Published online Mar 27, 2014. doi: 10.4254/wjh.v6.i3.144
Revised: January 27, 2014
Accepted: February 20, 2014
Published online: March 27, 2014
The use of medicinal plants in treating illnesses has been reported since ancestral times. In the case of hepatic diseases, several species such as Silybum marianum, Phyllanthus niruri, and Panus giganteus (Berk.) have been shown to ameliorate hepatic lesions. Silymarin is a natural compound derived from the species Silybum marianum, which is commonly known as Milk thistle. This plant contains at least seven flavoligands and the flavonoid taxifolin. The hepatoprotective and antioxidant activity of silymarin is caused by its ability to inhibit the free radicals that are produced from the metabolism of toxic substances such as ethanol, acetaminophen, and carbon tetrachloride. The generation of free radicals is known to damage cellular membranes and cause lipoperoxidation. Silymarin enhances hepatic glutathione and may contribute to the antioxidant defense of the liver. It has also been shown that silymarin increases protein synthesis in hepatocytes by stimulating RNA polymerase I activity. A previous study on humans reported that silymarin treatment caused a slight increase in the survival of patients with cirrhotic alcoholism compared with untreated controls.
Core tip: One of the mechanisms of liver damage caused by alcohol is the generation of free radicals formed by the metabolism of this xenobiotic. Silymarin is an antioxidant that protects the liver from the free radical damage produced by alcohol metabolism. Silymarin is the most used natural compound for the treatment of hepatic diseases worldwide due to its antioxidant, anti-inflammatory, and anti-fibrotic activities. Silymarin functions by stabilizing biological membranes and increasing protein synthesis.
- Citation: Vargas-Mendoza N, Madrigal-Santillán E, Morales-González &, Esquivel-Soto J, Esquivel-Chirino C, González-Rubio MGLY, Gayosso-de-Lucio JA, Morales-González JA. Hepatoprotective effect of silymarin. World J Hepatol 2014; 6(3): 144-149
- URL: https://www.wjgnet.com/1948-5182/full/v6/i3/144.htm
- DOI: https://dx.doi.org/10.4254/wjh.v6.i3.144
The liver is an important organ that has a key role in the maintenance of homeostasis. The liver is responsible for multiple metabolic functions and physiological processes such as bile production, energy generation, vitamin storage, and the metabolism of carbohydrates, proteins, and lipids. After intestinal absorption is complete the blood is rich in nutrients and xenobiotics. The blood is then transported to the liver via the portal vein, which carries multiple toxic substances including ethanol (Et-OH), drugs, pharmaceuticals, and toxins to the liver. As a result, the liver is susceptible to toxicity and damage. Many people have been afflicted with some type of liver lesion. Examples of liver lesions include fatty liver, non-alcoholic steatosis, hepatitis A, B, or C, cirrhosis, and hepatocellular carcinoma (the third leading cause of cancer-related mortality worldwide)[1]. Hepatic diseases are primary causes of morbidity and mortality worldwide. The most recent surveillance report published by the National Institute on Alcohol Abuse and Alcoholism showed that liver cirrhosis was the 12th leading cause of death in the United States[2]. Liver disease is exacerbated by unhealthy lifestyles, obesity, and the excessive consumption of alcohol and drugs[3].
The use of medicinal plants has been reported since ancestral times. In the case of hepatic diseases, several species such as Silybum marianum[4], Phyllanthus niruri[5], and Panus giganteus (Berk.) have been shown to ameliorate hepatic lesions[6].
Flavonoids are polyphenol compounds that are also considered essential nutrients. Their basic chemical structure consists of two benzene rings bound by a three-atom heterocyclic carbon chain. The oxidation of the structure generates several families of flavonoids (flavones, flavonoles, flavanones, anthocyanins, flavanoles, and isoflavones). Chemical modifications of each family can lead to > 5000 individual compounds with different properties[7].
Silybum marianum is the scientific name for Milk thistle or St. Mary’s thistle. It is a plant native to the Mediterranean region and belongs to the Asteraceae family. It is characterized by thorny branches and a milky sap, with its oval leaves reaching up to 30 cm. The flowers are bright pink and can measure up to 8 cm in diameter[8]. Milk thistle grows in its wild form in southern Europe, northern Africa, and the Middle East. The plant is cultivated in Hungary, China, and South American countries such as Argentina, Venezuela, and Ecuador. In Mexico, Milk thistle is consumed as a supplementary food[9].
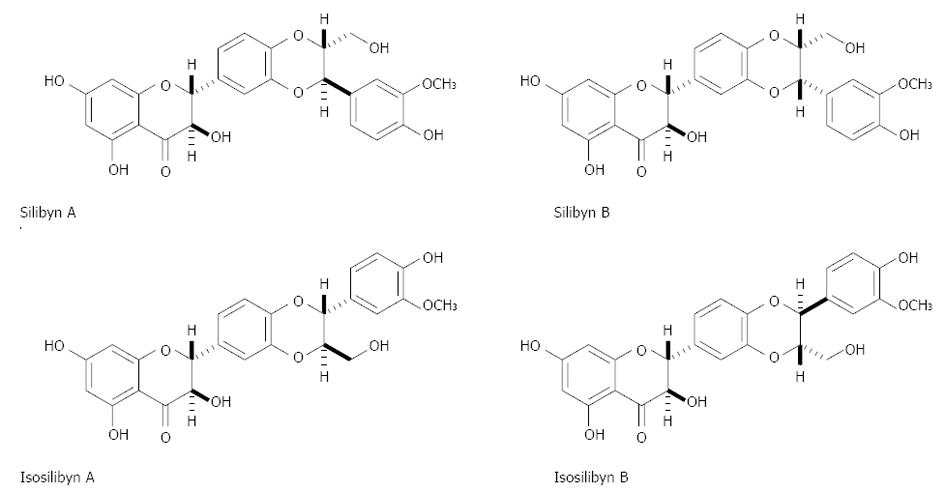
Silymarin is a natural compound that is present in species derived from Silybum marianum, which is commonly known as Milk thistle. The plant contains at least seven flavolignans and the flavonoid taxifolin. The most important flavolignans present include silybin, silydianin, and silychristine. Silybin represents between 50% and 70% of the extract from silymarin. The following flavolignan isoforms are known (Figure 1): silybin A, silybin B, isosilybin A, and isosilybin B[10]. Silymarin has been used worldwide for many years as a complementary alternative medicine because of the beneficial effects associated with the treatment of hepatic diseases. Silymarin belongs to the Aster family (Asteraceae or Compositae). The mature plant has large brilliant-purple flowers and abundant thorns. The plant grows in places with sufficient sun exposure[11].
The low level of bioavailable flavolignans is known. For example, the level of silymarin absorption is between 20% and 50%. Silybin is the major compound of silymarin and limiting factors such as low solubility in water, low bioavailability, and poor intestinal absorption reduce its efficacy. New soluble silybin-derived biocompounds (silybin bis-hemisuccinate, β-cyclodextrin complex, silybin-N-methyl-glucamine, silybin 11-O-phosphate, and silybin-phosphatidylcholine) have thus been designed[10]. Chronic inflammation occurs in patients with hepatic damage. Thus, for patients with compensatory cirrhosis, hepatitis C, and non-alcoholic hepatic steatosis, the bioavailability of compounds present in silymarin may be affected, which may also explain the low effectiveness of treatment with flavonoids in these patients[12,13].
Sy-Cordero et al[14] isolated four key flavolignans and diastereoisomers (silybin A, silybin B, isosilybin A, and isosilybin B) from S. marianum on a gram-scale. These compounds and two other related analogues are present in extremely minute quantities. The compounds were evaluated for their antiproliferative/cytotoxic activity against human prostate cancer cell lines. Silymarin reduces the incidence of certain cancers[15]. Su et al[16] used silymarin on nasopharyngeal carcinoma cells (NPC-TW01) and found an increase in Bcl-2 expression and a decrease in the activated caspase-3 or apoptosis-inducing factor (AIF) with low-dose (80 μmol/L) treatment.
The molecular targets of silymarin for cancer prevention have been studied. Milk thistle interferes with the expression of the cell cycle regulators and proteins involved in apoptosis. Thus, it can modulate the balance between cell survival and apoptosis. Lee et al[17] reported that silybin inhibited the kinase activity of mitogen-activated protein kinase (MEK)-1/2 and ribosomal S6 kinase (RSK)-2 in melanoma cells. The treatment of melanoma cells with silybin attenuated the phosphorylation of extracellular signal-regulated kinase (ERK)-1/2 and RSK2, which is regulated by the upstream kinases MEK1/2. The blockade of MEK1/2-ERK1/2-RSK2 signaling by silybin resulted in the reduced activation of nuclear factor-kappa B (NF-κB), activator protein-1, and STAT3. These proteins are transcriptional regulators of several proliferative genes in melanomas. Silybin blocks the activation of these transcription factors and induces cell-cycle arrest at the G1 phase, which inhibits melanoma cell growth in vitro and in vivo. Silymarin suppresses ultraviolet radiation A-induced oxidative stress (OS), which can induce skin damage. Thus, the topical application of silymarin can be a useful strategy for protecting against skin cancer[18].
In previous studies, the inherent hepatoprotective and antioxidant activity of silymarin was shown to be caused by its control of free radicals (FR), which are produced by the hepatic metabolism of toxic substances such as Et-OH, acetaminophen (Paracetamol), or carbon tetrachloride. The FR damage cellular membranes and cause lipoperoxidation (LPO)[19]. The cytoprotective effect in liver is also caused by the inhibition of the cyclooxygenase cycle, leukotrienes, and the production of FR in Kupffer cells in mice. These affects reduce inflammation[20], and it has been suggested that silymarin also performs the following functions: protecting against genomic injury, increasing hepatocyte protein synthesis, decreasing the activity of tumor promoters, stabilizing mast cells, chelating iron, and slowing calcium metabolism, among other activities that have been described in the literature[21].
Silymarin has been reported to have antioxidant, immunomodulatory, anti-fibrotic, anti-proliferative, and antiviral properties. It also affects the synthesis of RNA and DNA. Furthermore, silymarin maintains the integrity of the hepatocyte membrane and impedes the entrance of toxic substances or xenobiotics. Due to its phenolic nature, it is capable of donating electrons to stabilize FR and reactive oxygen species (ROS). Silymarin also affects intracellular glutathione, which prevents lipoperoxidation of membranes[22].
Pure compounds extracted from silymarin have been examined in cell lines infected with the hepatitis C virus (HCV). Polyak et al[23] showed that silymarin inhibits the replication of an infectious HCV genotype 2a strain (JFH1) in hepatoma cell cultures. The most effective compounds were isosilbin A, taxifolin, and silybinin, and these compounds reduced virus infection. The OS level induced by HCV, the tumor necrosis factor (TNF)-α level, and the transcription factor NF-κB were affected by silbyn A and silbyn B treatment. In general, all of the compounds showed antiviral activity and reduced the OS level caused by HCV infection[24].
The use of a silymarin extract in 72 patients with non-alcoholic hepatic steatosis (non-alcoholic fatty liver disease, NAFLD) on a controlled diet led to significantly reduced levels of alanine aminotransferase (ALT) and aspartame aminotransferase (AST) (AST/ALT < 1). Another parameter evaluated was γ-glutamyl transpeptidase (γ-GT). In NAFLD patients, γ-GT is high because of obesity, hyperinsulinemia, inflammation, and changes in the membrane permeability of the hepatocytes. The level of γ-GT decreased due to the silymarin-mediated inhibition of toxins entering the cells. Additionally, silymarin permits the stabilization of hepatocyte membranes. It also reduced the level of TNF-α, which reduces inflammation. A favorable change in the hepatorenal clearance index was also observed, which suggests a reduction in the accumulation of lipids in the liver. All of these results were visible after 6 mo of treatment[4].
Silymarin has both hepatoprotective and regenerative actions. The mechanism of action is a reduction of the FR formed by toxins that damage the cell membranes (LPO) and competitive inhibition through hepatocyte external cell membrane modification. Silymarin forms a complex that impedes the entrance of toxins into the interior of liver cells. Additionally, silymarin metabolically stimulates hepatic cells and activates the RNA iosynthesis of ribosomes to stimulate protein formation[25-27]. In a study published by Sandoval et al[28], the authors observed a silymarin protection effect in rat hepatic cells when they used it as a comparison factor to measure liver weight/animal weight % (hepatomegaly). The hepatomegaly was reduced compared to other groups that were administered antioxidant substances. There was no significant difference observed between the silymarin group and the silymarin-alcohol group. This result suggests liver protection by silymarin. Silymarin enhances hepatic glutathione generation by elevating cysteine availability and inducing cysteine synthesis while inhibiting its catabolism to taurine. The regulation of cysteine synthesis may subsequently contribute to the antioxidant defense[29]. Silymarin reduced collagen accumulation by 30% in biliary fibrosis induced in rats[30]. A study in humans reported a slight increase in the survival of patients with cirrhotic alcoholism compared with untreated controls[31]. Silymarin is perhaps the most frequently used natural compound for the treatment of hepatic diseases worldwide due to its antioxidant, anti-inflammatory, and anti-fibrotic activities[32].
Study conducted with guinea pigs (Cavia porcellus) examining hepatic fibrosis induced through the administration of Et-OH (4/kg of weight/d) for 90 d revealed a significant reduction of lesion markers such as ALT, AST, and γ-glutamyl after silymarin treatment. The gene expressions of cytochrome 450 2E1 (CYP2E1), TNF-α, transforming growth factor beta-1 (TGF-β1), and nuclear factor kappa-light-chain-enhancer of activated B cells-1 were also reduced. There was also a reduction in FR and reduced markers of fibrosis such as alpha smooth muscle actin, collagen α1(I), and in the caspase cytotoxicity marker. However, silymarin was less effective than vitamin C in this study. This result indicates that vitamin C is more effective in reducing the markers of damage and the production of ROS during Et-OH-induced lesions[33]. Another study evaluated the hepatoprotective effect by measuring the level of antioxidants and the effect of body weight (bw) in rats exposed to Et-OH (1.6 g/kg of bw for 4 wk). The results revealed that intoxication by Et-OH influences the bw of rats and the levels of thiobarbituric acid reactive substances (TBARS). The activity of the enzymes superoxide dismutase (SOD) and glutathione-S-transferase (GST) increased significantly. Conversely, glutathione (GSH), the activity of glutathione reductase (GR), glutathione peroxidase, and catalase (CAT) were reduced by exposure to Et-OH. The rats that received silybin and ascorbic acid had attenuated lesion markers, although the effect was greater in the group that received ascorbic acid than in the group treated with silybin. The study also concluded that stopping alcohol intake favors hepatic regeneration. Thus, it is more effective to take preventive measures than to implement curative treatment[34]. A mouse study examining the antioxidant, immunomodulatory activity and vascular function of mice showed a significant increase in OS levels in animals that received ethanol (1.6 g/kg per bw/d during 12 wk). Ethanol increased the production of TBARS, nitrite levels, and the activity of GST. Ethanol also significantly diminished the content of GSH and the activity of SOD, CAT, GPX, and GR. Mice that received Et-OH plus silymarin (250 mg/kg of bw/d for 12 wk) normalized the altered parameters. In addition, the silymarin-treated mice had reduced levels of interleukin-10 (IL-10), TNF-α, interferon (IFN), IFN-γ, vascular endothelial growth factor-A, and TGF-1β. The treatment also reduced the levels of IL-4 in the blood. The results of silymarin treatment were similar to mice that received vitamin C treatment[35].
The use of Sylubim β-cyclodextrin has been studied in non-insulin-dependent patients with diabetes and alcoholic hepatopathy. Treatment with a 135-mg/d dose did not influence insulin secretion but did significantly reduce the glucose (P < 0.03) and serum levels of triglycerides (P < 0.01) compared with the placebo. These results suggest that this treatment improves the response to insulin[36].
Clinical study conducted in 170 cirrhosis patients treated with 140 mg of silymarin three times daily for 41 months showed significant improvement, especially in the subgroups with alcoholic cirrhosis and initial “Child A” hepatic disease[31]. However, the results are controversial. A meta-analysis of 13 randomized clinical assays evaluated the beneficial or detrimental effects of Milk thistle and included patients with alcoholic and/or hepatitis B and C hepatic disease. The authors concluded that according to the data, Milk thistle did not significantly influence the improvement of these diseases. Conversely, it may have negatively affected the pathological condition[37].
There is substantial evidence suggesting that silymarin treatment improves hepatic diseases. However, some of the data are contradictory. Therefore, additional molecular studies investigating the mechanisms of action for these compounds are needed. It is known that silymarin does not possess adverse effects at high doses. Thus, it is a natural compound that is widely utilized in traditional medicine and has been investigated in formal scientific studies. Diverse hepatic damage models and ethanol injury have been utilized to study silymarin because ethanol is responsible for many cases of liver damage worldwide. The current data demonstrate that the use of silymarin treatment in alcoholic cirrhosis patients may attenuate the damage. However, silymarin treatment does not affect mortality.
P- Reviewers: Liu EQ, Pallottini V, Pajares MA, Yu DY S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Kim MN, Kim BK, Han KH. Hepatocellular carcinoma in patients with chronic hepatitis C virus infection in the Asia-Pacific region. J Gastroenterol. 2013;48:681-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1244] [Cited by in F6Publishing: 1342] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 3. | Ramírez-Farías C, Madrigal-Santillán E, Gutiérrez-Salinas J, Rodríguez-Sánchez N, Martínez-Cruz M, Valle-Jones I, Gramlich-Martínez I, Hernández-Ceruelos A, Morales-Gonzaléz JA. Protective effect of some vitamins against the toxic action of ethanol on liver regeneration induced by partial hepatectomy in rats. World J Gastroenterol. 2008;14:899-907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 24] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Cacciapuoti F, Scognamiglio A, Palumbo R, Forte R, Cacciapuoti F. Silymarin in non alcoholic fatty liver disease. World J Hepatol. 2013;5:109-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 5. | Pramyothin P, Ngamtin C, Poungshompoo S, Chaichantipyuth C. Hepatoprotective activity of Phyllanthus amarus Schum. et. Thonn. extract in ethanol treated rats: in vitro and in vivo studies. J Ethnopharmacol. 2007;114:169-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 51] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 6. | Wong WL, Abdulla MA, Chua KH, Kuppusamy UR, Tan YS, Sabaratnam V. Hepatoprotective Effects of Panus giganteus (Berk.) Corner against Thioacetamide- (TAA-) Induced Liver Injury in Rats. Evid Based Complement Alternat Med. 2012;2012:170303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Morales González JA. Oxidative stress and chronic degenerative diseases-a role for antioxidants. Rijeka: Croatia InTech 2013; 500. [Cited in This Article: ] |
| 8. | Madrigal-Santillán E, Madrigal-Bujaidar E, Cruz-Jaime S, Valadez-Vega MC, Sumaya-Martínez MT, Pérez-Ávila KG, Morales-González JA. The Chemoprevention of Chronic Degenerative Disease Through Dietary Antioxidants: Progress, Promise and Evidences. Oxidative stress and chronic degenerative diseases-a role for antioxidants. Rijeka: Croatia InTech 2013; 155-185. [Cited in This Article: ] |
| 9. | Morazzoni P, Bombardelli E. Silybum marianum (Cardusarianum). Fitoterapia. 1995;66:3-42. [Cited in This Article: ] |
| 10. | Loguercio C, Festi D. Silybin and the liver: from basic research to clinical practice. World J Gastroenterol. 2011;17:2288-2301. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 233] [Cited by in F6Publishing: 223] [Article Influence: 17.2] [Reference Citation Analysis (2)] |
| 11. | Morales-González JA, Gayosso-Islas E, Sánchez-Moreno C, Valadez-Vega C, Morales-González A, Esquivel-Soto J, Esquivel-Chirino C, García-Luna y González-Rubio M, Madrigal-Santillán E. Protective effect of silymarin on liver damage by xenobiotics. Rijeka: Croatia InTech 2013; . [Cited in This Article: ] |
| 12. | Hawke RL, Schrieber SJ, Soule TA, Wen Z, Smith PC, Reddy KR, Wahed AS, Belle SH, Afdhal NH, Navarro VJ. Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J Clin Pharmacol. 2010;50:434-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 97] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 13. | Schrieber SJ, Wen Z, Vourvahis M, Smith PC, Fried MW, Kashuba AD, Hawke RL. The pharmacokinetics of silymarin is altered in patients with hepatitis C virus and nonalcoholic Fatty liver disease and correlates with plasma caspase-3/7 activity. Drug Metab Dispos. 2008;36:1909-1916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Sy-Cordero A, Graf TN, Nakanishi Y, Wani MC, Agarwal R, Kroll DJ, Oberlies NH. Large-scale isolation of flavonolignans from Silybum marianum extract affords new minor constituents and preliminary structure-activity relationships. Planta Med. 2010;76:644-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Deep G, Oberlies NH, Kroll DJ, Agarwal R. Isosilybin B and isosilybin A inhibit growth, induce G1 arrest and cause apoptosis in human prostate cancer LNCaP and 22Rv1 cells. Carcinogenesis. 2007;28:1533-1542. [PubMed] [Cited in This Article: ] |
| 16. | Su CH, Chen LJ, Liao JF, Cheng JT. Dual effects of silymarin on nasopharyngeal carcinoma cells (NPC-TW01). Forsch Komplementmed. 2013;20:261-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Lee MH, Huang Z, Kim DJ, Kim SH, Kim MO, Lee SY, Xie H, Park SJ, Kim JY, Kundu JK. Direct targeting of MEK1/2 and RSK2 by silybin induces cell-cycle arrest and inhibits melanoma cell growth. Cancer Prev Res (Phila). 2013;6:455-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Svobodová A, Zdarilová A, Walterová D, Vostálová J. Flavonolignans from Silybum marianum moderate UVA-induced oxidative damage to HaCaT keratinocytes. J Dermatol Sci. 2007;48:213-224. [PubMed] [Cited in This Article: ] |
| 19. | Trouillas P, Marsal P, Svobodová A, Vostálová J, Gazák R, Hrbác J, Sedmera P, Kren V, Lazzaroni R, Duroux JL. Mechanism of the antioxidant action of silybin and 2,3-dehydrosilybin flavonolignans: a joint experimental and theoretical study. J Phys Chem A. 2008;112:1054-1063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 126] [Article Influence: 7.9] [Reference Citation Analysis (1)] |
| 20. | Dehmlow C, Erhard J, de Groot H. Inhibition of Kupffer cell functions as an explanation for the hepatoprotective properties of silibinin. Hepatology. 1996;23:749-754. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 44] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Flora K, Hahn M, Rosen H, Benner K. Milk thistle (Silybum marianum) for the therapy of liver disease. Am J Gastroenterol. 1998;93:139-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 512] [Cited by in F6Publishing: 443] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 22. | Karimi G, Vahabzadeh M, Lari P, Rashedinia M, Moshiri M. “Silymarin”, a promising pharmacological agent for treatment of diseases. Iran J Basic Med Sci. 2011;14:308-317. [PubMed] [Cited in This Article: ] |
| 23. | Polyak SJ, Morishima C, Shuhart MC, Wang CC, Liu Y, Lee DY. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology. 2007;132:1925-1936. [PubMed] [Cited in This Article: ] |
| 24. | Polyak SJ, Morishima C, Lohmann V, Pal S, Lee DY, Liu Y, Graf TN, Oberlies NH. Identification of hepatoprotective flavonolignans from silymarin. Proc Natl Acad Sci USA. 2010;107:5995-5999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 197] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 25. | Abou Zid S. Silymarin, Natural Flavonolignans from Milk Thistle. Phytochemicals-A Global Perspective of Their Role in Nutrition and Health. Rijeka: Croatia InTech 2012; 255-272. [Cited in This Article: ] |
| 26. | Pietrangelo A, Borella F, Casalgrandi G, Montosi G, Ceccarelli D, Gallesi D, Giovannini F, Gasparetto A, Masini A. Antioxidant activity of silybin in vivo during long-term iron overload in rats. Gastroenterology. 1995;109:1941-1949. [PubMed] [Cited in This Article: ] |
| 27. | Sonnenbichler J, Goldberg M, Hane L, Madubunyi I, Vogl S, Zetl I. Stimulatory effect of Silibinin on the DNA synthesis in partially hepatectomized rat livers: non-response in hepatoma and other malign cell lines. Biochem Pharmacol. 1986;35:538-541. [PubMed] [Cited in This Article: ] |
| 28. | Sandoval M, Lazarte K, Arnao I. Antioxidant liver protection of Vitis vinifera L. (grape) skin and seed. Available from: http://www.scielo.org.pe/scielo.php?pid=S1025-55832008000400006&script=sci_arttext. [Cited in This Article: ] |
| 29. | Kwon do Y, Jung YS, Kim SJ, Kim YS, Choi DW, Kim YC. Alterations in sulfur amino acid metabolism in mice treated with silymarin: a novel mechanism of its action involved in enhancement of the antioxidant defense in liver. Planta Med. 2013;79:997-1002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Boigk G, Stroedter L, Herbst H, Waldschmidt J, Riecken EO, Schuppan D. Silymarin retards collagen accumulation in early and advanced biliary fibrosis secondary to complete bile duct obliteration in rats. Hepatology. 1997;26:643-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 193] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Ferenci P, Dragosics B, Dittrich H, Frank H, Benda L, Lochs H, Meryn S, Base W, Schneider B. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105-113. [PubMed] [Cited in This Article: ] |
| 32. | Bergheim I, McClain CJ, Arteel GE. Treatment of alcoholic liver disease. Dig Dis. 2005;23:275-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 51] [Article Influence: 2.7] [Reference Citation Analysis (1)] |
| 33. | Abhilash PA, Harikrishnan R, Indira M. Ascorbic acid is superior to silymarin in the recovery of ethanol-induced inflammatory reactions in hepatocytes of guinea pigs. J Physiol Biochem. 2013;69:785-798. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Das SK, Vasudevan DM. Protective effects of silymarin, a milk thistle (Silybium marianum) derivative on ethanol-induced oxidative stress in liver. Indian J Biochem Biophys. 2006;43:306-311. [PubMed] [Cited in This Article: ] |
| 35. | Das SK, Mukherjee S. Biochemical and immunological basis of silymarin effect, a milk thistle (Silybum marianum) against ethanol-induced oxidative damage. Toxicol Mech Methods. 2012;22:409-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 36. | Lirussi F, Beccarello A, Zanette G, De Monte A, Donadon V, Velussi M, Crepaldi G. Silybin-beta-cyclodextrin in the treatment of patients with diabetes mellitus and alcoholic liver disease. Efficacy study of a new preparation of an anti-oxidant agent. Diabetes Nutr Metab. 2002;15:222-231. [PubMed] [Cited in This Article: ] |
| 37. | Rambaldi A, Jacobs BP, Iaquinto G, Gluud C. Milk thistle for alcoholic and/or hepatitis B or C virus liver diseases. Cochrane Database Syst Rev. 2005;CD003620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |