Copyright
©The Author(s) 2024.
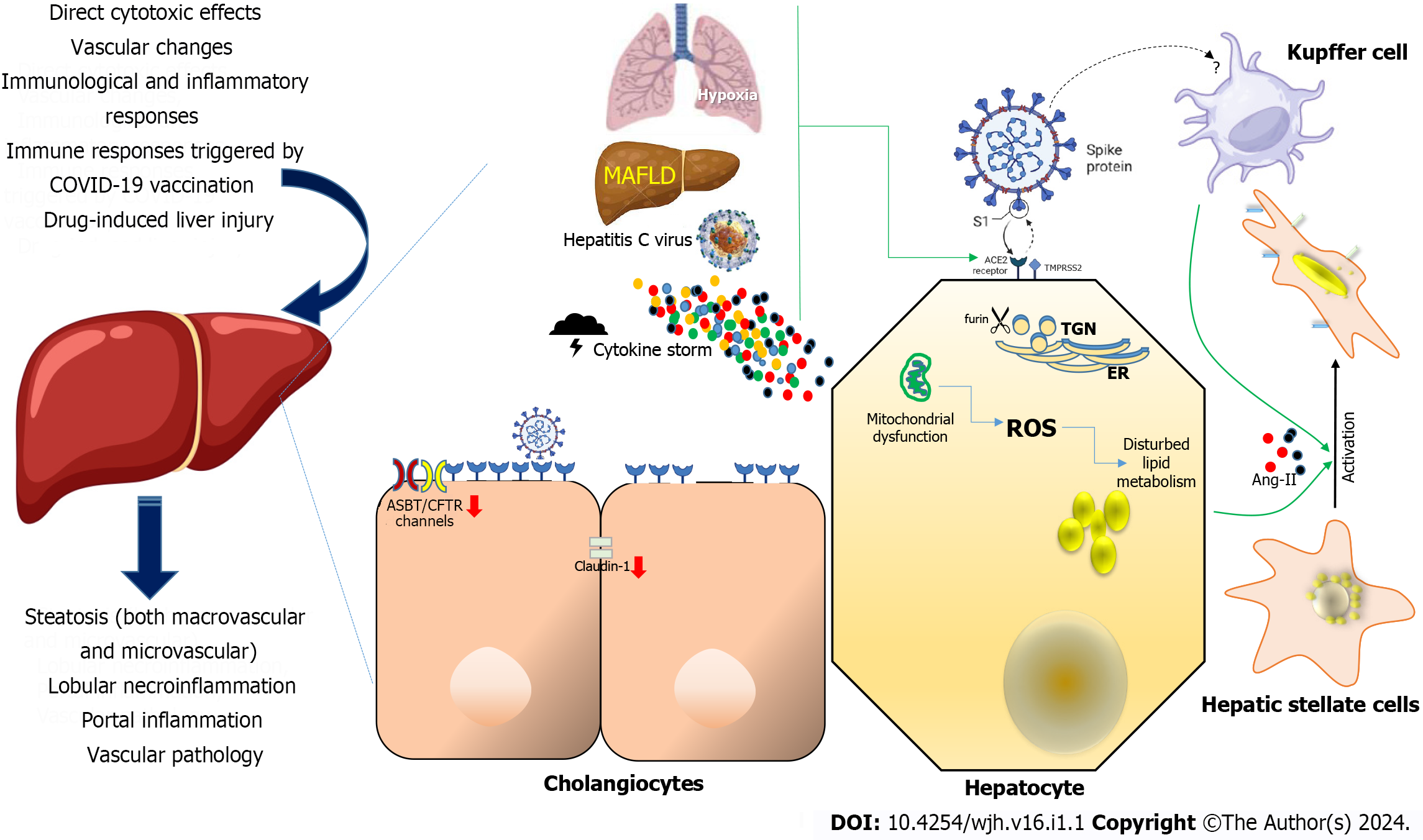
Figure 1 Mechanisms of severe acute respiratory syndrome coronavirus-2 disease-induced liver injury and their consequences at organ level (left).
Severe acute respiratory syndrome coronavirus-2 cellular targets involved in liver damage (center and right). Various factors have been postulated to contribute to liver injury in the context of coronavirus disease 2019 (COVID-19), including direct cytotoxic effects, vascular changes, immunological and inflammatory responses associated with COVID-19, immune responses triggered by COVID-19 vaccination, and drug-induced liver injury. In the context of liver injury associated with COVID-19, the histological patterns encompass features such as steatosis (both macrovascular and microvascular), lobular necroinflammation, portal inflammation, and vascular pathology. At the cellular level, hypoxia, metabolic dysfunction-associated fatty liver disease, and concomitant hepatitis C virus infection, and the cytokine storm may upregulate the Angiotensin-converting enzyme-2 (ACE2), transmembrane serine protease 2 and furin expression in hepatocytes. Mitochondrial dysfunction has been affected directly by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection of hepatocytes which in turn may be connected to pre-existing inflammation and the adverse impacts of excessive and dysfunctional adipose tissue. In cholangiocytes, SARS-CoV-2 Leads to a decrease in the mRNA expression of Claudin-1 and downregulates the expression of hepatobiliary transporters, such as ASBT and the chloride channel CFTR. The ACE-2 expression in Kupffer cells is still controversial. Hepatic stellate cells appear do not express ACE2 in any activation state. Their activation is a pivotal event in the progression of chronic liver disease, as these cells serve as the primary source of fibrosis, and it is induced by proinflammatory and profibrotic signals, including angiotensin II, which is generated by the catalytic action of ACE as part of the profibrotic branch of the renin-angiotensin system. Liver and Kupffer cell are created with BioRender.com. ROS: Reactive oxygen species; ACE2: Angiotensin-converting enzyme-2; TMPRSS2: Transmembrane serine protease 2; MAFLD: metabolic dysfunction-associated fatty liver disease; ER: Endoplasmic reticulum; TGN: Trans-Golgi network.
- Citation: Quarleri J, Delpino MV. Molecular mechanisms underlying SARS-CoV-2 hepatotropism and liver damage. World J Hepatol 2024; 16(1): 1-11
- URL: https://www.wjgnet.com/1948-5182/full/v16/i1/1.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i1.1