Published online May 27, 2013. doi: 10.4254/wjh.v5.i5.288
Revised: April 9, 2013
Accepted: April 13, 2013
Published online: May 27, 2013
Primary biliary cirrhosis is a slowly progressive cholestatic autoimmune liver disease that mainly affects middle-aged women with an estimated prevalence ranging from 6.7 to 402 cases per million. Hereditary hemorrhagic telangiectasia, or Rendu-Osler-Weber disease, is an autosomal dominant disorder characterized by angiodysplastic lesions (telangiectases and arteriovenous malformations) that can affect many organs, including liver, with a prevalence of 1-2 cases per 10000. We describe the coexistence, for the first time to our knowledge, of these two rare diseases in a 50-year old Caucasian woman. In this setting, the relevance of an accurate medical history, the role of liver histology and the characterization of liver involvement through dynamic imaging techniques can be emphasized.
Core tip: This case report shows the coexistence of two rare diseases, primary biliary cirrhosis and hemorrhagic hereditary telangiectasia, in a single patient. We think that this case would be worthwhile to publish because this is the first manuscript, to our knowledge, in which the coexistence of these two rare diseases has been reported. In this setting, the relevance of an accurate medical history, the role of liver histology and the characterization of liver involvement through dynamic imaging techniques can be emphasized.
- Citation: Macaluso FS, Maida M, Alessi N, Cabibbo G, Cabibi D. Primary biliary cirrhosis and hereditary hemorrhagic telangiectasia: When two rare diseases coexist. World J Hepatol 2013; 5(5): 288-291
- URL: https://www.wjgnet.com/1948-5182/full/v5/i5/288.htm
- DOI: https://dx.doi.org/10.4254/wjh.v5.i5.288
Primary biliary cirrhosis (PBC) is a slowly progressive cholestatic autoimmune liver disease that mainly affects middle-aged women with an estimated prevalence ranging from 6.7 to 402 cases per million[1]. Hereditary hemorrhagic telangiectasia (HHT), or Rendu-Osler-Weber disease, is an autosomal dominant disorder characterized by angiodysplastic lesions (telangiectases and arteriovenous malformations) that can affect many organs, including liver, with a prevalence of 1-2 cases per 10000[2]. This condition may lead to portal hypertension due to the presence of intrahepatic shunts between hepatic artery and portal vein[3], and to ischemic lesions of the biliary ducts causing the development of strictures and/or dilatation[4,5]. Liver function tests (LFTs) abnormalities commonly observed in HHT are elevation of alkaline phosphatase (ALP) and gamma glutamyl transpeptidase (GGT)[6], thus resembling those observed in cholestatic liver diseases such as PBC. We describe the coexistence of these two rare diseases in a 50-year old Caucasian woman.
A 50-year-old Caucasian woman with PBC stage I according to Scheuer’s classification [antimitochondrial antibodies (AMA) type M2 positivity, titer 1:320, and histological diagnosis five years earlier] was admitted to our Unit in September 2011 for the evaluation of a hypoechoic focal liver lesion (segment VII, longest diameter 20 mm) detected with routine abdominal ultrasound.
At the time of PBC diagnosis, GGT and ALP levels were approximately five times the upper limit of normal, while other LFTs were normal. Subsequent therapy with ursodeoxycholic acid (UDCA, 15 mg/kg per day) had obtained a rapid reduction, up to normalization, of cholestatic liver enzymes. Furthermore, a thorough medical history revealed recurrent epistaxis and two episodes of gastrointestinal bleeding with negativity of both esofagogastroduodenoscopy and colonoscopy in 2003 and 2004. The causes of death of her parents were unknown.
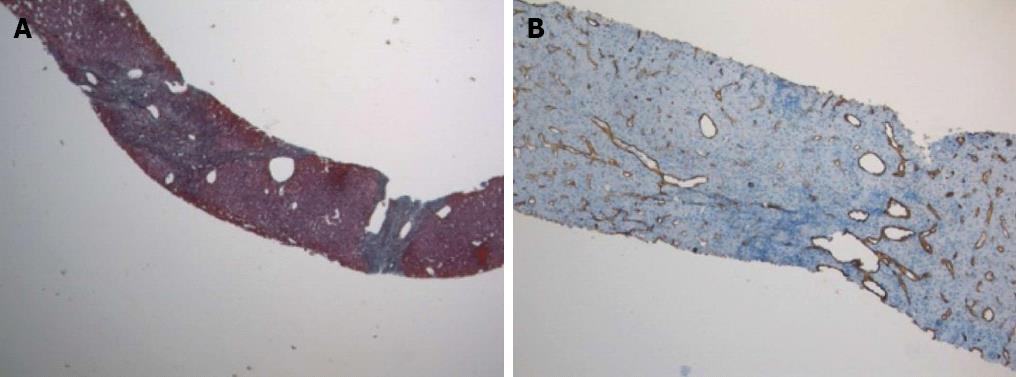
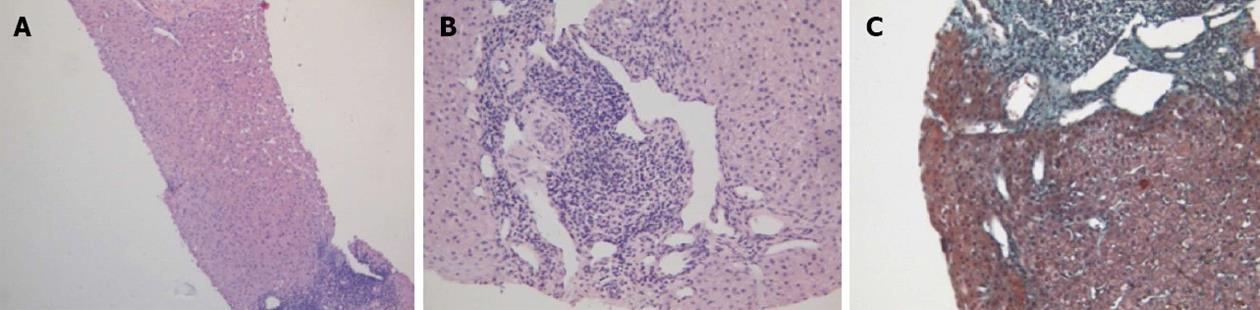
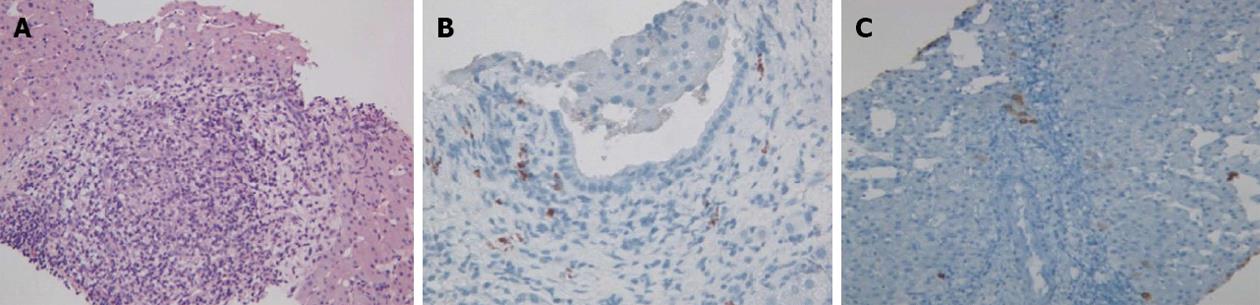
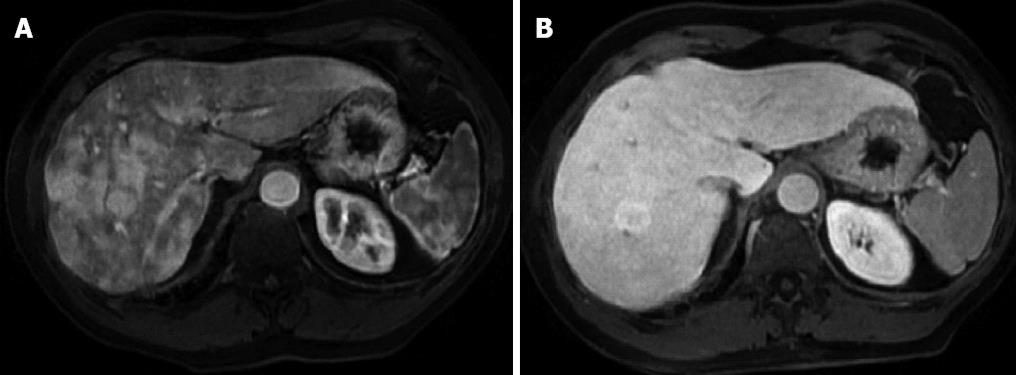
Physical examination upon admission was unremarkable, except for two clearly visible telangiectasias, one on the lower lip and the other one on the tongue. All routine laboratory tests, including LFTs, were normal. Non-organ specific autoantibodies evaluation (tested by indirect immunofluorescence for antinuclear antibodies, AMA, smooth muscle antigens and by immunoblotting for AMA type M2, type I liver-kidney microsomes, lebercytosol 1, soluble liver antigen/liver-pancreas antigen) confirmed AMA (title 1:1280) and AMA type M2 positivity. Esofagogastroduodenoscopy and colonoscopy did not show any lesion. Multiphasic contrast-enhanced helical computed tomography (CT) scans of the liver revealed multiple focal hypervascular areas (the greater in segment VII) without a fully exhaustive radiological characterization. Ultrasonically guided fine needle biopsy of segment VII focal liver lesion showed fibrous septa intersecting the parenchyma, giving an appearance of nodular liver. Staining the fibrous septa in green through Masson’s trichrome, the nodular architecture was highlighted (Figure 1A). In addition, CD34 immunohistochemical assay showed diffuse CD34 positive staining in the sinusoids (Figure 1B). These features were limited to the hypoechoic focal liver lesion, in keeping with a diagnosis of focal nodular hyperplasia (FNH). Biopsy of the adjacent liver parenchyma was devoid of fibrous intra-parenchimal septa but showed widespread vascular ectasias in portal and peri-portal areas (Figure 2). Furthermore, an intra-portal granuloma, portal immunoglobulin M (IgM) lymphoplasmacytic infiltrate (polyclonal anti-IgM; Novocastra, Newcastle, United Kingdom), bile duct damage and absence of cytokeratin 7 positive bile ducts in some portal tracts were evidenced both in the focal lesion and in the adjacent parenchyma (Figure 3). These features were all consistent with the previous diagnosis of PBC. No complications occurred after the procedure. Afterwards, multiphasic abdominal magnetic resonance (MR) showed hypervascular areas mainly in the right liver lobe, due to arteriovenous shunts, a clearly visible 3 cm lesion consistent with FNH and enlarged hepatic artery and hepatic veins (Figure 4). MR cholangiography did not show any alteration of the biliary tract. Finally, a CT angiography of the chest was negative for pulmonary arteriovenous malformations.
UDCA therapy was continued at the same dosage. At the last follow-up visit (January 2012) the patient was still asymptomatic, and all LFTs were persistently normal.
PBC is a rare chronic autoimmune disease whose diagnosis is mainly based on AMA type M2 positivity and liver histology; it primarily affects middle-aged women with a prevalence of less than 1/2000[7]. HHT is an autosomal dominant disease characterized by angiodysplastic lesions that can affect many organs, including liver, with an estimated prevalence of 1/5000[2].
The so-called Curaçao criteria (Table 1) are widely used in clinical practice for the diagnosis of HHT[8]. In our case, HHT diagnosis may be labeled as “definite”, because at least three out of four criteria (epistaxis, telangiectases and visceral involvement) were present, while the fourth criterion (family history) was not applicable.
| Criteria | Description |
| Epistaxis | Spontaneous and recurrent |
| Telangiectases | Multiple, at characteristic sites: lips, oral cavity, fingers, nose |
| Visceral lesions | Gastrointestinal telangiectasias, pulmonary, hepatic, cerebral or spinal arteriovenous malformations |
| Family history | A first-degree relative with hereditary hemorrhagic telangiectasia according to these criteria |
The prevalence of hepatic involvement in HHT has been estimated to range between 32% and 72% by ultrasonography or CT scan[4], even if about 90% of patients are reported to be asymptomatic. High output heart failure is the most common manifestation[3], even if our patient did not show any sign or symptom of cardiovascular disfunction nor abdominal angina, which may rarely occur due to mesenteric arterial steal by vascular liver malformations[6]. Furthermore, ischemic lesions of the biliary tree may cause the development of strictures and/or dilatation[9], but MR-colangiography was negative. LFTs abnormalities commonly observed in HHT patients are elevation of ALP and GGT[6], which were normal on admission but elevated before the PBC diagnosis and the subsequent introduction of UDCA therapy five years before. Thus, overall clinical picture could be related to the coexistence of HHT and PBC, whose diagnosis was stated again by AMA type M2 positivity and liver histology. In fact, even if bile duct abnormalities and ductopenia may also be secondary to ischemic bile duct injury due to HHT liver involvement[10], the presence of the portal granuloma and the increase of IgM plasma cell number in the portal tract confirmed a definitive diagnosis of PBC[11].
Consequently, even if generally not recommended in a clinical setting of HTT due to a theoretical risk of bleeding[12], liver biopsy was decisive to confirm the diagnosis of PBC.
Finally, the prevalence of FNH in patients with HHT is 100-fold greater than general population[13]. In our case, FNH lesion showed a CD34 immunostaining pattern resembling the one usually observed in hepatocellular carcinoma. Nevertheless, the presence of fibrous septa, highlighted by Masson‘s trichrome staining, and the absence of cell atypias led to a conclusive diagnosis of FNH, which, as previously reported[14], can show the above mentioned CD34 immunohistochemical pattern. Its histological finding raised the suspicion of HHT liver involvement, which was furtherly confirmed by RM detection of the typical liver vascular abnormalities of HHT and by histologically evident portal and peri-portal angiectases.
To our knowledge, this is the first case in which the coexistence of PBC and HHT has been reported. In this setting, the relevance of an accurate medical history, the role of liver histology and the characterization of liver involvement through dynamic imaging techniques can be emphasized.
P- Reviewer Qin JM S- Editor Gou SX L- Editor A E- Editor Li JY
| 1. | Prince MI, James OF. The epidemiology of primary biliary cirrhosis. Clin Liver Dis. 2003;7:795-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 99] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Kjeldsen AD, Vase P, Green A. Hereditary haemorrhagic telangiectasia: a population-based study of prevalence and mortality in Danish patients. J Intern Med. 1999;245:31-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 269] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 3. | Garcia-Tsao G. Liver involvement in hereditary hemorrhagic telangiectasia (HHT). J Hepatol. 2007;46:499-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Buscarini E, Plauchu H, Garcia Tsao G, White RI, Sabbà C, Miller F, Saurin JC, Pelage JP, Lesca G, Marion MJ. Liver involvement in hereditary hemorrhagic telangiectasia: consensus recommendations. Liver Int. 2006;26:1040-1046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Mendoza A, Oliff S, Elias E. Hereditary haemorrhagic telangiectasia and secondary biliary cirrhosis. Eur J Gastroenterol Hepatol. 1995;7:999-1002. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Senzolo M, Riggio O, Primignani M. Vascular disorders of the liver: recommendations from the Italian Association for the Study of the Liver (AISF) ad hoc committee. Dig Liver Dis. 2011;43:503-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Poupon R. Primary biliary cirrhosis: a 2010 update. J Hepatol. 2010;52:745-758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 215] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 8. | Shovlin CL, Guttmacher AE, Buscarini E, Faughnan ME, Hyland RH, Westermann CJ, Kjeldsen AD, Plauchu H. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet. 2000;91:66-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 4] [Reference Citation Analysis (0)] |
| 9. | McInroy B, Zajko AB, Pinna AD. Biliary necrosis due to hepatic involvement with hereditary hemorrhagic telangiectasia. AJR Am J Roentgenol. 1998;170:413-415. [PubMed] [Cited in This Article: ] |
| 10. | Blewitt RW, Brown CM, Wyatt JI. The pathology of acute hepatic disintegration in hereditary haemorrhagic telangiectasia. Histopathology. 2003;42:265-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Cabibi D, Tarantino G, Barbaria F, Campione M, Craxì A, Di Marco V. Intrahepatic IgG/IgM plasma cells ratio helps in classifying autoimmune liver diseases. Dig Liver Dis. 2010;42:585-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Faughnan ME, Palda VA, Garcia-Tsao G, Geisthoff UW, McDonald J, Proctor DD, Spears J, Brown DH, Buscarini E, Chesnutt MS. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet. 2011;48:73-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 649] [Cited by in F6Publishing: 652] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 13. | Ianora AA, Memeo M, Sabba C, Cirulli A, Rotondo A, Angelelli G. Hereditary hemorrhagic telangiectasia: multi-detector row helical CT assessment of hepatic involvement. Radiology. 2004;230:250-259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 159] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Ahmad I, Iyer A, Marginean CE, Yeh MM, Ferrell L, Qin L, Bifulco CB, Jain D. Diagnostic use of cytokeratins, CD34, and neuronal cell adhesion molecule staining in focal nodular hyperplasia and hepatic adenoma. Hum Pathol. 2009;40:726-734. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |