Published online Jul 27, 2013. doi: 10.4254/wjh.v5.i7.372
Revised: May 19, 2013
Accepted: June 1, 2013
Published online: July 27, 2013
AIM: To study the antineoplastic efficacy of 10% aspirin intralesional injection on VX2 hepatic tumors in a rabbit model.
METHODS: Thirty-two male rabbits (age: 6-9 wk; body weight: 1700-2500 g) were inoculated with VX2 hepatic tumor cells (104 cells/rabbit) via supra-umbilical median laparotomy. On day 4 post-implantation, when the tumors were about 1 cm in diameter, the rabbits were randomly divided into the following groups (n = 8 each group) to assess early (24 h) and late (7 d) antineoplastic effects of intratumoral injection of 10% bicarbonate aspirin solution (experimental groups) in comparison to intratumoral injection of physiological saline solution (control groups): group 1, 24 h control; group 2, 24 h experimental; group 3, 7 d control; group 4, 7 d experimental. The serum biochemistry profile (measurements of glycemia, alkaline phosphatase, gamma-glutamyl transferase, aspartate aminotransferase, and alanine aminotransferase) and body weight measurements were obtained for all animals at the following time points: D0, before tumor implant; D4, day of treatment; D5, day of sacrifice for groups 1 and 2; D11, day of sacrifice for groups 3 and 4. Gross assessments of the abdominal and thoracic cavities were carried out upon sacrifice. The resected liver tissues, including hepatic tumors, were qualitatively (general morphology, signs of necrosis) and quantitatively (tumor area) assessed by histopathological analysis.
RESULTS: Gross examination showed no alterations, besides the left hepatic lobe tumors, had occurred in the thoracic and abdominal cavities of any animal at any time point evaluated. However, the features of the tumor foci were distinctive between the groups. Compared to the control groups, which showed normal unabated tumor progression, the aspirin-treated groups showed imprecise but limited tumor boundaries and a general red-white coloration (indicating hemorrhaging) at 24 h post-treatment, and development of yellow-white areas of a cicatricial aspect at 7 d after treatment. At all time points evaluated, all except one biochemical parameters tested within the reference range (P > 0.05); a significant increase was detected in the alkaline phosphatase level of the control group 3 on D11 (P < 0.05). At 24 h post-treatment, the aspirin-treated groups showed extensive coagulation necrosis accompanied by a remarkable absence of viable tumor foci; at 7 d after treatment, the tumors had completely disappeared in these animals and fibrous necrotic nodules had developed. In contrast, throughout the study course, the tumors of the control groups remained unchanged, showing tumor nodules without necrosis at the time point corresponding to 24 h post-treatment and increased amounts of tumor nodules at the time point corresponding to 7 d post-treatment. Quantitative analysis of the remaining tumor area revealed that the aspirin-treated groups had significantly smaller tumor foci at 24 h post-treatment (8.5% ± 0.7%) and at 7 d after treatment (11.0% ± 4.2%), compared to those in the control groups (24 h: 98.5% ± 1.5% and 7 d: 94.0% ± 2.7%; both, P < 0.005).
CONCLUSION: Intralesional injection of a 10% aspirin solution causes destruction of VX2 hepatic tumors in rabbits without evidence of relapse at 7 d after treatment administration.
Core tip: This experimental study employed the well-established VX2 hepatic tumor rabbit model to assess the antineoplastic efficacy of intratumoral aspirin injection. Analysis of early (24 h post-treatment) and late (7 d post-treatment) effects indicated that the therapy caused early tumor destruction, as evidenced by significant necrotic areas in histopathological analysis, without late recurrence, as demonstrated by hepatic tissue regeneration and restoration of liver function biochemical parameters.
-
Citation: Saad-Hossne R, Teixeira FV, Denadai R.
In vivo assessment of intratumoral aspirin injection to treat hepatic tumors. World J Hepatol 2013; 5(7): 372-378 - URL: https://www.wjgnet.com/1948-5182/full/v5/i7/372.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i7.372
Cancer is the leading cause of death in economically developed nations and the second leading cause of death in developing nations[1]. Myriad advances in detection and treatment modalities have led to decreases in the mortality rates for the most common cancers in the United States and other western countries (i.e., lung, colorectal, female breast, and prostate); yet, many of these technologies have not yet reached the less developed and economically transitioning countries[2], where the rates of cancers are actually increasing. Thus, there remains a need for development of simple and effective therapeutic approaches; moreover, such novel therapies will be based upon the convenient and practical methodologies to determine a patient’s prognosis that are currently in practice in the poorer and less technologically advanced clinics, such as histological detection of lymphatic compromise, local recurrence, tumor staging, and presence of distant metastases[3].
In general, cases of distant metastases frequently involve the liver, and these patients account for approximately 40% of the population diagnosed with terminal cancer[4]. Furthermore, colorectal cancer (a leading public health concern worldwide) is associated with high risk of liver metastasis; it has been estimated that nearly 50% of colorectal cancer patients develop liver metastasis at some point during the course of their disease[5].
Although many therapies targeting liver metastases are available[6], surgical resection remains the treatment option with the highest cure rate[6-9]. However, the curative efficacy is influenced by several features related to the metastasis itself (i.e., number, location, and extent), the patient condition (i.e., comorbidities, fitness for surgery/anesthesia), and the healthcare setting (i.e., physician expertise, availability of technical and financial resources)[7,8]. As a consequence, curative surgery is not a feasible option for all patients; indeed, it has been estimated that up to 80% of patients with colorectal cancer liver metastases are not viable candidates for surgical removal[10].
Alternative non-surgical approaches are available for treating such patients[11,12]; the most common being physical ablative techniques (cryotherapy, radiotherapy, laser, and microwave) and chemotherapy[7-9], which have shown appreciable efficacy and safety profiles. However, clinical application of these approaches is still impacted by somewhat prohibitive cost and extent of involvement required of the patients (e.g., several return visits for serial chemotherapy administrations), as well as adverse side effects (e.g., emesis and anemia), some with life-threatening potential (e.g., immune system suppression and anaphylactic shock). Thus, the need for a low-cost, simple antineoplastic treatment with good efficacy and low side effect profile has yet to be fulfilled[13].
Over the past few years, our research group has evaluated the cytolytic and antineoplastic potentials of acetylsalicylic acid (aspirin) and its derivatives[14-16]. The collective results from our in vitro (cultured tumor cell systems) and in vivo (animal-implanted tumors) analyses suggest that injecting aspirin directly into liver tumors may destroy the lesion with minimal or no adverse effects, either locally or systemically. Therefore, the current experimental study was designed to evaluate the therapeutic efficacy and safety of intratumoral aspirin injection using the well-established VX2 tumor rabbit model of hepatic metastases.
The study was conducted with pre-approval by the Ethics Committee of Botucatu Medical School at São Paulo State University (UNESP), Brazil. All procedures involving animals were carried out in accordance with the standards of published in the Care and Use of Laboratory Animals by the Institute for Laboratory Animal Research (1996) and the ethical principles of the Brazilian College on Animal Experimentation (COBEA).
Thirty-two male New Zealand albino rabbits (6-9 wk-old, weighing 1700-2500 g) were housed under 12/12 h light-dark cycles with unrestricted access to standard rabbit chow (Coelhil R®- Socil: Belo Horizonte, MG, Brazil) and water. Six hours prior to the tumor inoculation, the animals were fasted.
The rabbits were administered general anesthesia by intravenous injection of 3% sodium pentobarbital (30 mg/kg body weight). VX2 tumor cell suspension containing 104 cells (Boston University, MA, United States) were injected slowly into the left hepatic lobe using a 27-gauge needle via supra-umbilical median laparotomy, as previously described[17]. The laparotomy incision was closed by suturing with non-dissolving stitches (Ethicon mononylon 4-0; Johnson and Johnson, São José dos Campos, SP, Brazil).
Four days after the VX2 inoculation, when the tumors had reached about 1 cm in diameter[17], the rabbits were randomly divided into experimental and control groups (n = 16 each) for a second laparotomy to receive intratumoral injection of 10% aspirin or physiological saline solution, respectively. The 10% aspirin solution (pH: 7.27) was generated by diluting 5000 mg of acetylsalicylic acid (Pharma Nostra, Brazil) in 50 mL of 10% sodium bicarbonate solution. Treatments were administered as 0.5 mL aliquots of the experimental or control solution, as this volume was sufficient to infiltrate the entire hepatic lesion.
The experimental and control groups were further sub-divided into equal groups (n = 8 each) for analysis of early (24 h post-treatment) and late (7 d post-treatment) effects[14-16]. Thus, the four study groups were: group 1, 24 h non-treated VX2 tumor control; group 2, 24 h aspirin-injected VX2 tumor experimental; group 3, 7 d non-treated VX2 tumor control; group 4, 7 d aspirin-injected VX2 tumor experimental. At each group’s end-of-treatment time, the animals were sacrificed by intravenous anesthesia overdose.
All animals underwent clinical evaluation to assess the disease evolution using objective parameters of post-surgical recuperation, such as resumption of feeding and activity. Effects on liver function were assessed by biochemical analysis of serum markers, including alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST) and alanine aminotransferase (ALT). In addition, changes in body weight and glycemic status were recorded. Assessments were made at the following time points: D0, before tumor implant; D4, day of treatment; D5, day of sacrifice for groups 1 and 2; D11, day of sacrifice for groups 3 and 4.
Immediately upon anesthesia overdose, a third laparotomy was performed for specimen collection (all lesions were removed) and gross evaluation of the abdominal and thoracic cavities. The specimens were sectioned and prepared for histopathological analysis by bright field optical microscopy with hematoxylin-eosin staining, which was conducted by an experienced pathologist who was blinded to the study. Qualitative analysis was performed by analyzing the morphological features of tumor specimens. Quantitative analysis was performed by measuring the percentage of total liver tissue that was represented by tumor cells using the Optimas® 6.1 imaging software.
The significance of between-group differences in tumor tissue area (in mm2) over time (in days) was assessed by the two-factor repeated measure ANOVA F test. Percentage data was analyzed using the non-parametric test for repeated measures. All statistical analyses were carried out by the SAS statistical software (version 9.2 for Windows; SAS Institute, United States). Statistical significance was indicated by 95%CI or P value of < 0.05.
Clinical evolution, weight, glycemia, and liver function: At 24 h post-treatment, all animals in groups 1 and 2 presented good clinical evolution without any deaths. All biochemical parameters were within the normal range, and the differences between the control and experimental treatment groups did not reach statistical significance (P > 0.05).
Gross features of tumors and proximal tissues: Unlike the thoracic cavity, the abdominal cavity appeared to be remarkably affected by the experimental treatment. The animals in group 1 showed well-defined, solid, yellowy-white hepatic lesions, measuring between 0.9 and 1.2 cm in diameter, occurring as singlets in all rabbits. The animals in group 2 also developed singlet solid lesions, measuring between 0.8 and 0.9 cm, but with the distinctive gross features of imprecise but limited borders, red-white coloration (indicating hemorrhaging), more extensive involvement of the hepatic tissue, and a cystic aspect.
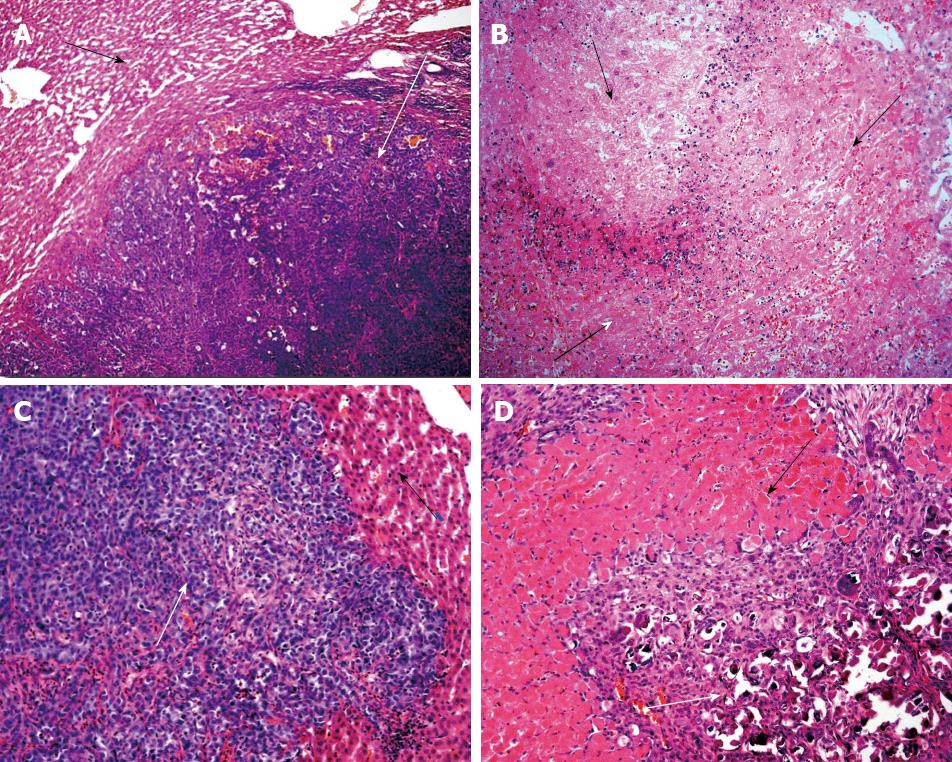
Histopathogical features of tumors: The livers from group 1 animals showed tumor nodules embedded throughout the normal hepatic tissue, and no necrotic areas (Figure 1A). The livers from group 2 animals also showed tumor nodules throughout the organ, but the hepatic parenchyma also showed extensive necrotic areas and hemorrhaging. In addition, intraparenchymal inflammatory infiltrates were observed, and there was a remarkable absence of viable tumor foci (Figure 1B).
The mean tumor area in livers from group 1 animals was significantly higher than that in the group 2 animals (98.5% vs 8.5%, P = 0.0036) (Table 1). This result clearly demonstrates the cytolytic effect of intratumoral aspirin injection.
| Analysis | Groups | P value | |
| Group 1 (control) | Group 2 (aspirin) | ||
| mean ± SD | 98.5% ± 1.512% | 8.5% ± 0.707% | 0.0036 |
| Median | 98.5% | 0% | |
| 25 percentile | 97.75% | 0% | |
| 75 percentile | 100% | 2% | |
Clinical evolution, weight, glycemia, and liver function: Similar to the results at 24 h post-treatment, all animals in groups 3 and 4 presented good clinical evolution without any deaths at 7 d post-treatment. In additional, all animals experienced weight gain. All of the biochemical parameters measured were also within normal range, with the notable exception of ALP (Table 2). In group 3, the ALP level was enhanced over time (D4: 129 vs D11 172, P < 0.05). This effect was not observed in group 4, indicating that the antineoplastic effects of intratumoral aspirin injection also helped to restore liver function.
Gross features of tumors and proximal tissues: Similar to the results at 24 h post-treatment, the thoracic cavity appeared to be unaffected but the abdominal cavity appeared to be remarkably affected by the experimental treatment. The animals in group 3 showed solid, yellowy-white, nodular tumoral lesions, measuring between 1.2 and 1.6 cm in diameter; however, unlike the results at the early time point, each animal had developed multiple small punctiform lesions around the nodular tumoral lesion. These multiple lesions were restricted to the left hepatic lobe, and no other lesions were observed in the right lobe or in the rest of the abdominal cavity. The animals in group 4 showed small, superficial, yellowy-white lesions with cicatricial characteristics, measuring between 0.2 and 0.4 cm.
Histopathogical features of tumors: Similar to the results at 24 h post-treatment, the livers of group 3 animals showed well-defined tumor nodules throughout the hepatic tissues, and no necrotic areas (Figure 1C). In stark contrast to both the livers of group 3 and those from group 2 (at the 24 h post-treatment time point), the livers of group 4 showed no tumor nodules; only a few isolated tumor cells associated with the presence of fibrous necrotic nodules and actively proliferating normal hepatic ducts and cells were observed (Figure 1D).
The mean tumor area in livers from group 3 animals was significantly lower than that in the group 4 animals (94.0% vs 11.0%, P = 0.0035) (Table 3). This result clearly demonstrates the maintenance of the cytolytic effect of intratumoral aspirin injection.
| Analysis | Groups | P value | |
| Group 3 (control) | Group 4 (aspirin) | ||
| mean ± SD | 94% ± 2.726% | 11% ± 4.243% | 0.0035 |
| Median | 94.5% | 0% | |
| 25 percentile | 91.75% | 0% | |
| 75 percentile | 96.25% | 2% | |
The VX2 hepatic tumor rabbit model is a sufficiently accurate tool for experimental investigations of newly developed anti-tumor treatments, and has been successfully applied to research of adriamycin[18,19], microwave ablation[20], angiogenesis inhibitor[21], oxaliplatin[22,23], and interventional radiology[24,25]. To the best of our knowledge, however, the study described herein represents the first usage of this rabbit model to study the antineoplastic effects of intratumoral 10% aspirin injection.
The intratumoral aspirin injection produced good clinical and weight evolution in all animals, without any deaths, suggesting not only good therapeutic efficacy but also a good safety profile. In particular, no toxic or detrimental effects (either local or systemic) were observed. The absence of early effects on glycemia or liver function markers indicates that neither the implanted tumor cells nor the intratumoral aspirin treatment elicited any major functional alterations (that would be otherwise detectable by biochemical tests). However, a late effect on ALP levels was observed in untreated rabbits with hepatic tumors, suggesting that the tumorigenesis may induce intrahepatic cholestasis and bile duct compression[26]. The fact that this effect was absent in the aspirin-treated rabbits provides further evidence of this therapy’s anti-tumor efficacy.
The lack of gross changes in the thoracic cavity (organs and serous membranes) of control animals suggests that the inoculated tumor cells did not undergo extensive or aggressive metastasis. In addition, the lack of gross changes (no signs of hemorrhaging or pulmonary condensation) in the thoracic cavity of experimental animals indicated that the intratumoral aspirin injection did not cause any damage to the proximal pulmonary tissues.
Obvious early differences in the gross features of livers with and without the aspirin treatment, including extensive coagulation necrosis in the treated hepatic parenchyma, minimal viable tumor foci, and quantifiable decrease in tumor cells, demonstrated rapid therapeutic efficacy. The low level of viable tumor cell foci present in the aspirin-treated livers may reflect usage of an insufficient injection volume or sub-optimal perfusion. Obvious late differences in the gross features of livers with and without the aspirin treatment indicated treatment-induced relief of tumoral lesions without evidence of recurrence. However, the aspirin-treated livers showed signs of fibrosis, suggesting that the remaining tumor tissue may differentiate to fibrotic scar tissue.
Recent studies with cultured human colorectal cancer cells have demonstrated the inhibitive activities of aspirin on proliferation and its inductive activities on apoptosis[27,28]. Still other in vitro studies have shown that aspirin can inhibit the growth of endometrial cancer cells[29], and induce apoptosis in human oral cancer cells[30] and in B cell chronic lymphocytic leukemia cells, via activation of caspases[31]. Moreover, aspirin pretreatment was found to augment TRAIL-induced apoptotic death in the human prostate adenocarcinoma line, LNCaP, and in the human colorectal carcinoma line, CX-1[32].
Regular intake of nonsteroidal anti-inflammatory drugs and cyclooxygenase (COX) inhibitors, such as aspirin, can reduce the risk of developing some cancers[33-35]. Considering that COX-2 overexpression is a frequent finding of many cancer specimens[36], we are intrigued by the idea that the direct application of aspirin to tumors may stimulate apoptosis and destroy the cancer cells through a mechanism involving inhibition of COX proteins.
Some limitations inherent to this study design may have impacted our results and must be considered with interpreting our findings. First, our study focused solely on one therapeutic agent, and no comparisons were made with similar substances, such as acetic acid. However, we previously demonstrated that aspirin has less toxicity than either aqueous phenol, acetic acid, or glycerine[14-16], and therefore we have focused our subsequent research on aspirin[37-39]. Second, we did not evaluate the pharmacological parameters of the aspirin treatment. Since acetylsalicylic acid is one of the best studied therapeutic substances[40-42], we chose to focus our current study on its antineoplastic benefit and safety as an intratumorally-delivered agent for liver cancer. Future experimental studies should not only be designed to overcome these limitations but also to include further long-term effects of this solution and delivery method prior to extending the analysis to humans in a clinical environment.
In conclusion, the rabbit VX2 hepatic tumor model was used to show that intratumoral injection of 10% aspirin can induce tumor destruction within 24 h after delivery, and that the antineoplastic effects were maintained out to 7 d post-treatment, with no signs of necrotic areas or tumor nodules but with signs of hepatic tissue regeneration and fibrosis foci.
Colorectal cancer remains a major public health concern, especially in developing countries. Moreover, it is estimated that approximately one-half of patients with colorectal cancer will develop liver metastases during the disease course, yet only 20% of these individuals are good candidates for curative liver resection. As such, there is an urgent need for new treatment modalities that are simple, cost-effective, and efficacious.
The current non-surgical treatment options for liver metastases have limited therapeutic efficacy, and are cost-prohibitive, inconvenient, and associated with detrimental side effects. Development of a new treatment modality that is technically simple, minimally-invasive, and able to be performed in a single (or minimal) application(s), such as intratumoral aspirin injection, will improve disease outcome among those patients who are not fit for curative resection.
Using the rabbit VX2 tumor model, intratumoral injection of aspirin was shown to safely, rapidly, and effectively induce tumor destruction followed by hepatic tissue regeneration and differentiation to fibrotic scar tissue.
These findings indicate the promise of a new therapeutic approach for managing unresectable liver metastases, which may be developed as a technically simple, low-cost, efficacious therapy for future clinical application.
This is an interesting in vivo analysis demonstrating the antineoplastic effects of 10% aspirin in hepatic tumors, which was based on the VX2 rabbit hepatic tumor model. The content is of scientific interest has potential clinical relevance, but further research is needed prior to its application in humans in the clinical setting.
P- Reviewers Cidon EU, Dragoteanu M, Vyslouzil K S- Editor Wen LL L- Editor A E- Editor Li JY
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23762] [Cited by in F6Publishing: 25182] [Article Influence: 1937.1] [Reference Citation Analysis (3)] |
| 2. | Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19:1893-1907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1754] [Cited by in F6Publishing: 1807] [Article Influence: 129.1] [Reference Citation Analysis (0)] |
| 3. | Gospodarowicz M, O’Sullivan B. Prognostic factors in cancer. Semin Surg Oncol. 2003;21:13-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 28] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Beckingham IJ, Krige JE. ABC of diseases of liver, pancreas, and biliary system. BMJ. 2001;322:477-480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Stangl R, Altendorf-Hofmann A, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343:1405-1410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 509] [Cited by in F6Publishing: 474] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | Kobayashi A, Miyagawa S. Advances in therapeutics for liver metastasis from colorectal cancer. World J Gastrointest Oncol. 2010;2:380-389. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Benoist S, Nordlinger B. The role of preoperative chemotherapy in patients with resectable colorectal liver metastases. Ann Surg Oncol. 2009;16:2385-2390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Tomlinson JS, Jarnagin WR, DeMatteo RP, Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH, D’Angelica M. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007;25:4575-4580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 845] [Cited by in F6Publishing: 854] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 9. | Bertolini F, Malavasi N, Scarabelli L, Fiocchi F, Bagni B, Del Giovane C, Colucci G, Gerunda GE, Depenni R, Zironi S. FOLFOX6 and bevacizumab in non-optimally resectable liver metastases from colorectal cancer. Br J Cancer. 2011;104:1079-1084. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 10. | Simmonds PC, Primrose JN, Colquitt JL, Garden OJ, Poston GJ, Rees M. Surgical resection of hepatic metastases from colorectal cancer: a systematic review of published studies. Br J Cancer. 2006;94:982-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 610] [Cited by in F6Publishing: 589] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 11. | Pathak S, Jones R, Tang JM, Parmar C, Fenwick S, Malik H, Poston G. Ablative therapies for colorectal liver metastases: a systematic review. Colorectal Dis. 2011;13:e252-e265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 12. | Tanaka K, Ichikawa Y, Endo I. Liver resection for advanced or aggressive colorectal cancer metastases in the era of effective chemotherapy: a review. Int J Clin Oncol. 2011;16:452-463. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Kadry Z, Clavien PA. New treatments with curative intent for metastatic colorectal liver cancer. Expert Opin Pharmacother. 2002;3:1191-1197. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 14. | Saad-Hossne R, Hossne WS, Prado RG. Efeito da solução aquosa de fenol, ácido acético e glicerina sobre o tumor ascítico de Erlich. Estudo experimental in vitro. Acta Cir Bras. 2004;19:54-58. [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Saad-Hossne R, Hossne WS, Prado RG. Ascite neoplásica. Efeito da solução aquosa de fenol, ácido acético e glicerina sobre o tumor ascítico de Erlich. Acta Cir Bras. 2003;18:534-536 [DOI : 10.1590/S0102-86502003000600007]. [Cited in This Article: ] |
| 16. | Saad-Hossne R, Prado RG, Hossne WS. Efeito da solução de ácido acetilsalicílico e de ácido acético em fígado de coelhos. Acta Cir Bras. 2004;19:677-686. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Hossne RS. Tumor hepático experimental (VX-2) em coelho: implantação do modelo no Brasil. Acta Cir Bras. 2002;17:208-210. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Ridge JA, Collin C, Bading JR, Hancock C, Conti PS, Daly JM, Raaf JH. Increased adriamycin levels in hepatic implants of rabbit Vx-2 carcinoma from regional infusion. Cancer Res. 1988;48:4584-4587. [PubMed] [Cited in This Article: ] |
| 19. | Swistel AJ, Bading JR, Raaf JH. Intraarterial versus intravenous adriamycin in the rabbit Vx-2 tumor system. Cancer. 1984;53:1397–1404. [Cited in This Article: ] |
| 20. | Kigure T, Harada T, Yuri Y, Satoh Y. Ultrasound-guided microwave thermotherapy on a VX-2 carcinoma implanted in rabbit kidney. Ultrasound Med Biol. 1995;21:649-655. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Kamei S, Okada H, Inoue Y, Yoshioka T, Ogawa Y, Toguchi H. Antitumor effects of angiogenesis inhibitor TNP-470 in rabbits bearing VX-2 carcinoma by arterial administration of microspheres and oil solution. J Pharmacol Exp Ther. 1993;264:469-474. [PubMed] [Cited in This Article: ] |
| 22. | Dzodic R, Gomez-Abuin G, Rougier P, Bonnay M, Ardouin P, Gouyette A, Rixe O, Ducreux M, Munck JN. Pharmacokinetic advantage of intra-arterial hepatic oxaliplatin administration: comparative results with cisplatin using a rabbit VX2 tumor model. Anticancer Drugs. 2004;15:647-650. [PubMed] [Cited in This Article: ] |
| 23. | She JJ, Wang ZM, Che XM, Pan CE. Research of beta-elemene interventional treatment on VX2 carcinoma transplanted on kidney in rabbits. Zhongxiyi Jiehe Xuebao. 2006;4:611-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Choi SH, Chung JW, Kim HC, Baek JH, Park CM, Jun S, Kim MU, Lee ES, Cho HR, Jae HJ. The role of perfusion CT as a follow-up modality after transcatheter arterial chemoembolization: an experimental study in a rabbit model. Invest Radiol. 2010;45:427-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Zhang J, Wang R, Lou H, Zou Y, Zhang M. Functional computed tomographic quantification of angiogenesis in rabbit VX2 soft-tissue tumor before and after interventional therapy. J Comput Assist Tomogr. 2008;32:697-705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Li X, Mortensen B, Rushfeldt C, Huseby NE. Serum gamma-glutamyltransferase and alkaline phosphatase during experimental liver metastases. Detection of tumour-specific isoforms and factors affecting their serum levels. Eur J Cancer. 1998;34:1935-1940. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Yu HG, Huang JA, Yang YN, Huang H, Luo HS, Yu JP, Meier JJ, Schrader H, Bastian A, Schmidt WE. The effects of acetylsalicylic acid on proliferation, apoptosis, and invasion of cyclooxygenase-2 negative colon cancer cells. Eur J Clin Invest. 2002;32:838-846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Yu HG, Huang JA, Yang YN, Luo HS, Yu JP, Meier JJ, Schrader H, Bastian A, Schmidt WE, Schmitz F. Inhibition of cytosolic phospholipase A2 mRNA expression: a novel mechanism for acetylsalicylic acid-mediated growth inhibition and apoptosis in colon cancer cells. Regul Pept. 2003;114:101-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Arango HA, Icely S, Roberts WS, Cavanagh D, Becker JL. Aspirin effects on endometrial cancer cell growth. Obstet Gynecol. 2001;97:423-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Ho CC, Yang XW, Lee TL, Liao PH, Yang SH, Tsai CH, Chou MY. Activation of p53 signalling in acetylsalicylic acid-induced apoptosis in OC2 human oral cancer cells. Eur J Clin Invest. 2003;33:875-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Bellosillo B, Piqué M, Barragán M, Castaño E, Villamor N, Colomer D, Montserrat E, Pons G, Gil J. Aspirin and salicylate induce apoptosis and activation of caspases in B-cell chronic lymphocytic leukemia cells. Blood. 1998;92:1406-1414. [PubMed] [Cited in This Article: ] |
| 32. | Kim KM, Song JJ, An JY, Kwon YT, Lee YJ. Pretreatment of acetylsalicylic acid promotes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by down-regulating BCL-2 gene expression. J Biol Chem. 2005;280:41047-41056. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Ye X, Fu J, Yang Y, Chen S. Dose-risk and duration-risk relationships between aspirin and colorectal cancer: a meta-analysis of published cohort studies. PLoS One. 2013;8:e57578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Luo T, Yan HM, He P, Luo Y, Yang YF, Zheng H. Aspirin use and breast cancer risk: a meta-analysis. Breast Cancer Res Treat. 2012;131:581-587. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 35. | Veitonmäki T, Tammela TL, Auvinen A, Murtola TJ. Use of aspirin, but not other non-steroidal anti-inflammatory drugs is associated with decreased prostate cancer risk at the population level. Eur J Cancer. 2013;49:938-945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Iwata C, Kano MR, Komuro A, Oka M, Kiyono K, Johansson E, Morishita Y, Yashiro M, Hirakawa K, Kaminishi M. Inhibition of cyclooxygenase-2 suppresses lymph node metastasis via reduction of lymphangiogenesis. Cancer Res. 2007;67:10181-10189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Batista RP, Denadai R, Saad-Hossne R. Effects of aspirin on mesenteric lymph nodes of rabbits as basis for its use on lymph nodes metastases. Acta Cir Bras. 2012;27:795-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Ioriatti ES, Rodrigues MAM, Siqueira JM, Saad-Hossne R. Efeitos da injeção de solução bicarbonatada de ácido acetilsalicílico em mucosa colorretal de coelhos, com vistas a aplicação no preparo pré-operatório do cólon. Rev Bras Colo-Proctol. 2007;27:439-445. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 39. | Siqueira JM, Barreto AB, Saad-Hossne R. Treatment of endometriosis with local acetylsalicylic acid injection: experimental study in rabbits. J Minim Invasive Gynecol. 2011;18:800-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 40. | Schrör K. 100 years of successful drug discovery. The history of aspirin. Pharm Unserer Zeit. 2009;38:306-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 41. | Patrono C, Rocca B. Aspirin, 110 years later. J Thromb Haemost. 2009;7 Suppl 1:258-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Fuster V, Sweeny JM. Aspirin: a historical and contemporary therapeutic overview. Circulation. 2011;123:768-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |