Copyright
©The Author(s) 2025.
World J Hepatol. Jul 27, 2025; 17(7): 106993
Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.106993
Published online Jul 27, 2025. doi: 10.4254/wjh.v17.i7.106993
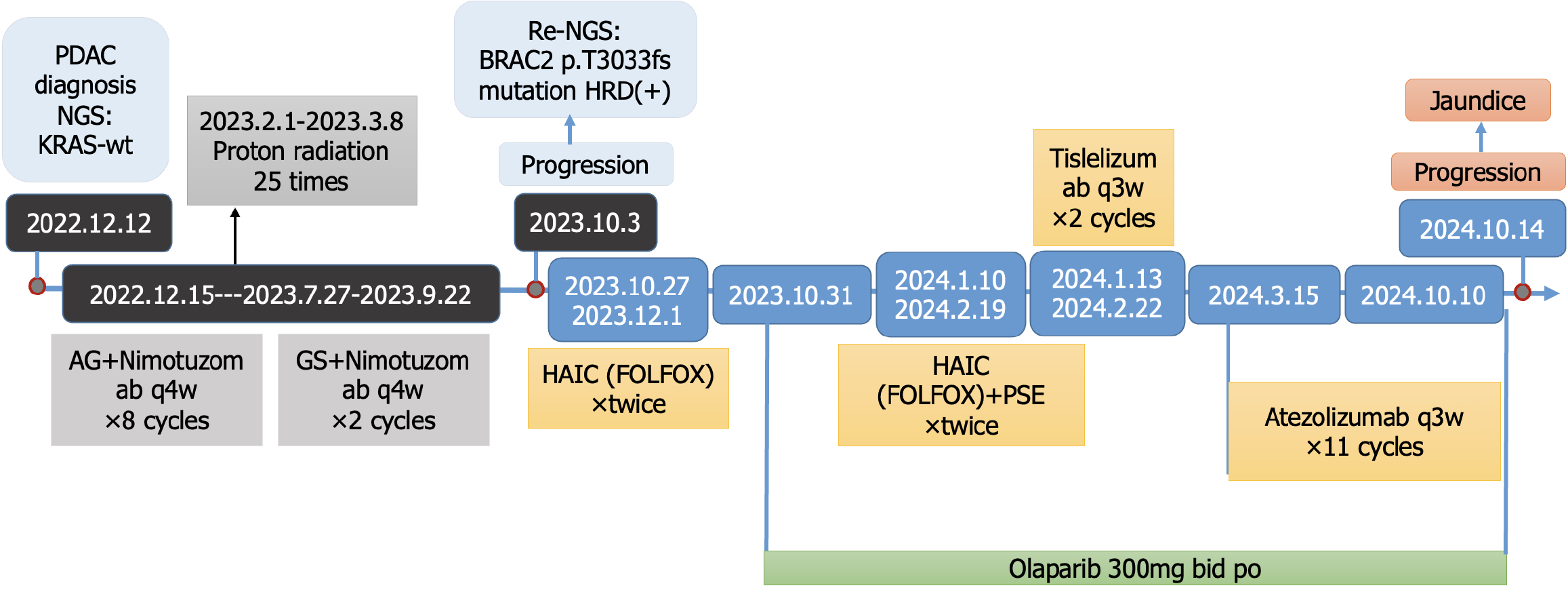
Figure 3 The overall treatment process of the case.
The patient was diagnosed with Pancreatic ductal adenocarcinoma, and next-generation sequencing on December 12, 2022, revealed wild-type KRAS. Subsequently, the patient was treated with gemcitabine + albumin-bound paclitaxel + nimotuzomab q4w × 8 cycles, proton radiation 25 times and gemcitabine + tegafur/gimeracil/oteracil potassium + nimotuzomab q4w × 2 sequential cycles. The tumor progressed on October 3, 2023. The patient was then visit our molecular tumor boards clinic. Repeat next-generation sequencing identified homologous recombination deficiency positivity and a pathogenic BRCA2 mutation, prompting the initiation of hepatic arterial infusion chemotherapy (5-fluorouracil + leucovorin + oxaliplatin) + immune checkpoint inhibitors + olaparib. On October 14, 2024, jaundice was observed, with a subsequent confirmation of disease progression. PDAC: Pancreatic ductal adenocarcinoma; NGS: Next-generation sequencing; AG: Gemcitabine + albumin-bound paclitaxel; GS: Gemcitabine + tegafur/gimeracil/oteracil potassium; HRD: Homologous recombination deficiency HAIC: Hepatic arterial infusion chemotherapy; FOLFOX: 5-fluorouracil + leucovorin + oxaliplatin; PSE: Partial splenic embolization.
- Citation: Yan Y, Ren ZZ, Wang WY, Tang J, Zhang YW. Molecular tumor boards in pancreatic cancer with liver metastasis: A case report. World J Hepatol 2025; 17(7): 106993
- URL: https://www.wjgnet.com/1948-5182/full/v17/i7/106993.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i7.106993