671-2677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 81. | Ueshima K, Kudo M, Takita M, Nagai T, Tatsumi C, Ueda T, Kitai S, Ishikawa E, Yada N, Inoue T. Des-γ-carboxyprothrombin may be a promising biomarker to determine the therapeutic efficacy of sorafenib for hepatocellular carcinoma. Dig Dis. 2011;29:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 82. | Kawaoka T, Aikata H, Kan H, Fujino H, Fukuhara T, Kobayashi T, Naeshiro N, Miyaki D, Hiramatsu A, Imamura M. Clinical outcome and prognostic factors of patients with hepatocellular carcinoma and extrahepatic metastasis treated with sorafenib. Hepatol Res. 2014;44:1320-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Hicklin DJ, Ellis LM. Role of the vascular endothelial growth factor pathway in tumor growth and angiogenesis. J Clin Oncol. 2005;23:1011-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2025] [Cited by in RCA: 2178] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 84. | Poon RT, Lau CP, Cheung ST, Yu WC, Fan ST. Quantitative correlation of serum levels and tumor expression of vascular endothelial growth factor in patients with hepatocellular carcinoma. Cancer Res. 2003;63:3121-3126. [PubMed] |
| 85. | Schoenleber SJ, Kurtz DM, Talwalkar JA, Roberts LR, Gores GJ. Prognostic role of vascular endothelial growth factor in hepatocellular carcinoma: systematic review and meta-analysis. Br J Cancer. 2009;100:1385-1392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 86. | Kaseb AO, Hanbali A, Cotant M, Hassan MM, Wollner I, Philip PA. Vascular endothelial growth factor in the management of hepatocellular carcinoma: a review of literature. Cancer. 2009;115:4895-4906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 87. | Kaseb AO, Morris JS, Hassan MM, Siddiqui AM, Lin E, Xiao L, Abdalla EK, Vauthey JN, Aloia TA, Krishnan S. Clinical and prognostic implications of plasma insulin-like growth factor-1 and vascular endothelial growth factor in patients with hepatocellular carcinoma. J Clin Oncol. 2011;29:3892-3899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 88. | Ebos JM, Lee CR, Bogdanovic E, Alami J, Van Slyke P, Francia G, Xu P, Mutsaers AJ, Dumont DJ, Kerbel RS. Vascular endothelial growth factor-mediated decrease in plasma soluble vascular endothelial growth factor receptor-2 levels as a surrogate biomarker for tumor growth. Cancer Res. 2008;68:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 89. | Kemik O, Sumer A, Kemik SA, Purisa S, Tuzun S. Circulating levels of VEGF family and their receptors in hepatocellular carcinoma. Bratisl Lek Listy. 2010;111:485-488. [PubMed] |
| 90. | Peña C, Lathia C, Shan M, Escudier B, Bukowski RM. Biomarkers predicting outcome in patients with advanced renal cell carcinoma: Results from sorafenib phase III Treatment Approaches in Renal Cancer Global Evaluation Trial. Clin Cancer Res. 2010;16:4853-4863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 91. | Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS, Lahdenranta J. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27:3027-3035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 365] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 92. | Faivre SJ, Bouattour M, Dreyer C, Raymond E. Sunitinib in hepatocellular carcinoma: redefining appropriate dosing, schedule, and activity end points. J Clin Oncol. 2009;27:e248-e250; author reply e251-e252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 93. | Harmon CS, DePrimo SE, Raymond E, Cheng AL, Boucher E, Douillard JY, Lim HY, Kim JS, Lechuga MJ, Lanzalone S. Mechanism-related circulating proteins as biomarkers for clinical outcome in patients with unresectable hepatocellular carcinoma receiving sunitinib. J Transl Med. 2011;9:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1299] [Cited by in RCA: 1246] [Article Influence: 62.3] [Reference Citation Analysis (0)] |
| 95. | Deprimo SE, Bello CL, Smeraglia J, Baum CM, Spinella D, Rini BI, Michaelson MD, Motzer RJ. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med. 2007;5:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 258] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 96. | Rini BI, Michaelson MD, Rosenberg JE, Bukowski RM, Sosman JA, Stadler WM, Hutson TE, Margolin K, Harmon CS, DePrimo SE. Antitumor activity and biomarker analysis of sunitinib in patients with bevacizumab-refractory metastatic renal cell carcinoma. J Clin Oncol. 2008;26:3743-3748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 330] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 97. | Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci USA. 2007;104:17069-17074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 307] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 98. | Boige V, Malka D, Bourredjem A, Dromain C, Baey C, Jacques N, Pignon JP, Vimond N, Bouvet-Forteau N, De Baere T. Efficacy, safety, and biomarkers of single-agent bevacizumab therapy in patients with advanced hepatocellular carcinoma. Oncologist. 2012;17:1063-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 99. | Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, Chen LL, Podoloff DA, Benjamin RS. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007;25:1753-1759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1094] [Cited by in RCA: 1115] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 100. | Ronot M, Bouattour M, Wassermann J, Bruno O, Dreyer C, Larroque B, Castera L, Vilgrain V, Belghiti J, Raymond E. Alternative Response Criteria (Choi, European association for the study of the liver, and modified Response Evaluation Criteria in Solid Tumors [RECIST]) Versus RECIST 1.1 in patients with advanced hepatocellular carcinoma treated with sorafenib. Oncologist. 2014;19:394-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (1)] |
| 101. | Keyvanjah K, DePrimo SE, Harmon CS, Huang X, Kern KA, Carley W. Soluble KIT correlates with clinical outcome in patients with metastatic breast cancer treated with sunitinib. J Transl Med. 2012;10:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 102. | Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1354] [Article Influence: 71.3] [Reference Citation Analysis (0)] |
| 103. | Breuhahn K, Longerich T, Schirmacher P. Dysregulation of growth factor signaling in human hepatocellular carcinoma. Oncogene. 2006;25:3787-3800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 104. | Chau GY, Lui WY, Chi CW, Chau YP, Li AF, Kao HL, Wu CW. Significance of serum hepatocyte growth factor levels in patients with hepatocellular carcinoma undergoing hepatic resection. Eur J Surg Oncol. 2008;34:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 105. | Vejchapipat P, Tangkijvanich P, Theamboonlers A, Chongsrisawat V, Chittmittrapap S, Poovorawan Y. Association between serum hepatocyte growth factor and survival in untreated hepatocellular carcinoma. J Gastroenterol. 2004;39:1182-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 106. | Mizuguchi T, Nagayama M, Meguro M, Shibata T, Kaji S, Nobuoka T, Kimura Y, Furuhata T, Hirata K. Prognostic impact of surgical complications and preoperative serum hepatocyte growth factor in hepatocellular carcinoma patients after initial hepatectomy. J Gastrointest Surg. 2009;13:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 107. | Goyal L, Muzumdar MD, Zhu AX. Targeting the HGF/c-MET pathway in hepatocellular carcinoma. Clin Cancer Res. 2013;19:2310-2318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 249] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 108. | Mitsuhashi N, Shimizu H, Ohtsuka M, Wakabayashi Y, Ito H, Kimura F, Yoshidome H, Kato A, Nukui Y, Miyazaki M. Angiopoietins and Tie-2 expression in angiogenesis and proliferation of human hepatocellular carcinoma. Hepatology. 2003;37:1105-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 109. | Zhang ZL, Liu ZS, Sun Q. Expression of angiopoietins, Tie2 and vascular endothelial growth factor in angiogenesis and progression of hepatocellular carcinoma. World J Gastroenterol. 2006;12:4241-4245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 110. | Torimura T, Ueno T, Kin M, Harada R, Taniguchi E, Nakamura T, Sakata R, Hashimoto O, Sakamoto M, Kumashiro R. Overexpression of angiopoietin-1 and angiopoietin-2 in hepatocellular carcinoma. J Hepatol. 2004;40:799-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 111. | Kuboki S, Shimizu H, Mitsuhashi N, Kusashio K, Kimura F, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Miyazaki M. Angiopoietin-2 levels in the hepatic vein as a useful predictor of tumor invasiveness and prognosis in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2008;23:e157-e164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 112. | Miyahara K, Nouso K, Tomoda T, Kobayashi S, Hagihara H, Kuwaki K, Toshimori J, Onishi H, Ikeda F, Miyake Y. Predicting the treatment effect of sorafenib using serum angiogenesis markers in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1604-1611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 113. | Wang J, Yang J, Yuan D, Wang J, Zhao J, Wang L. Effects of basic fibroblast growth factor on angiogenin expression and cell proliferation in H7402 human hepatoma cells. J Genet Genomics. 2009;36:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 114. | Mise M, Arii S, Higashituji H, Furutani M, Niwano M, Harada T, Ishigami S, Toda Y, Nakayama H, Fukumoto M. Clinical significance of vascular endothelial growth factor and basic fibroblast growth factor gene expression in liver tumor. Hepatology. 1996;23:455-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 214] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 115. | Poon RT, Ng IO, Lau C, Yu WC, Fan ST, Wong J. Correlation of serum basic fibroblast growth factor levels with clinicopathologic features and postoperative recurrence in hepatocellular carcinoma. Am J Surg. 2001;182:298-304. [PubMed] |
| 116. | Becker G, Schmitt-Graeff A, Ertelt V, Blum HE, Allgaier HP. CD117 (c-kit) expression in human hepatocellular carcinoma. Clin Oncol (R Coll Radiol). 2007;19:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 117. | Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1562] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 118. | LeRoith D, Roberts CT. The insulin-like growth factor system and cancer. Cancer Lett. 2003;195:127-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 788] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 119. | Shao YY, Huang CC, Lin SD, Hsu CH, Cheng AL. Serum insulin-like growth factor-1 levels predict outcomes of patients with advanced hepatocellular carcinoma receiving antiangiogenic therapy. Clin Cancer Res. 2012;18:3992-3997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 120. | Capillo M, Mancuso P, Gobbi A, Monestiroli S, Pruneri G, Dell’Agnola C, Martinelli G, Shultz L, Bertolini F. Continuous infusion of endostatin inhibits differentiation, mobilization, and clonogenic potential of endothelial cell progenitors. Clin Cancer Res. 2003;9:377-382. [PubMed] |
| 121. | Goon PK, Lip GY, Boos CJ, Stonelake PS, Blann AD. Circulating endothelial cells, endothelial progenitor cells, and endothelial microparticles in cancer. Neoplasia. 2006;8:79-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 122. | Ho JW, Pang RW, Lau C, Sun CK, Yu WC, Fan ST, Poon RT. Significance of circulating endothelial progenitor cells in hepatocellular carcinoma. Hepatology. 2006;44:836-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 123. | Bertolini F, Shaked Y, Mancuso P, Kerbel RS. The multifaceted circulating endothelial cell in cancer: towards marker and target identification. Nat Rev Cancer. 2006;6:835-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 446] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 124. | Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1439] [Article Influence: 68.5] [Reference Citation Analysis (0)] |
| 125. | Norden-Zfoni A, Desai J, Manola J, Beaudry P, Force J, Maki R, Folkman J, Bello C, Baum C, DePrimo SE. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13:2643-2650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 165] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 126. | Shao YY, Lin ZZ, Chen TJ, Hsu C, Shen YC, Hsu CH, Cheng AL. High circulating endothelial progenitor levels associated with poor survival of advanced hepatocellular carcinoma patients receiving sorafenib combined with metronomic chemotherapy. Oncology. 2011;81:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 127. | Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, De Greve J, Douillard JY, Lathia C, Schwartz B. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293-4300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 906] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 128. | Ozenne V, Paradis V, Pernot S, Castelnau C, Vullierme MP, Bouattour M, Valla D, Farges O, Degos F. Tolerance and outcome of patients with unresectable hepatocellular carcinoma treated with sorafenib. Eur J Gastroenterol Hepatol. 2010;22:1106-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 129. | Shao YY, Chen CL, Ho MC, Huang CC, Tu HC, Hsu CH, Cheng AL. Dissimilar immunohistochemical expression of ERK and AKT between paired biopsy and hepatectomy tissues of hepatocellular carcinoma. Anticancer Res. 2012;32:4865-4870. [PubMed] |
| 130. | Baker AF, Dragovich T, Ihle NT, Williams R, Fenoglio-Preiser C, Powis G. Stability of phosphoprotein as a biological marker of tumor signaling. Clin Cancer Res. 2005;11:4338-4340. [PubMed] |
| 131. | Hagiwara S, Kudo M, Nagai T, Inoue T, Ueshima K, Nishida N, Watanabe T, Sakurai T. Activation of JNK and high expression level of CD133 predict a poor response to sorafenib in hepatocellular carcinoma. Br J Cancer. 2012;106:1997-2003. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 132. | Park YN, Kim YB, Yang KM, Park C. Increased expression of vascular endothelial growth factor and angiogenesis in the early stage of multistep hepatocarcinogenesis. Arch Pathol Lab Med. 2000;124:1061-1065. [PubMed] |
| 133. | Poon RT, Lau CP, Ho JW, Yu WC, Fan ST, Wong J. Tissue factor expression correlates with tumor angiogenesis and invasiveness in human hepatocellular carcinoma. Clin Cancer Res. 2003;9:5339-5345. [PubMed] |
| 134. | Peng S, Wang Y, Peng H, Chen D, Shen S, Peng B, Chen M, Lencioni R, Kuang M. Autocrine vascular endothelial growth factor signaling promotes cell proliferation and modulates sorafenib treatment efficacy in hepatocellular carcinoma. Hepatology. 2014;60:1264-1277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 135. | Yang XR, Xu Y, Yu B, Zhou J, Qiu SJ, Shi GM, Zhang BH, Wu WZ, Shi YH, Wu B. High expression levels of putative hepatic stem/progenitor cell biomarkers related to tumour angiogenesis and poor prognosis of hepatocellular carcinoma. Gut. 2010;59:953-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 136. | Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee JE, Cho JY, Yoo JE, Choi JS, Park YN. Human hepatocellular carcinomas with “Stemness”-related marker expression: keratin 19 expression and a poor prognosis. Hepatology. 2011;54:1707-1717. [PubMed] |
| 137. | Kim H, Yoo JE, Cho JY, Oh BK, Yoon YS, Han HS, Lee HS, Jang JJ, Jeong SH, Kim JW. Telomere length, TERT and shelterin complex proteins in hepatocellular carcinomas expressing “stemness”-related markers. J Hepatol. 2013;59:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 138. | Kim H, Park YN. Hepatocellular carcinomas expressing ‘stemness’-related markers: clinicopathological characteristics. Dig Dis. 2014;32:778-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 139. | Wang X, Ren H, Zhao T, Chen J, Sun W, Sun Y, Ma W, Wang J, Gao C, Gao S. Stem cell factor is a novel independent prognostic biomarker for hepatocellular carcinoma after curative resection. Carcinogenesis. 2014;35:2283-2290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 140. | Jubb AM, Hurwitz HI, Bai W, Holmgren EB, Tobin P, Guerrero AS, Kabbinavar F, Holden SN, Novotny WF, Frantz GD. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J Clin Oncol. 2006;24:217-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 304] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 141. | Li Y, Ma X, Zhang J, Liu X, Liu L. Prognostic value of microvessel density in hepatocellular carcinoma patients: a meta-analysis. Int J Biol Markers. 2014;29:e279-e287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 142. | Santoro A, Rimassa L, Borbath I, Daniele B, Salvagni S, Van Laethem JL, Van Vlierberghe H, Trojan J, Kolligs FT, Weiss A. Tivantinib for second-line treatment of advanced hepatocellular carcinoma: a randomised, placebo-controlled phase 2 study. Lancet Oncol. 2013;14:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 461] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 143. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4491] [Article Influence: 224.6] [Reference Citation Analysis (0)] |
| 144. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4506] [Article Influence: 346.6] [Reference Citation Analysis (2)] |
| 145. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2583] [Cited by in RCA: 3260] [Article Influence: 217.3] [Reference Citation Analysis (36)] |
| 146. | Faivre S, Zappa M, Vilgrain V, Boucher E, Douillard JY, Lim HY, Kim JS, Im SA, Kang YK, Bouattour M. Changes in tumor density in patients with advanced hepatocellular carcinoma treated with sunitinib. Clin Cancer Res. 2011;17:4504-4512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 147. | Edeline J, Boucher E, Rolland Y, Vauléon E, Pracht M, Perrin C, Le Roux C, Raoul JL. Comparison of tumor response by Response Evaluation Criteria in Solid Tumors (RECIST) and modified RECIST in patients treated with sorafenib for hepatocellular carcinoma. Cancer. 2012;118:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 148. | Liu L, Wang W, Chen H, Zhao Y, Bai W, Yin Z, He C, Jia J, Yang M, Xia J. EASL- and mRECIST-evaluated responses to combination therapy of sorafenib with transarterial chemoembolization predict survival in patients with hepatocellular carcinoma. Clin Cancer Res. 2014;20:1623-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 149. | Salvaggio G, Furlan A, Agnello F, Cabibbo G, Marin D, Giannitrapani L, Genco C, Midiri M, Lagalla R, Brancatelli G. Hepatocellular carcinoma enhancement on contrast-enhanced CT and MR imaging: response assessment after treatment with sorafenib: preliminary results. Radiol Med. 2014;119:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 150. | Provenzale JM. Imaging of angiogenesis: clinical techniques and novel imaging methods. AJR Am J Roentgenol. 2007;188:11-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 151. | Shiozawa K, Watanabe M, Kikuchi Y, Kudo T, Maruyama K, Sumino Y. Evaluation of sorafenib for hepatocellular carcinoma by contrast-enhanced ultrasonography: a pilot study. World J Gastroenterol. 2012;18:5753-5758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 152. | Sugimoto K, Moriyasu F, Saito K, Rognin N, Kamiyama N, Furuichi Y, Imai Y. Hepatocellular carcinoma treated with sorafenib: early detection of treatment response and major adverse events by contrast-enhanced US. Liver Int. 2013;33:605-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 153. | Zocco MA, Garcovich M, Lupascu A, Di Stasio E, Roccarina D, Annicchiarico BE, Riccardi L, Ainora ME, Ponziani F, Caracciolo G. Early prediction of response to sorafenib in patients with advanced hepatocellular carcinoma: the role of dynamic contrast enhanced ultrasound. J Hepatol. 2013;59:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 154. | Piscaglia F, Cucchetti A, Dietrich CF, Salvatore V. Towards new tools for refined management of patients with advanced hepatocellular carcinoma under systemic therapy: some enthusiasm with a word of caution. J Hepatol. 2013;59:924-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 155. | O’Connor JP, Jackson A, Parker GJ, Jayson GC. DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer. 2007;96:189-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 415] [Cited by in RCA: 377] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 156. | Hsu CY, Shen YC, Yu CW, Hsu C, Hu FC, Hsu CH, Chen BB, Wei SY, Cheng AL, Shih TT. Dynamic contrast-enhanced magnetic resonance imaging biomarkers predict survival and response in hepatocellular carcinoma patients treated with sorafenib and metronomic tegafur/uracil. J Hepatol. 2011;55:858-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 157. | Siemerink EJ, Mulder NH, Brouwers AH, Hospers GA. 18F-Fluorodeoxyglucose positron emission tomography for monitoring response to sorafenib treatment in patients with hepatocellular carcinoma. Oncologist. 2008;13:734-735; author reply 736-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 158. | Fartoux L, Decaens T. Contribution of biomarkers and imaging in the management of hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2011;35 Suppl 1:S21-S30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 159. | Lee JH, Park JY, Kim do Y, Ahn SH, Han KH, Seo HJ, Lee JD, Choi HJ. Prognostic value of 18F-FDG PET for hepatocellular carcinoma patients treated with sorafenib. Liver Int. 2011;31:1144-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 160. | Shim JH, Park JW, Choi JI, Park BJ, Kim CM. Practical efficacy of sorafenib monotherapy for advanced hepatocellular carcinoma patients in a Hepatitis B virus-endemic area. J Cancer Res Clin Oncol. 2009;135:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 161. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [PubMed] |