Published online Sep 27, 2014. doi: 10.4254/wjh.v6.i9.660
Revised: June 30, 2014
Accepted: August 27, 2014
Published online: September 27, 2014
AIM: To assess, in a routine practice setting, the sustained virologic response (SVR) to telaprevir (TPV) or boceprevir (BOC) in hepatitis C virus (HCV) null-responders or relapsers with severe liver fibrosis.
METHODS: One hundred twenty-five patients were treated prospectively for 48 wk with TPV or BOC + pegylated-interferon (peg-INF) α2a + ribavirin (PR) according to standard treatment schedules without randomization. These patients were treated in routine practice settings in 10 public or private health care centers, and the data were prospectively collected. Only patients with severe liver fibrosis (Metavir scores of F3 or F4 upon liver biopsy or liver stiffness assessed by elastography), genotype 1 HCV and who were null-responders or relapsers to prior PR combination therapy were included in this study.
RESULTS: The Metavir fibrosis scores were F3 in 35 (28%) and F4 in 90 (72%) of the patients. In total, 62.9% of the patients were null-responders and 37.1% relapsers to the previous PR therapy. The overall SVR rate at 24 wk post-treatment withdrawal was 59.8%. The SVR was 65.9% in the TPV group and 44.1% in the BOC group. Independent predictive factors of an SVR included a response to previous treatment, relapsers vs null-responders [OR = 3.9; (1.4, 10.6), P = 0.0084], a rapid virological response (RVR) [OR 6.9 (2.6, 18.2), P = 0.001] and liver stiffness lower than 21.3 kPa [OR = 8.2 (2.3, 29.5), P = 0.001]. During treatment, 63 patients (50.8%) had at least one severe adverse event (SAE) of grade 3 or 4. A multivariate analysis identified two factors associated with SAEs: female gender [OR = 2.4 (1.1, 5.6), P = 0.037] and a platelet count below 150 × 103/ mm3 [OR = 5.3 (2.3, 12.4), P≤ 0.001].
CONCLUSION: More than half of these difficult-to-treat patients achieved an SVR and had SAEs in an actual practice setting. The SVR rate was influenced by the response to previous PR treatment, the RVR and liver stiffness.
Core tip: To the best of our knowledge, this study marks the first time that a significant link has been shown between a sustained virological response to triple therapy and the liver stiffness measured by elastography at baseline. We also demonstrate that triple therapy is poorly tolerated. Two factors predict the development of serious adverse events: female gender and an initial platelet count of less than 150000/mm3; these factors facilitate the identification of at-risk patients.
- Citation: Bonnet D, Guivarch M, Bérard E, Combis JM, Remy AJ, Glibert A, Payen JL, Metivier S, Barange K, Desmorat H, Palacin A, Nicot F, Abravanel F, Alric L. Telaprevir- and boceprevir-based tritherapies in real practice for F3-F4 pretreated hepatitis C virus patients. World J Hepatol 2014; 6(9): 660-669
- URL: https://www.wjgnet.com/1948-5182/full/v6/i9/660.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i9.660
Approximately 80% of patients infected with the hepatitis C virus (HCV) develop chronic infections that could lead to cirrhosis and hepatocellular carcinoma[1]. Combination therapy with pegylated-interferon (peg-INF) α2a + ribavirin (PR) was the first demonstrably effective treatment[2,3]. The current combination of PR with protease inhibitors (PIs) such as telaprevir (TPV) or boceprevir (BOC) is clearly more beneficial for HCV genotype 1 patients[4-7]. The majority of HCV genotype 1 patients demonstrate a sustained virological response (SVR) to TPV (69% to 75%) and BOC triple therapy (68% to 75%)[4-7]. However, only 65% of genotype 1 HCV patients who were previously unresponsive to PR therapy produced an SVR to TPV triple therapy; only 66% of these unresponsive patients produce an SVR to BOC triple therapy[8-10]. Some predictive factors, such as high baseline viral load, HCV genotype 1a, IL-28B T/T polymorphism and severe liver fibrosis, have been associated with a poor response to antiviral treatment with PI[6,10]. Moreover, the data on the benefit of retreating HCV genotype 1 cirrhotic patients who did not respond to a standard PR regimen with triple therapy are inconclusive[8-10]. A study on small subgroups of null-responder patients with severe liver fibrosis given TPV triple therapy determined that 39% of Metavir F3 patients and only 14% of patients with Metavir F4 produced an SVR[8]. Therefore, guidelines and new treatment strategies are required that consider the cost and adverse effects of TPV and BOC combined with PR for these difficult-to-treat patients. In these pivotal studies performed exclusively in academic centers, many patients experienced adverse effects despite restricting inclusion criteria and strict observance of treatment rules[5-10]. In addition, because very few non-responder patients with severe liver fibrosis were included in these studies, the occurrence of severe adverse effects (SAEs) in this specific population remains unclear. Therefore, we need to evaluate (in actual practice settings) the efficacy and safety profile of PI triple therapy in pretreated HCV genotype 1 patients with severe liver fibrosis.
This study assesses the SVR and safety profiles of triple therapy with TPV or BOC combined with PR in HCV genotype 1 patients with severe liver fibrosis (Metavir F3 or F4) who had previously failed to adequately respond to the standard PR treatment. This observational non-randomized prospective cohort study was performed in actual practice settings.
This study used a non-randomized multicenter prospective observational cohort from a Midi-Pyrénées network (HEPATOMIP) of hepatogastroenterology practitioners working in the Toulouse University hospital and in 9 general hospitals or private clinics. The cohort included 125 consecutive HCV genotype 1 null-responder or relapser patients with severe liver fibrosis who were seen between February 2011 and January 2012. Only those patients with severe liver fibrosis having a Metavir fibrosis score of F3 or F4 were included.
All of the patients were infected with HCV genotype 1 and did not achieve an SVR with previous standard treatments with peg-IFN α2a or 2b + ribavirin, described as follows[11]: (1) relapsers were defined as patients who achieved undetectable HCV RNA levels at the end of 48 wk of PR treatment and then subsequently relapsed; and (2) null-responders failed to achieve a decrease of at least 2 log HCV RNA IU/mL during PR treatment given for at least 24 wk.
Partial responders to previous therapy were not included in the study. After an interval of at least 6 mo, patients were given either 12 wk of TPV (750 mg every 8 h, Janssen-Cilag, Issy les Moulineaux, France) combined with PR (Roche, Meylan, France) followed by 36 wk of PR or 4 wk (lead-in phase) of PR followed by 44 wk of PR and BOC (800 mg every 8 h (MSD, Courbevoie, France) according to French label guidelines[12] (French National Agency of drugs and health products security, ANSM cohort temporary use authorization n° 324 and n° 330). In this observational cohort, TPV or BOC triple therapy was selected by each physician; however, all of the patients received the same schedule of PR, as follows: peg-IFN α2a (180 g/wk) + ribavirin (1000 to 1200 mg/d, depending on body weight). The Toulouse University review board approved this cohort, and all of the patients provided written informed consent.
A quantification of the HCV RNA level was performed at baseline, then every 4 wk during triple therapy and at 12 and 24 wk following treatment withdrawal using real-time polymerase chain reaction (COBAS Amplicor/TaqMan, Roche Diagnostics, Basel, Switzerland) with a lower detection limit of 15 IU/mL. Fibrosis was evaluated by a liver biopsy or by measuring the liver stiffness (LS) according to the manufacturer’s instructions (Fibroscan, Echosens). The results were expressed in kilopascals (kPa). Metavir F3 was defined by a liver stiffness of 9.5-12.4 kPa and Metavir F4 cirrhotic patients were defined by values of up to 12.5 kPa.
The response to triple therapy could be summarized as: (1) A rapid virological response (RVR), i.e., negative for HCV RNA after 4 weeks of triple therapy (defined as week 4 for the TPV group and week 8 for the BOC group); (2) A virological response (VR), i.e., negative for HCV RNA at the end of triple therapy; and (3) or a sustained virological response (SVR), i.e., negative for HCV RNA 24 wk after the end of treatment.
All of the patients were seen by their physicians at baseline, every 2 wk during the first 2 mo, every 4 wk during the following phase of therapy and then every 4, 12, and 24 wk after treatment withdrawal. Adverse events were graded by investigators according to a modified World Health Organization grading system. Non-life-threatening adverse events and hematological disorders were managed according to the French association of the study of the liver (AFEF) by reducing the ribavirin dose and/or giving erythropoietin (EPO) at the discretion of the physician[11]. EPO was recommended when the patient’s hemoglobin (Hb) level dropped to less than 10 g/dL, despite a previous reduction in the ribavirin dose by 200 mg/d.
Statistical analyses were performed using STATA software, release 11.2 (STATA Corporation, College Station, TX, United States). Numbers and frequencies were used for the described qualitative data, and means ± SD or medians (inter-quartile range: IQR) were used when the normality assumption was not met for quantitative data. The qualitative variables were compared between groups (TPV and BOC groups; SVR and no-SVR groups; SAEs and no-SAEs groups) using the χ2 test (or Fisher’s exact test for small expected numbers). Student’s t test was used to compare the distribution of quantitative data. Alternatively, the Mann-Whitney test was used when the distribution was not normal or when homoscedasticity was rejected. We assessed the accuracy of liver stiffness to predict an SVR according to receiver operating characteristic (ROC) curves (plotting sensitivity vs 1-specificity at various cut-off settings), and we defined the optimal liver stiffness cut-off value of 21.3 kPa according to the best rate of correctly classified subjects {[(true positives + true negatives)/total]; 69.2%}. Odds ratios (ORs) for SVR or SAE and 95%CIs were assessed using a logistic regression model. The variables initially included in the model were those associated with SVR or SAE in the univariate analysis (P value < 0.20). A backward procedure was applied to assess variables that were significantly and independently associated with SVR (or SAE) (P value < 0.05). Because the linearity hypothesis was not fully respected, the following continuous variables were transformed into ordered data: liver stiffness (< 21.3 kPa vs≥ 21.3 kPa) for the SVR model, platelet count (< 150 × 103/mm3vs≥ 150 × 103/mm3) for the SAE model. Interactions between independent covariates were tested in the final regression models, and none of these interactions was significant. All of the reported P values are two-sided, and the significance threshold was set at < 0.05.
This prospective cohort included 125 HCV genotype 1 patients (Table 1). None of the patients had responded to previous treatment with standard PR combination therapy. There were 46/124 (37.1%) previous relapsers and 78/124 (62.49%) null-responders, and there were more men (64.8%) than women (35.2%). HCV subtype 1b (56.8%) infections were more frequent than HCV subtype 1a (31.2%) infections, although the HCV genotype was not defined as 1b or 1a in 12% of cases. As expected in this population of relapsers and null-responders to prior antiviral therapy, only 15.4% of the patients had an IL-28B genotype C/C. All of the patients had severe liver fibrosis: 28% were Metavir F3 and 72% were cirrhotic, with a Metavir F4 score. All except 2 of the cirrhotic patients were classified as Child-Pugh class A. Triple therapy was not randomized; TPV or BOC was selected by the patient’s physician, with 72% of the patients treated with TPV and 28% treated with BOC. We observed no difference in the subsequent parameters for the two groups.
| Triple therapy: Peg-IFNα2a + Ribavirin +Protease inhibitor, | 125 |
| Telaprevir | 90 (72) |
| Boceprevir | 35 (28) |
| Ribavirin dosage mg/kg, mean (SD) | 14.4 (2.1) |
| Age, yr, mean (SD) | 56.2 (9.7) |
| Gender | |
| Male | 81 (64.8) |
| Female | 44 (35.2) |
| HCV genotype | |
| 1a | 39 (31.2) |
| 1b | 71 (56.8) |
| Undetermined subtype 1 | 15 (12) |
| IL28B genotype (rs12979860) | |
| C/T or T/T | 88 (84.6) |
| Viral load, mean (log10 IU/ mL) | 6.3 (0.7) |
| Prior response to anti-viral therapy | |
| Relapsers | 46 (37.1) |
| Null-responders | 78 (62.9) |
| Liver fibrosis grade | |
| Metavir F3 | 35 (28) |
| Metavir F4 | 90 (72) |
| Child-Pugh score | |
| A | 123 (98.4) |
| B | 1 (0.8) |
| C | 1 (0.8) |
| Liver stiffness values (kPa) | |
| Mean (SD) | 17.5 (10.3) |
| Median (IQR) | 14.3 (10.4-20.6) |
| Oesophageal varices | |
| None | 92 (75.4) |
| Grade 1 | 16 (13.1) |
| Grade 2 or 3 | 14 (11.5) |
| Mean hemoglobin level (g/dL, mean (SD) | 15.1 (1.6) |
| Mean platelet count × 103/mm3, mean (SD) | 165.69 (64.9) |
| Mean neutrophil count × 103/mm3, mean (SD) | 3.4 (1.2) |
The overall SVR rate (Table 2) was 59.8% (73/122 patients). From the overall population, 92 patients (75.4%) had undetectable HCV RNA levels at the end of triple therapy, and 19 patients (20.6%) relapsed during the post-treatment follow-up. Three of the 57 patients (5.2%) with negative HCV RNA levels at 12 wk after the end of triple therapy suffered a late relapse after the twelfth week. These three patients were null-responders to previous PR treatment, and one of these patients had a RVR during triple therapy. The remaining 73 patients maintained their SVR until the end of the follow-up period, 24 wk after triple therapy withdrawal. The SVR rate was higher (Table 2) in the TPV group (65.9%) than in the BOC group [44.1%; P = 0.0276, OR = 2.49 (1.1, 5.5), univariate analysis]. The SVR rate was not significantly influenced by the HCV subtype (1a or 1b), IL-28B genotype or viral load at baseline. Non-cirrhotic patients tended to have a better SVR than the patients with cirrhosis (Table 2); however, this difference did not reach statistical significance (68.6% for Metavir F3 and 56.3% for Metavir F4, P = 0.212). Only 23 of the 52 patients (44%) in the subgroup of very difficult-to-treat patients (those with cirrhosis and a null-response to prior therapy) achieved an SVR. Among these SVR patients, 20/23 (87%) were given TPV triple therapy and 3/23 (13%) were given BOC triple therapy. Neither decreasing the ribavirin dosage nor anemia was associated with a loss of SVR (Table 2).
| No sustained virological responsen = 49 | Sustained virological responsen = 73 | P valueunivariate analysis | P valuemultivariate analysisOR(95%CI) | |
| Protease inhibitor | ||||
| Telaprevir | 30 (34.1) | 58 (65.9) | 0.0276 | NS1 |
| Boceprevir | 19 (55.9) | 15 (44.1) | ||
| Gender | ||||
| Male | 27 (34.2) | 52 (65.8) | 0.0675 | NS1 |
| Female | 22 (51.2) | 21 (48.8) | ||
| HCV genotype 1 subtype | ||||
| 1a | 17 (45.9) | 20 (54.1) | 0.8051 | - |
| 1b | 26 (37.1) | 44 (62.9) | ||
| IL28B genotype, rs12979860, n (%) | ||||
| C/C | 4 (25) | 12 (75) | 0.4420 | - |
| C/T or T/T | 40 (45.5) | 48 (54.5) | ||
| Response to prior therapy | 0.008 | |||
| Null-responders | 40 (52.6) | 36 (47.4) | 0.0004 | 1.0 |
| Relapsers | 9 (20) | 36 (80) | 3.9 (1.4-10.6) | |
| Grade of liver fibrosis | ||||
| Metavir F3 | 11 (31.4) | 24 (68.6) | 0.2118 | - |
| Metavir F4 | 38 (43.7) | 49 (56.3) | ||
| Liver stiffness value (kPa) | ||||
| Median, kPa (IQR) | 17.3 (11.5-28.8) | 13.9 (9.4-19.7) | 0.0296 | 0.001 |
| < 21.3 kPa | 21 (30) | 49 (70) | 0.002 | 8.2 (2.3-29.5) |
| ≥ 21.3 kPa | 14 (66.7) | 7 (33.3) | 1 | |
| Rapid virological response | 18 (23.7) | 58 (76.3) | ≤ 0.0001 | ≤ 0.001 |
| No rapid virological response | 26 (68.4) | 12 (31.6) | 6.9 (2.6-18.2) 1.0 | |
| Decrease of ribavirin dosage | 18 (37.5) | 30 (62.5) | 0.6287 | - |
| No decrease of ribavirin dosage | 31 (41.9) | 43 (58.1) | ||
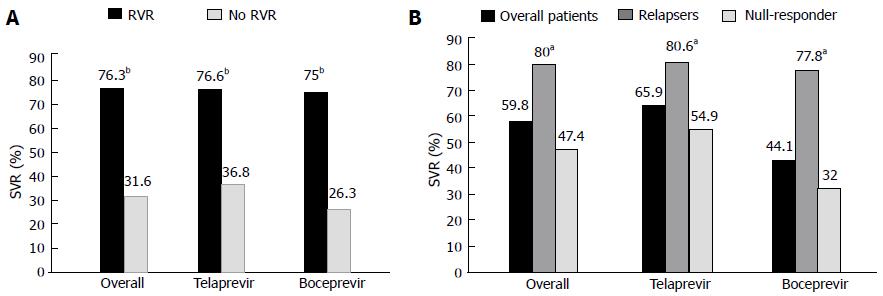
Patients who exhibited a RVR (Figure 1A), defined as a negative viral load 4 wk after the initiation of PI therapy, had a better SVR than the patients who did not have this rapid drop in the HCV RNA load (76.3% vs 36.4%, respectively, P < 0.0001). In the overall population (Figure 1B), the relapsers to prior PR therapy had a better SVR than the null-responders (80% vs 47.4%, P = 0.0004). This higher rate of SVR observed in the prior PR relapsers compared with the null-responders remained significant in the TPV or BOC subgroups (80.6% vs 54.9%, 77.8% vs 32%, respectively, P < 0.05).
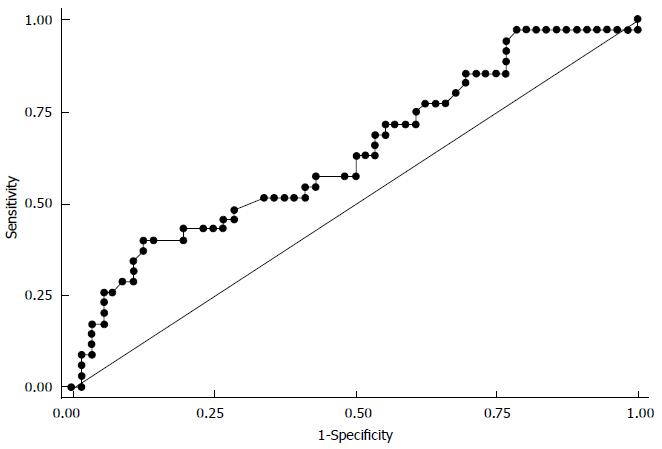
Overall, in the triple therapy population (Table 2), the liver stiffness (LS) values were significantly lower (P = 0.0296) in the patients with an SVR [median: 13.9 kPa, IQR (9.4-19.7)] than in the patients who failed to have an SVR [median: 17.3 kPa, IQR (11.5-28.8)]. The corresponding area under the ROC curve (Figure 2) predicting SVR was 0.64 (0.52-0.76). The optimal LS cut-off value associated with an SVR was 21.3 kPa, which had a predictive positive value of 66.7% (43, 85) and a negative predictive value of 70% (57, 80). An SVR occurred in 70% of the patients with a LS below 21.3 kPa and in 33.3% of the patients with a LS of up to 21.3 kPa (P = 0.002).
The logistic regression analysis (Table 2) showed that only three factors were independently associated with SVR. These factors were a relapse after PR treatment, the LS value, and a RVR to triple therapy. The SVR rate was greater in the prior relapsers than in the prior null-responders to PR therapy [OR = 3.9 (1.4, 10.6), P = 0.004]. A LS of less than 21.3 kPa was associated with an improved response to triple therapy [OR = 8.2 (2.3, 29.5), P = 0.001]. An SVR was associated with a RVR under triple therapy [OR = 6.9 (2.48, 18.2), P = 0.001], defined as HCV RNA-negative after 4 wk of antiviral treatment (week 4 in the TPV group and week 8 in the BOC group). The multivariate analysis revealed no difference in the SVRs of the TPV and BOC groups.
Adverse events ≥ grade 1 occurred in 102/124 patients (82.2%) and were significantly more frequent in the patients receiving TPV (n = 79/89, 88.8%) compared with the patients receiving BOC (n = 23/35, 65.7%) [OR = 4.12 (1.4; 12), P = 0.0059]. Approximately half of the patients (63: 50.8%) suffered a SAE ≥ grade 3 during treatment (Table 3). These grade 3 or 4 SAEs were as follows: thrombocytopenia (n = 42, 66%), neutropenia (n = 21, 33%), anemia (n = 18, 28.5%), severe infection (n = 4, 6.3%), fatigue (n = 3, 4.7%), skin rash (n = 2, 3.2%), and hepatic failure (n = 2, 3.2 %). The total percentage exceeds 100% because some subjects had several grade 3 or 4 SAEs. None of the patients died during treatment. Neither the fibrosis stage (F3 or F4) nor the protease inhibitor used (TPV or BOC) influenced the occurrence of SAEs. EPO use and blood transfusions were analyzed among the 125 patients. A total of 17 patients (13.6%) were given blood transfusions, and 65 patients (52%) received EPO. The frequencies of EPO use and blood transfusions in the TPV and BOC groups were not significantly different. Treatment was discontinued because of SAEs in 11 patients (8.9%).
| Overall patients(n = 124) | Telaprevir(n = 89) | Boceprevir(n = 35) | P valueunivariateanalysis | |
| Premature discontinuation due to SAE | 11 (8) | 10 (11.2) | 1 (2.9) | 0.178 |
| Death | 0 | 0 | 0 | - |
| Severe adverse events grade 3/4 | 63 (50.8) | 46 (51.7) | 17 (48.6) | 0.75 |
| Infection | 4 (3.2) | 3 (3.4) | 1 (2.9) | 1 |
| Liver decompensation | 2 (1.6) | 2 (2.2) | 0 | 1 |
| Fatigue | 3 (2.4) | 3 (3.4) | 0 | 0.558 |
| Skin rash | 2 (1.6) | 2 (2.2) | 0 | 1 |
| Kidney failure | 1 (0.8) | 1 (1.1) | 0 | 1 |
| Digestive adverse events | 1 (0.8) | 1 (1.1) | 0 | 1 |
| Thromboembolic events | 1 (0.8) | 1 (1.1) | 0 | 1 |
| Anemia | 18 (14.5) | 16 (18) | 2 (5.7) | 0.081 |
| Neutropenia | 21 (16.8) | 14 (15.7) | 7 (20) | 0.568 |
| Thrombocytopenia | 42 (33.6) | 32 (36) | 10 (28.6) | 0.434 |
| Erythropoietin use1 | 65 (52) | 45 (90) | 20 (57.1) | 0.473 |
| Blood transfusion1 | 17 (13.6) | 15 (16.7) | 2 (5.7) | 0.149 |
The univariate analysis (Table 4) showed four factors associated with an SAE during triple therapy. Women had SAEs more frequently (62.8%) than men (44.4%, P = 0.05). The platelet counts (mean ± SD) were lower in the patients who had a SAE (143.5 ± 65.4 × 103/mm3) than in the patients with no SAE 191.1 ± 54.9 × 103/mm3, P≤ 0.0001). SAEs were more frequent in patients with low levels of serum albumin (median: 39.4 ± 4.9 and 42 ± 4.9 g/L, P = 0.02) or with a high bilirubin concentration [median: 13.1, IQR (9.1-19.1) and 10.8 (8-13.5) M/L, P = 0.036]. The two factors that remained independently associated with SAE occurrence (Table 4) were being female [OR = 2.4 (1.1, 5.6), P = 0.037] and a platelet count lower than 150 × 103/mm3 [OR 5.3 (2.3, 12.4), P≤ 0.001]. A greater number of the patients with platelet counts lower than 150 × 103/mm3 (75.6%) experienced an SAE than did those with platelet counts higher than this cutoff (37.5%; P = 0.0001).
| Severeadverse eventsn = 63 | No severeadverse eventsn = 61 | P valueunivariate analysis | P valuemultivariate AnalysisOR (95%CI) | |
| Protease inhibitor | ||||
| Telaprevir | 47 (51.7) | 43 (48.3) | 0.7548 | - |
| Boceprevir | 17 (48.6) | 18 (51.4) | ||
| Genre | 0.037 | |||
| Male | 36 (44.4) | 45 (55.6) | 1.0 | |
| Female | 27 (62.8) | 16 (37.2) | 0.0518 | 2.4 (1.1-5.6) |
| Liver fibrosis | ||||
| Metavir F3 | 15 (42.9) | 20 (57.1) | 0.2667 | - |
| MetavirF4 | 48 (53.9) | 41 (46.1) | ||
| Platelets | ||||
| Mean × 10³/mm³ (SD) | 143.5 (65.43) | 191.1 (54.9) | ≤ 0.0001 | ≤ 0.001 |
| < 150 × 10³/mm³ | 34 (75.6) | 11 (24.4) | 0.0001 | 1.0 |
| ≥ 150 × 10³/mm³ | 27 (37.5) | 45 (62.5) | 5.3 (2.3-12.4) | |
| Albumin, mean, g/L, (SD) | 39.4 (4.9) | 42 (54.9) | 0.0196 | - |
| Bilirubin, median μM/L, (IQR) | 13.1 (9.1-19.1) | 10.8 (8-13.5) | 0.0359 | - |
PI therapy has rarely been used to treat patients with severe liver fibrosis who failed to respond to prior treatment with PR. The few Metavir F3 or F4 patients treated were selected from within larger studies and do not always reflect the population seen in routine clinical practice[5-10]. The main objective of this study was to assess the effectiveness of triple therapy for difficult-to-treat patients with severe liver fibrosis who were null-responders or relapsers to prior PR treatment. It is necessary to understand how patients tolerate these treatments to identify the patients most at risk of suffering severe adverse side effects.
The overall SVR rate of 59.8% at 24 wk post-treatment was satisfactory in this difficult-to-treat population. This high rate shows that PI could be used successfully in routine clinical practice, including for patients with severe liver fibrosis, with an efficacy equivalent to results obtained in controlled trials. The SVR rate for patients in the RESPOND-2 trial[10] with severe liver fibrosis (Metavir F3 or F4) who failed to respond to previous treatment and were retreated with BOC was 55.5%. This study, however, was carried out on a limited number of patients. The SVR rate for our patients treated with BOC was 44.1%. The lower response rate for our BOC-treated patients might be due to the type of response of these patients to the prior treatment. The majority of patients in the RESPOND-2 study[10] were relapsers (64%), whereas the majority (73.5%) of our patients were null-responders. The SVR rate for the patients in the REALIZE trial[8] who had severe liver fibrosis (Metavir F3 and F4) and who were given TPV triple therapy was 56%. The SVR for our patients given TPV triple therapy was 65.9%, which was better than that of the REALIZE patients. However, the SVR rate for those REALIZE patients[8] classified as Metavir F3 was similar (66.4%) to the rate for our patients.
We identified elements predicting an SVR. One of the main predictive factors of the virological response to triple therapy was the type of response to the previous PR treatment. Relapsers on the previous treatment had very high SVR rates (80%), whereas the SVR of null-responders was only 47.4%. In the overall population, the type of response to a previous treatment was independently associated with the SVR to triple therapy. This influence of the type of virological response to PR treatment on the SVR to triple therapy was also described in phase III studies for patients treated with both TPV and BOC[8-10]. We observed an unexpectedly high SVR (54.9%) with TPV triple therapy in our previous null-responders (28 of 51 patients). This rate is approximately twofold higher than the SVR rate observed in the REALIZE study in the same population of null-responders with severe liver fibrosis[8]. A more detailed comparison of the characteristics of cirrhotic patients in phase III studies and our patients in the current study should provide a better understanding of this difference in the SVR rate. We confirmed that the patients who relapse after a previous double-therapy benefit more from PI treatment than null-responders. However, we also determined that an encouraging SVR could be obtained for greater than one-third of previous null-responder cirrhotic patients; therefore, triple therapy with TPV or BOC should be offered to these patients, especially to the patients who could not be included in new drug trials[13]. The rationale for treating null-responders to PR therapy with severe liver fibrosis is that the second generation of direct-acting antiviral drugs (DAAs) would be used in the near future to obtain a higher SVR[14].
We have shown an overall difference in the SVR of patients treated with TPV (65.9%) and BOC (44.1%). This difference was significant in the univariate analysis; however, it does not appear to be an independent value in the multivariate analysis. There were more null-responders to PR treatment in our BOC subgroup (73.5%) than in the TPV subgroup (58.6%). An analysis of the response to triple therapy (in terms of the previous response) indicated that the SVRs of relapsers in the TPV (80.6%) and BOC (77.8%) subgroups were similar. BOC tended to be less effective for the null-responders to prior PR therapy, although this difference was not significant after the multivariate analysis. Our study was not a randomized study; each clinician could choose to use TPV or BOC, although all of the patients were given the same treatment with the same doses of peg-IFN α2a + ribavirin, unlike those in the CUPIC trial[15], which used peg-IFN α2a or α b. More of our patients were treated with TPV than BOC because TPV was approved for use in France in January 2011, and BOC was approved at a later date. The possible difference between the SVR of the TPV and BOC groups should be confirmed in a randomized trial that includes more patients. However, taking into account the development of new DAAs, such a randomized study is unlikely to be completed. Two meta-analyses[16,17] compared the efficacy of TPV and BOC. Both studies determined that the SVR for TPV was superior. These meta-analyses included only a few trials on heterogeneous populations. The results could not be extrapolated to routine clinical practice, although they are compatible with our findings. In the overall population, a RVR was observed in 66.7% of individuals. A RVR was observed for 76.3% of the patients treated with TPV and for 31.6% of the patients treated with BOC. A Spanish study found that the RVR was significantly higher in the TPV patients, suggesting that the drug acted more rapidly[18]. Close monitoring of the viral load, especially at the start of treatment, could lead to the selection of a sub-group of patients very likely to have an SVR[19-21]. This information could strongly motivate these difficult-to-treat patients on prolonged treatment for 48 wk to adhere more closely to the treatment protocol.
We found no link between the degree of liver fibrosis, Metavir F3 or Metavir F4, and the SVR. However, we demonstrated a statistically significant link between the SVR and the LS measured by Fibroscan® at inclusion. The SVR rate was significantly higher in the patients with an LS under 21.3 kPa. This LS value of 21.3 kPa is well above the value of 12.5 kPa that is typically used to diagnose cirrhosis[22], suggesting that the population of patients with Child-Pugh class A cirrhosis is heterogeneous. The prognosis for the patients with a high LS value is likely different from the prognosis for the patients with lower LS values. A previous study[23] of patients treated with PR showed that the liver LS values were significantly lower in patients having an SVR than in non-responders. The LS value could be used to identify portal hypertension[24-27]. Various studies have given thresholds from 13.6 kPa to 48 kPa for portal hypertension. One study on patients with various liver diseases observed that a threshold of 21 kPa is useful for predicting significant portal hypertension[25]. Other studies specifically included patients with hepatitis C for diagnosing non-invasive portal hypertension. A LS threshold of 19.8 kPa[26] in one study and of 21.5 kPa in two others[22,27] enabled to accurately predict the presence of esophageal varices in this population. These threshold values assessed in studies on heterogeneous populations are similar to the thresholds that we identified as predictive of an SVR. Our finding of a link between the LS value and the SVR suggests that there is a subgroup of patients with severe liver fibrosis who have high LS values and a diminished response to triple therapy with PI. The threshold identified in this study should be confirmed by studies on larger populations of patients.
One of our secondary objectives was to assess how well PI treatment was tolerated by difficult-to-treat patients in the context of routine clinical practice. Because the great majority of patients suffered from at least one side effect, we focused on the development of severe side effects and the factors predicting their development. We determined that 50.8% of patients treated with PI developed these severe adverse reactions, causing 8.9% of the patients to abandon their treatment early. The preliminary results of the CUPIC trial[15] demonstrated that 40% of patients suffered an SAE after 16 wk, and 14.7% of these patients abandoned treatment prematurely. Published studies indicate that 57% of patients on TPV[8,9] suffered an SAE, as did 40.4% of those on BOC[28]. In phase III trials[28], SAEs were observed in only 16% of patients with severe liver fibrosis, which was a much lower percentage than in our findings. This type of difference between initial studies and routine clinical practice is not uncommon; 24% of our patients had at least one criterion that would have excluded them from a phase III trial. Our patients were also older than the patients included in phase III trials and were likely more fragile, making them more susceptible to an SAE. None of our patients died during follow-up, unlike the patients in the CUPIC trial.
We had to administer EPO to 52% of the patients in this study and had to reduce the ribavirin dose for 38.7% of the patients. In addition, 13.6% of the patients required a blood transfusion. Reducing the ribavirin dose had no effect on the SVR for our patients. Anemia was treated in the SPRINT-2 trial[7], similarly to our study, with a reduction in the ribavirin dose for only 8% of patients; EPO was used for 38% of patients, and a combination of the reducing the ribavirin dose and EPO was used for 44% of patients. TPV appeared to cause anemia more frequently than BOC among our patients; however, this difference was not significant. TPV treatment was not an independent factor suggesting the development of SAEs in our study. We have confirmed that patients with severe liver fibrosis, whether Metavir F3 or F4, do not readily tolerate triple therapy with PI and suffer from a high incidence of SAEs. We determined that two factors were independently associated with the development of an SAE. One factor was the platelet count at inclusion, which was significantly lower in the patients who developed an SAE. The threshold for development of an SAE was 150000 platelets/mm³. Data from the CUPIC cohort[15] indicate that a threshold of 100000/mm³ was predictive of death and severe complications. Some of our patients had Metavir F3 or F4 scores, whereas the CUPIC cohort[15] included only patients with cirrhosis; this difference could account for the lower SVR rate in the CUPIC study. Female gender is the second factor independently associated with the development of an SAE. The PI dose should not be scaled to the patient’s body weight; however, further studies on men and women are needed to define any differences in body mass index in men and women to assess whether this factor influences tolerance.
In conclusion, we studied a large cohort of patients with genotype 1 HCV infection and severe liver fibrosis (Metavir F3 or F4) who had failed to respond to an earlier PR treatment. Our data obtained in routine clinical practice confirm the satisfactory efficacy of PI triple therapy. We have also demonstrated that a threshold value of 21.3 kPa of LS is associated with an SVR. However, triple therapy with PI is rather poorly tolerated. We should use better methods to select patients for PI treatment and should be able to offer the patients at the greatest risk of treatment failure (as well as those with an intolerance for other therapies) a second generation of new DAAs.
We would like to thanks for Julie Laurent for technical assistance in statistical analysis and Dr. Owen Parks for English corrections.
The addition of telaprevir (TPV) or boceprevir (BOC) in the treatment of genotype 1 hepatitis C virus (HCV) patients has significantly increased the sustained virologic response rate of pegylated-interferon (peg-INF) α2a + ribavirin (PR). Data on these new therapeutic options are limited in the setting of very difficult-to-treat patients, although these patients are in the highest priority for achieving viral clearance.
The research goal is to assess the efficacy and safety of telaprevir- and boceprevir-based triple therapies in a multicentric cohort of previously treated HCV-genotype 1 patients with severe liver fibrosis.
In the clinical setting, the efficacy of TPV and BOC-based triple therapies in previously treated HCV-genotype 1 patients with severe liver fibrosis is similar or even better than the results obtained in controlled trials [overall sustained virological response (SVR) 59.8%]. The SVR is inversely correlated to liver stiffness, as assessed by elastography with a cut-off of 21.3 kPa, which is predictive of a poor response rate. These treatments are poorly tolerated, and half of all patients experience at least one grade 3-4 adverse event.
This study suggests that telaprevir and boceprevir-based triple therapies could be used in clinical practice in the subset of very difficult-to-treat patients; these triple therapies resulted in a viral clearance rate similar to or even better than the rates obtained in controlled trials. An SVR was achieved even in cirrhosis patients. However, patients with the most advanced stage of fibrosis should be considered for other treatments because these treatments are significantly less efficient when the liver stiffness is higher than 21.3 kPa and are significantly less tolerated in the presence of biological markers of advanced liver fibrosis (platelet count < 150 × 10³/mm³).
SVR: undetectable levels of viral RNA at 24 wk following treatment completion; Rapid virological response (RVR): undetectable levels of viral RNA at week 4 or week 8 after initiation of telaprevir- or boceprevir-based triple therapies, respectively.
This manuscript by Bonnet et al described the efficacy and safety of Telaprevir or Boceprevir therapy for treatment experienced patients with advanced fibrosis. The majority of patients were cirrhosis (F4 72%) and null-responder to prior therapy (63%), thus reflecting most difficult-to-treat patients. The results are encouraging showing high SVR rate in prior relapsers (80%) and even in null-responders (47%) with low rate of premature discontinuation due to SAE (8%) and no death. This information in real-life setting may be of value for physicians treating hepatitis C.
P- Reviewer: Kurosaki M, Liu J S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Dienstag JL, Ghany MG, Morgan TR, Di Bisceglie AM, Bonkovsky HL, Kim HY, Seeff LB, Szabo G, Wright EC, Sterling RK. A prospective study of the rate of progression in compensated, histologically advanced chronic hepatitis C. Hepatology. 2011;54:396-405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 2. | Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975-982. [PubMed] [Cited in This Article: ] |
| 3. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [PubMed] [Cited in This Article: ] |
| 4. | Cammà C, Cabibbo G, Bronte F, Enea M, Licata A, Attanasio M, Andriulli A, Craxì A. Retreatment with pegylated interferon plus ribavirin of chronic hepatitis C non-responders to interferon plus ribavirin: a meta-analysis. J Hepatol. 2009;51:675-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Kwo PY, Lawitz EJ, McCone J, Schiff ER, Vierling JM, Pound D, Davis MN, Galati JS, Gordon SC, Ravendhran N. Efficacy of boceprevir, an NS3 protease inhibitor, in combination with peginterferon alfa-2b and ribavirin in treatment-naive patients with genotype 1 hepatitis C infection (SPRINT-1): an open-label, randomised, multicentre phase 2 trial. Lancet. 2010;376:705-716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 519] [Cited by in F6Publishing: 567] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 6. | Hézode C, Forestier N, Dusheiko G, Ferenci P, Pol S, Goeser T, Bronowicki JP, Bourlière M, Gharakhanian S, Bengtsson L. Telaprevir and peginterferon with or without ribavirin for chronic HCV infection. N Engl J Med. 2009;360:1839-1850. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 837] [Cited by in F6Publishing: 888] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 7. | Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, Jacobson IM, Reddy KR, Goodman ZD, Boparai N. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1195-1206. [PubMed] [Cited in This Article: ] |
| 8. | Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, Focaccia R, Younossi Z, Foster GR, Horban A. Telaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417-2428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1205] [Cited by in F6Publishing: 1194] [Article Influence: 91.8] [Reference Citation Analysis (0)] |
| 9. | Liu J, Jadhav PR, Amur S, Fleischer R, Hammerstrom T, Lewis L, Naeger L, O’Rear J, Pacanowski M, Robertson S. Response-guided telaprevir therapy in prior relapsers? The role of bridging data from treatment-naïve and experienced subjects. Hepatology. 2013;57:897-902. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Bacon BR, Gordon SC, Lawitz E, Marcellin P, Vierling JM, Zeuzem S, Poordad F, Goodman ZD, Sings HL, Boparai N. Boceprevir for previously treated chronic HCV genotype 1 infection. N Engl J Med. 2011;364:1207-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1287] [Cited by in F6Publishing: 1288] [Article Influence: 99.1] [Reference Citation Analysis (0)] |
| 11. | Wedemeyer H, Jensen DM, Godofsky E, Mani N, Pawlotsky JM, Miller V. Recommendations for standardized nomenclature and definitions of viral response in trials of hepatitis C virus investigational agents. Hepatology. 2012;56:2398-2403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Leroy V, Serfaty L, Bourlière M, Bronowicki JP, Delasalle P, Pariente A, Pol S, Zoulim F, Pageaux GP. Protease inhibitor-based triple therapy in chronic hepatitis C: guidelines by the French Association for the Study of the Liver. Liver Int. 2012;32:1477-1492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Liang TJ, Ghany MG. Current and future therapies for hepatitis C virus infection. N Engl J Med. 2013;368:1907-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 344] [Cited by in F6Publishing: 325] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 14. | Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, Schultz M, Davis MN, Kayali Z, Reddy KR. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878-1887. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1322] [Cited by in F6Publishing: 1287] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 15. | Hézode C, Fontaine H, Dorival C, Larrey D, Zoulim F, Canva V, de Ledinghen V, Poynard T, Samuel D, Bourlière M. Triple therapy in treatment-experienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) - NCT01514890. J Hepatol. 2013;59:434-441. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 370] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 16. | Sitole M, Silva M, Spooner L, Comee MK, Malloy M. Telaprevir versus boceprevir in chronic hepatitis C: a meta-analysis of data from phase II and III trials. Clin Ther. 2013;35:190-197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Cure S, Diels J, Gavart S, Bianic F, Jones E. Efficacy of telaprevir and boceprevir in treatment-naïve and treatment-experienced genotype 1 chronic hepatitis C patients: an indirect comparison using Bayesian network meta-analysis. Curr Med Res Opin. 2012;28:1841-1856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Benito JM, Sánchez-Parra C, Maida I, Aguilera A, Rallón NI, Rick F, Labarga P, Fernández-Montero JV, Barreiro P, Soriano V. Triple combination therapy for hepatitis C with telaprevir exhibits greater early antiviral activity than with boceprevir. Antivir Ther. 2013;18:709-715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 19. | Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, Fried MW, Adler M, Reesink HW, Martin M. Response-guided telaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 592] [Cited by in F6Publishing: 628] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 20. | Foster GR, Zeuzem S, Andreone P, Pol S, Lawitz EJ, Diago M, Roberts S, Pockros PJ, Younossi Z, Lonjon-Domanec I. Sustained virologic response rates with telaprevir by response after 4 weeks of lead-in therapy in patients with prior treatment failure. J Hepatol. 2013;58:488-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Poordad F, Bronowicki JP, Gordon SC, Zeuzem S, Jacobson IM, Sulkowski MS, Poynard T, Morgan TR, Molony C, Pedicone LD. Factors that predict response of patients with hepatitis C virus infection to boceprevir. Gastroenterology. 2012;143:608-618.e1-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 157] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 22. | Castéra L, Le Bail B, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, Couzigou P, de Lédinghen V. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol. 2009;50:59-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 272] [Cited by in F6Publishing: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 23. | Patel K, Friedrich-Rust M, Lurie Y, Grigorescu M, Stanciu C, Lee CM, Schiff ER, Häussinger D, Manns MP, Gerken G. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol. 2011;17:4581-4589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 16] [Cited by in F6Publishing: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Forestier N, Gaus A, Herrmann E, Sarrazin C, Bojunga J, Poynard T, Albert J, Gerber L, Schneider MD, Dultz G. Acoustic radiation force impulse imaging for evaluation of antiviral treatment response in chronic hepatitis C. J Gastrointestin Liver Dis. 2012;21:367-373. [PubMed] [Cited in This Article: ] |
| 25. | Bureau C, Metivier S, Peron JM, Selves J, Robic MA, Gourraud PA, Rouquet O, Dupuis E, Alric L, Vinel JP. Transient elastography accurately predicts presence of significant portal hypertension in patients with chronic liver disease. Aliment Pharmacol Ther. 2008;27:1261-1268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 260] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 26. | Pritchett S, Cardenas A, Manning D, Curry M, Afdhal NH. The optimal cut-off for predicting large oesophageal varices using transient elastography is disease specific. J Viral Hepat. 2011;18:e75-e80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Vizzutti F, Arena U, Romanelli RG, Rega L, Foschi M, Colagrande S, Petrarca A, Moscarella S, Belli G, Zignego AL. Liver stiffness measurement predicts severe portal hypertension in patients with HCV-related cirrhosis. Hepatology. 2007;45:1290-1297. [PubMed] [Cited in This Article: ] |
| 28. | Bruno S, Vierling JM, Esteban R, Nyberg LM, Tanno H, Goodman Z, Poordad F, Bacon B, Gottesdiener K, Pedicone LD. Efficacy and safety of boceprevir plus peginterferon-ribavirin in patients with HCV G1 infection and advanced fibrosis/cirrhosis. J Hepatol. 2013;58:479-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |