Published online Oct 27, 2018. doi: 10.4254/wjh.v10.i10.719
Peer-review started: May 23, 2018
First decision: June 14, 2018
Revised: July 12, 2018
Accepted: August 6, 2018
Article in press: August 7, 2018
Published online: October 27, 2018
To determine the influence of the construction design over the biological component’s performance in an experimental bio-artificial liver (BAL) device.
Two BAL models for liver microorgans (LMOs) were constructed. First, we constructed a cylindrical BAL and tested it without the biological component to establish its correct functioning. Samples of blood and biological compartment (BC) fluid were taken after 0, 60, and 120 min of perfusion. Osmolality, hematocrit, ammonia and glucose concentrations, lactate dehydrogenase (LDH) release (as a LMO viability parameter), and oxygen consumption and ammonia metabolizing capacity (as LMO functionality parameters) were determined. CPSI and OTC gene expression and function were measured. The second BAL, a “flat bottom” model, was constructed using a 25 cm2 culture flask while maintaining all other components between the models. The BC of both BALs had the same capacity (approximately 50 cm3) and both were manipulated with the same perfusion system. The performances of the two BALs were compared to show the influence of architecture.
The cylindrical BAL showed a good exchange of fluids and metabolites between blood and the BC, reflected by the matching of osmolalities, and glucose and ammonia concentration ratios after 120 min of perfusion. No hemoconcentration was detected, the hematocrit levels remained stable during the whole study, and the minimal percentage of hemolysis (0.65% ± 0.10%) observed was due to the action of the peristaltic pump. When LMOs were used as biological component of this BAL they showed similar values to the ones obtained in a Normothermic Reoxygenation System (NRS) for almost all the parameters assayed. After 120 min, the results obtained were: LDH release (%): 14.7 ± 3.1 in the BAL and 15.5 ± 3.2 in the NRS (n = 6); oxygen consumption (μmol/min·g wet tissue): 1.16 ± 0.21 in the BAL and 0.84 ± 0.15 in the NRS (n = 6); relative expression of Cps1 and Otc: 0.63 ± 0.12 and 0.67 ± 0.20, respectively, in the BAL, and 0.86 ± 0.10 and 0.82 ± 0.07, respectively, in the NRS (n = 3); enzymatic activity of CPSI and OTC (U/g wet tissue): 3.03 ± 0.86 and 222.0 ± 23.5, respectively, in the BAL, and 3.12 ± 0.73 and 228.8 ± 32.8, respectively, in the NRS (n = 3). In spite of these similarities, LMOs as a biological component of the cylindrical BAL were not able to detoxify ammonia at a significant level (not detected vs 35.1% ± 7.0% of the initial 1 mM NH4+ dose in NRS, n = 6). Therefore, we built a second BAL with an entirely different design that offers a flat base BC. When LMOs were placed in this “flat bottom” device they were able to detoxify 49.3% ± 8.8% of the initial ammonia overload after 120 min of perfusion (n = 6), with a detoxification capacity of 13.2 ± 2.2 μmol/g wet tissue.
In this work, we demonstrate the importance of adapting the BAL architecture to the biological component characteristics to obtain an adequate BAL performance.
Core tip: This work describes the adaptation of a simplified bio-artificial liver (BAL) prototype to make it suitable to house rat liver microorgans (LMOs) as a biological component, and the evaluation of the performance in this new model. We demonstrate that the modification in the design of the artificial parts employed allows a good performance of LMOs, thus showing the importance of architecture and model configuration on the design of these devices. Besides its application as BAL, this mini bioreactor could serve as a suitable laboratory tool to evaluate the behavior and functionality of LMOs subjected to different incubation conditions due to its simple design and the utilization of standard materials.
- Citation: Pizarro MD, Mamprin ME, Daurelio LD, Rodriguez JV, Mediavilla MG. Experimental bio-artificial liver: Importance of the architectural design on ammonia detoxification performance. World J Hepatol 2018; 10(10): 719-730
- URL: https://www.wjgnet.com/1948-5182/full/v10/i10/719.htm
- DOI: https://dx.doi.org/10.4254/wjh.v10.i10.719
Bio-artificial liver (BAL) devices are extracorporeal systems that contain functional hepatic tissue or cells (the biological component) seeded into a man-made bioreactor (the artificial component) and separated from blood flow by semipermeable membranes[1]. The aim of these devices is to fulfill the necessary hepatic functions to keep patients with hepatic failure stabilized until the regeneration of their own livers or until the appearance of a compatible donor[1,2].
Though the majority of liver functions are performed by hepatocytes, the other hepatic cellular types (Kupffer, Pit, stellate or Ito, and sinusoidal epithelial cells) are also involved and exert some influence over the different functions in vivo[2]. Therefore, we became interested in studying a biological component possessing all these different liver constituent cells in search of a better performance of our BAL prototype with respect to a previous one designed in our laboratory to house isolated hepatocytes[3]. In this sense, liver microorgans (LMOs) are an appropriate choice mostly because they are thin slices of tissue that keep the liver structure and its physiological characteristics and allow a good exchange of substances with the surrounding liquid and gaseous environment[4,5].
Ammonia elimination from the blood of patients with liver failure is a key metabolic reaction that any biological component of an efficient BAL should accomplish in light of the correlation existing between this metabolite accumulation and the progression of hepatic failure[3,6]. Nonetheless, etiology of hyperammonemia can also be from non-hepatic situations, including sepsis[7,8], urea cycle inborn disorders[9], complications in a hepatic transplantation setting[10], complications from cirrhosis[11,12], and urinary tract infections[13]. Hyperammonemia should be quickly treated to avoid brain damage or even death, and simple hemofiltration is sometimes not sufficient to cope with it[10,11]. In this context, a device capable of efficiently detoxifying ammonia could be useful to ameliorate the condition of the patient. Also, it could be utilized in the non-hepatic situations until the origin of the complication can be determined and controlled[6].
When studying ammonia detoxification function displayed by LMOs, we found that these tissue slices had excellent performance when incubated in suspension in a shaker. This simple system was called the Normothermic Reoxygenation System (NRS)[14]. Surprisingly, they completely lost this detoxifying capacity in the BAL device when the blood was overloaded with ammonia.
In our laboratory we had previously designed a BAL consisting of a cylindrical shaped device to house isolated rat hepatocytes as the biological component[4]. This system showed a good performance and allowed the maintenance of adequate viability and functionality of fresh hepatocyte suspensions, and successfully fulfilling different in vitro tests that constitute the first step in the development of any BAL system[4]. In this work, we present the results obtained when we enlarged this original prototype to allow accommodation to the bulkier LMOs, hypothesizing that this was a straightforward path in the design. However, we showed that the LMOs were unable to detoxify NH4+, and therefore had to redesign the artificial component receptacle to make it suitable to this biological component. To the best of our knowledge, this is the first report that evaluates such an essential issue and is valuable information for scientists studying this field of research.
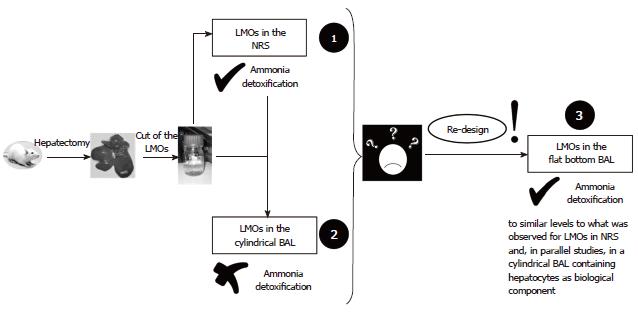
Figure 1 summarizes the path followed to develop a BAL prototype suitable for using LMOs as the biological component. Step 1: Evaluation of LMO inherent performance, mainly regarding ammonia detoxification capacity in the NRS[5] (Figure 2A); Step 2: Adaptation of the BAL, originally designed for hepatocytes[3], to house LMOs on its biological compartment (BC) (Figure 2B). Testing of this prototype without any biological component establishes its correct functioning. Testing of this prototype with LMOs as biological component to establish its performance as BAL, especially in relation to ammonia detoxification. Comparison with the results obtained in the NRS; Step 3: Due to the failure of LMOs to detoxify ammonia in the cylindrical BAL (in comparison with NRS): development of a “flat bottom” BAL (Figure 2C). Testing with LMOs as a biological component to determine the influence of the BAL configuration and design over the LMO ammonia detoxification capacity. Other parameters remained similar to the values encountered for the cylindrical BAL and were already published[15].
LMOs were obtained from livers of 60-day-old male Wistar rats with a weight of 250-300 g. Animals were given a fourteen day adaptation period to get used to the experimental laboratory environment, during which they could access standard rodent laboratory food and water ad libitum. Rats received care according to the principles and recommendations given by the National Academy of Sciences (Argentina). The School of Biochemical and Pharmaceutical Sciences Institutional Animal Care and Use Committee (Universidad Nacional de Rosario, Res No. 139/2011) approved all experimental procedures.
Rats were anesthetized (chloral hydrate, 500 mg/kg body weight, i.p.) and the liver was surgically removed. The right medial lobe was cut into blocks and LMOs were manually obtained from these blocks as thin slices (thickness: 338 ± 27 μm, n = 25), using a disposable microtome blade. We did all the manipulations over an ice-cooled cutting device to reduce liver deterioration, and on top of a piece of filter paper to avoid tissue slippage and ensure the accurate cutting of LMOs[14]. After slicing, LMOs were placed in KH-Base (KHB) solution (KHB, Table 1) and were pre-incubated for 15 min before loading into the NRS or the BAL prototype. The different KH solution compositions are shown in Table 1. KHB media was used for the LMO obtaining procedure and during the pre-incubation period.
| Components | KHB solution | KHA solution |
| NaCl | 114 mmol/L | 114 mmol/L |
| KH2PO4 | 1.2 mmol/L | 1.2 mmol/L |
| KCl | 4.8 mmol/L | 4.8 mmol/L |
| MgSO4 | 1.2 mmol/L | 1.2 mmol/L |
| CaCl2 | 1.5 mmol/L | 1.5 mmol/L |
| HEPES | 10 mmol/L | 10 mmol/L |
| NaHCO3 | 25 mmol/L | 25 mmol/L |
| Glucose | 25 mmol/L | 25 mmol/L |
| Allopurinol | 1 mmol/L | 1 mmol/L |
| Fructose | 5 mmol/L | 5 mmol/L |
| Glycine | - | 3 mmol/L |
| Adenosine | - | 10 µmol/L |
| Ornithine | - | 6 mmol/L |
| Sodium lactate | - | 10 mmol/L |
| pH | 7.4 | 7.4 |
| mOsm/kg H2O | 293 ± 6 (n = 10) | 328 ± 5 (n = 10) |
LMOs have been characterized regarding histology, water content, and viability (published results[5,14]). They were weighted for normalization of results and their weights were 0.070 ± 0.020 g (n = 25), showing size consistency.
To evaluate the biological component inherent performance before introducing it into our BAL, we tested LMOs in a NRS (Figure 2A). In the NRS, they were maintained in Krebs-Henseleit (KH) solution at 37 °C under carbogen atmosphere (95% O2: 5% CO2) using a six-well culture plate for 120 min[5]. To determine LMO NH4+ metabolizing capability, we added an ammonia overload (1 mmol/L approximate NH4+ final concentration[16]) to KH-ammonia solution (KHA, Table 1) from a concentrated ammonium chloride solution (approximate concentration: 350 mmol/L). This ammonia overload is far in excess of the level of this metabolite encountered in plasma of patients with liver failure (approximately 0.2 mmol/L). We chose this condition of work to challenge LMOs because they are supposed to deal with continuously infused plasma when applied to animal models of hepatic failure or patients in the future[16-18]. The exact amount of ammonia in KHA solution was then determined, as explained later in this section.
LMO disposition in the NRS is shown in Figure 2A. Two LMOs were placed on each well with 5 mL of KHA solution plus the ammonia overload, and the plate was then introduced in a Dubnoff metabolic shaker maintained at 37 °C and stirred at 60 cycles/min. Samples of tissue and the bathing solution were taken after 0, 60, and 120 min to evaluate lactate dehydrogenase (LDH) release, oxygen consumption, and ammonia metabolism[5]. This system is intended to evaluate the biological component performance and condition per se in a simpler and less stressing manner than in the BAL device.
The device designed for hepatocytes[4] was modified to use LMOs as biological component (Figure 2B). It was constructed with a cylindrical plastic cartridge that contained 120 to 140 hollow fibers (PolyamixTM, GAMBRO®) assembled to the ends of the cartridge by two Y-connectors (Nalgene, # 6152–0375) connected to S/P silicone tubes (6.4 mm i.d., 11.2 mm o.d., and 2.4 mm wall). Two Teflon large catheters (14 gauge, 2 mm i.d.) and an oxygenator (Ox) made of oxygen permeable tube (silicone tubing, 0.078 in. i.d., 0.125 in. o.d.; cat. No. T5715-9, Baxter Healthcare Corp., Deerfield, IL, United States) were assembled through the Y-connectors. LMOs were seeded through an access port (Lp, Figure 2B) in the space outside the fibers (BC capacity: approximately 50 cm3), while ram blood (obtained from animals in the animal house facility of our school) circulated along the inner space of the fibers (the blood compartment).
We constructed a device with a flat base BC using a standard 25 cm2 culture flask[15] as shown in Figure 2C. The culture flask was adapted introducing a Y-shape connector (Nalgene, # 6152–0375) onto its tap and a plain connector (S/P silicone tube, 6.4 mm i.d., 11.2 mm o.d., and 2.4 mm wall) on one side, to assemble the 140 hollow fibers (PolyamixTM, GAMBRO®). The loading port (Lp) was introduced on its top surface and the oxygenating tube (Ox) (silicone tubing, 0.078 in. i.d., 0.125 in. o.d.; cat. no. T5715-9, Baxter Healthcare Corp., Deerfield, IL, United States) access the BC through the free branch of the Y-connector. Both prototypes of BALs were tested using the perfusion system (Figure 2D). The peristaltic pump ensured ram blood recirculation from its reservoir passing through a clot filter/bubble trap before entering the hollow fibers. The perfusion system was kept at 37 °C by the thermostatic bath.
First of all, a sample of ram blood (basal blood) was taken before adding the ammonia overload and filling the blood perfusion system by the aid of a peristaltic pump (blood volume: approximately 35 mL). The BC was then filled with KHA alone (to test the bioreactor performance) or with KHA plus 1 g of LMO. Samples of blood and BC fluid were taken after 0, 60, and 120 min of perfusion to assay the different parameters mentioned below. Blood samples were centrifuged at 12000 × g for 3 min in a mini spin centrifuge.
During the experiments (BAL runs), the device was operated in horizontal position, immersed in the Dubnoff shaker bath at 37 °C stirred at 60 cycles/min. Carbogen gas pressure was kept at 85 mm Hg to introduce oxygen in the BC media via the silicone tube oxygenator, and in this way pH was maintained at 7.40 ± 0.10, which was controlled throughout the run. The blood maintained proper oxygenation, and its recirculation flow rate was maintained at 9 mL/min.
All the devices designed were subjected to two different types of studies: (1) without any biological component, to establish the adequate functioning of the device and the perfusion system[4]; and (2) with biological component, to determine if the chosen biological component could accomplish ammonia detoxification.
In experiments without LMOs, we first checked the correct system functioning by evaluating the following parameters: (1) hematocrit of blood samples after centrifugation (10000 × g for 3 min, Rolco CH24 centrifuge), as the percentage of volume that red cells represent from the total (blood cells + plasma). It was used to evaluate fluid exchange and the occurrence of hemolysis during perfusion; (2) osmolality of plasma and extra-fiber fluid was determined with a freezing point osmometer (Osmomat 030 Gonotec, GmbH, Berlin, Germany) to check the proper passage of fluids through the fiber walls; and (3) glucose and ammonia concentrations were assessed as described below and their mass balance was calculated to monitor the adequate exchange of metabolites between blood and the BC in the device.
The quantity of hemoglobin in plasma samples obtained after 0, 60, and 120 min of perfusion was assessed using the oxyhemoglobin method as previously described[19]. The equation proposed by Arnaud et al[20] was then used to calculate hemolysis percentage as follows:
Hemolysis (%) = 100 × [(HbS × (1 - Ht))/HbT]
where HbS represents the sample hemoglobin contents, in g/100 mL; HbT is the total hemoglobin content determined in whole blood, and Ht represents the respective hematocrit value.
This parameter was assessed with a commercially available kit (“Glicemia Enzimática AA”, Wiener Laboratories, Rosario, Argentina), as instructed in the information leaflet.
We used LDH release as a LMO viability parameter. This enzyme activity was determined in the KH incubation solution and in the liver slices as previously described[21]. Results are shown as the percentage of the total enzyme activity released to the bathing media.
Samples were stored in liquid nitrogen until ammonia quantification was done using the enzymatic method described by van Anken[22]. The reaction media (0.8 mL) was composed of 66.7 mmol/L phosphate buffer, pH = 8.30, 0.14 mmol/L NADPH, 6.5 mmol/L sodium α-ketoglutarate, 2.5 mmol/L ADP, and 120 UI/mL glutamate dehydrogenase (cat. #G2626, Sigma Aldrich, St. Louis, MO, United States).
In the BAL, ammonia mass balance was calculated using the equations described next:
QB,t = ([A]B,t × VB,t) – ([A]B,Bas × VB,t)
QBC,t = ([A]BC,t × VBC,t) - ([A]BC,Bas × VBC,t)
QT,t = QB,t + QBC,t
where: QB,t and QBC,t are the ammonia mass in blood and the BC solution at time t, respectively; [A]B,t and [A]BC,t represent respective ammonia concentrations in blood and BC fluid; VB,t and VBC,t are, respectively, the volumes of blood and the BC fluid at each time, and QT,t is the total ammonia mass at the different assayed times.
Then, we calculated the percentage of ammonia initial dose metabolized at each time with the subsequent equation:
Initial Dose Detoxified (%) = 100 – [QT,t × 100/ QT,0]
Also, we estimated the μmol of ammonia detoxified per gram of LMO as follows:
Ammonia Detoxification (μmol/g wet tissue) = (QT,0 – QT,t)/ PT
where PT represents the mass of LMOs in grams.
In the case of the NRS, ammonia concentration was determined in the LMO bathing solution and the equations applied were:
Initial Dose Detoxified (%) = 100 – [(Qt × 100)/ Qi]
Ammonia Detoxification (μmol/g wet tissue) = (Qi – Qt)/ PT
where Qi and Qt represent the amount of ammonia measured at the beginning of the experiments and after t minutes, respectively, and PT is the total weight of LMOs in grams.
As part of the LMO ammonia metabolism study, we determined the mRNA and activity levels of Carbamyl Phosphate Synthetase I (CPSI) and Ornithine Transcarbamylase (OTC) that catalyze the first and second steps of the urea cycle, respectively.
Total RNA extractions were performed using TriReagentTM (Sigma Chem. Co., St. Louis, United State) and following the instructions provided by the manufacturer. We carried out reverse transcription and semi-quantitative PCR as previously described[23]. In Table 2, the base sequences of the primers used for the study of each gene expression are listed. We applied the 2-ΔΔCT method to obtain relative expression, using Glyceraldehyde Phosphate Dehydrogenase (Gapdh), β-Actin (Actb) and rRNA 18S (Rn18S) as reporter mRNAs to normalize the values. Each sample was analyzed in triplicate, and its template cDNA initial quantity was expressed relative to a reference sample, considered 1X. This reference sample was taken at time 0.
| Primers | Fragment size | Genbank access number |
| Cps1 5’-ATCTGAGGAAGGAGCTGTCT-3’ (sense) Cps1 5’-AAAACCACTTGTCAATGGAT-3’ (anti-sense) | 120 bp | NM_017072 |
| Otc 5’-ATGACAGATGCAGTGTTAGC-3’ (sense) Otc 5’-CAGGATCTGGATAGGATGAT-3’ (anti-sense) | 120 bp | NM_013078 |
| Actb 5’-CAACCTTCTTGCCAGCTCCTC-3’ (sense) Actb 5’-GACGAGCGCAGCGATATC-3’ (anti-sense) | 79 bp | NM_031144.2 |
| Rn18S 5’-TAACCCGTTGAACCCCATT-3’ (sense) Rn18S 5’-CCATCCAATCGGTAGTAGCG-3’ (anti-sense) | 150 bp | X01117 |
| Gapdh 5’-CCATCACCATCTTCCAGGAG-3’ (sense) Gapdh 5’-CCTGCTTCACCACCTTCTTG-3’ (anti-sense) | 576 bp | NM_017008.4 |
To determine CPSI activity we performed the Pierson’s colorimetric test[24] that involves the reaction of carbamyl phosphate and hydroxylamine to obtain hydroxyurea. An enhanced colorimetric test for ureido compounds allows the quantification of the hydroxyurea produced due to the chromophore absorbance measurement at 458 nm. CPSI activity is expressed as U/g of wet tissue, being U the μmoles of carbamyl phosphate produced per minute at 37 °C.
OTC activity was measured with the method described by Ceriotti[25] as the rate of citrulline formation from ornithine and carbamyl phosphate is catalyzed by OTC. The quantity of citrulline produced was determined by the diacetyl monoxime-antipyrine reaction and OTC activity was also expressed as U/g of wet tissue, where U represents the μmoles of citrulline synthesized per minute at 37 °C.
Samples of LMOs were taken at different times (0, 60, and 120 min) from the NRS or the BAL prototype and were put into a thermostized oxygen electrode chamber constructed in our laboratory, filled with respiration media (KH plus 10 mmol/L HEPES and 2 mmol/Lm pyruvate, pH = 7.40 at 36 °C)[14]. The oxygen levels in the incubation media were measured using a Clark-type oxygen electrode (YSI 5300, Yellow Spring, OH, United States). After a 2 min stabilization period, the endogenous respiration rate was recorded and calculated over a 5 min period. Results are expressed as μmol O2/min/g wet tissue[14].
Results are expressed as mean ± SD. Data was analyzed using one-way or multifactor analysis of variance with Scheffe’s multiple range tests as post-test. Differences between means were considered statistically significant when P ≤ 0.05. Dr. Lucas D Daurelio (Estadística, Facultad de Cs. Bioquímicas y Farmacéuticas, UNR) has designed and supervised the statistical analysis performed in this work.
As was formerly explained, the first BAL constructed in our laboratory was designed to house hepatocyte suspensions as the biological component and showed a good performance[4]. In order to evaluate the use of LMOs as alternative biocomponent of our BAL prototype, we first studied their viability and functionality in a simpler model, the NRS, as shown in Figure 2A[5]. Once was established, their ability to metabolize ammonia (detoxification of 35.1% ± 7.0 %, or 14.3 ± 3.6 μmol/g wet tissue, after 120 min incubation, n = 6), we modified the cylindrical BAL, originally designed to contain hepatocytes, in order to accommodate LMOs on its BC.
The cylindrical shaped BAL for LMOs is shown in Figure 2B. The hollow fiber cartridge configuration is maintained: blood circulates inside the fibers and LMOs are placed in the compartment delimited between the plastic receptacle and the fiber outer surfaces, but this BC has a larger capacity (50 cm3vs 9 cm3 in the previous model). Also, a bigger loading port (Lp in Figure 2B) was assembled to the plastic cartridge. Both, Lp and BC capacity are larger than in the original BAL[4] to allow easy LMO loading and retrieval, and to accommodate the biological material, respectively. In both designs, approximately 140 hollow fibers are aligned inside the cartridge connected to two Y-shaped connectors.
We studied the functioning of the device and the perfusion system in experiments without a biological component. The values obtained for the different parameters assayed can be seen in Table 3 (n = 6 independent runs). Hematocrit values and osmolality ratios remained stable during the 120 min of perfusion and, though there was an increment in the percentage of hemolysis, it was minimal. Ammonia and glucose could readily cross the fiber walls because their concentrations in both compartments evened out after 120 min. Total ammonia mass (QNH4+) did not change during experiments.
| Perfusion time | OsmB/OsmBC | Ht (%) | Hemolysis (%) | [Glucose]B/[Glucose]BC | [NH4+]B/[NH4+]BC | QNH4+ (μmol) |
| 0 min | 0.95 ± 0.05 | 43 ± 3 | 0.12 ± 0.06 | 0.12 ± 0.01 | 23.2 ± 1.2 | 31.1 ± 3.9 |
| 60 min | 0.99 ± 0.01 | 42 ± 6 | 0.30 ± 0.08 | 0.81 ± 0.03 | 1.4 ± 0.4 | 31.1 ± 4.2 |
| 120 min | 1.00 ± 0.01 | 38 ± 2 | 0.65 ± 0.10 | 0.94 ± 0.02 | 1.2 ± 0.2 | 31.3 ± 3.8 |
After determining that the BAL was functioning correctly without a biological component, the modified device was tested with LMOs to establish its capacity to keep these tissue slices functional and viable for 120 min of perfusion.
As a viability parameter, we assayed the release of the cytosolic enzyme LDH. The values measured for LMOs in the BAL prototype are shown in Table 4 (n = 6 independent LMO preparations in separate runs), compared to the values obtained in the NRS[5] (n = 6 different LMO preparations). In our BAL, the amount of LDH release increased with perfusion time. A similar pattern was observed in the NRS and no difference was detected when both systems were compared, reaching almost the same percentages after 120 min (14.7% ± 3.1% for the BAL prototype and 15.5% ± 3.2% for the NRS).
Respiratory activity determination constitutes a very specific metabolic test to evaluate tissue functionality and notice the existence of hidden damages[26]. LMOs were taken out at different times and put into an oxygen chamber to test their oxygen consumption capacity (Table 4, n = 6 LMOs taken at each time, coming from different preparations in independent BAL-runs/NRS-incubations). This parameter remained stable during the 120 min of perfusion in the BAL and again no difference was observed compared to the NRS, even though in this system there was a significant decrease at 60 min and 120 min compared to the initial value.
Any biological component employed in a BAL must face and deal with the high levels of ammonia in the blood of the patients to be treated because the accumulation of this metabolite is associated with the progression of hepatic failure[3]. Therefore, we analyzed different parameters related to ammonia metabolism in LMOs. Relative gene expression and activity of CPSI and OTC, two key enzymes of the urea cycle, were studied and the results obtained are shown in Tables 5 and 6 (n = 3 different LMO preparations in independent BAL-runs/NRS-incubations). The levels of mRNA and activity for both enzymes did not significantly change after 120 min of perfusion in the BAL prototype. In addition, there were no significant differences in the levels measured for LMOs in the NRS[5], indicating proper enzyme quantities to perform ammonia detoxification. However, despite all these similarities, when the LMOs were used as the biological component of the cylindrical shaped BAL they were unable to metabolize ammonia (Table 7), while in the NRS they detoxified 22.2% ± 5.5% and 35.1% ± 7.0% of the initial dose after 60 min and 120 min, respectively (n = 6 different LMO preparations).
| Relative gene expression | Cps1 | Otc | |||||
| Time (min) | 0 | 60 | 120 | 0 | 60 | 120 | |
| Model | Cylindrical BAL | 1.05 ± 0.17 | 0.84 ± 0.09 | 0.63 ± 0.12 | 1.02 ± 0.26 | 0.87 ± 0.21 | 0.67 ± 0.20 |
| NRS | 1.00 ± 0.24 | 0.96 ± 0.12 | 0.86 ± 0.10 | 1.00 ± 0.20 | 0.87 ± 0.16 | 0.82 ± 0.07 | |
| Enzymatic activity | CPSI (U/g wet tissue) | OTC (U/g wet tissue) | |||||
| Time (min) | 0 | 60 | 120 | 0 | 60 | 120 | |
| Model | Cylindrical BAL | 2.48 ± 0.62 | 2.72 ± 0.61 | 3.03 ± 0.86 | 241.7 ± 12.6 | 235.1 ± 11.9 | 222.0 ± 23.5 |
| NRS | 2.47 ± 0.60 | 2.82 ± 0.76 | 3.12 ± 0.73 | 209.7 ± 33.2 | 251.8 ± 29.4 | 228.8 ± 32.8 | |
| Time (min) | Ammonia detoxification capacity (μmol/g wet tissue) | ||
| Flat bottom BAL | Cylindrical BAL | ||
| LMOs | LMOs | Hepatocytes | |
| 60 | 8.1 ± 1.2a | ND | 12.5 ± 1.8 |
| 120 | 13.2 ± 2.2 | ND | 18.6 ± 4.9 |
Cylindrical vs “flat bottom” shaped BAL prototypes: The importance of architecture
We hypothesized that the LMOs were unable to metabolize ammonia in the cylindrical shaped device was not related to the biological component because all the other functionality and viability parameters were similar to the NRS. Therefore, the problem could be due to the artificial part of the device, specifically the BC. To test this hypothesis, we decided to change the BC architecture of our BAL and mimic the configuration and disposition of the LMOs in the NRS (Figure 2A). The result was the design of a BAL prototype with a completely different shaped BC: The “flat bottom” BAL displayed in Figure 2C. Instead of using a cylindrical plastic cartridge, we utilized a 25 cm2 culture flask that offers a flat base BC where LMOs adopt a similar distribution as the one in the NRS. All the other components and materials utilized were the same in both devices, including the perfusion system. This “flat bottom” BAL performed well in experiments without a biological component[15].
Ammonia detoxification was the key task that LMOs could not perform in the cylindrical shape BAL. When they were evaluated in the “flat bottom” BAL, fresh LMOs were able to metabolize 32.1% ± 2.2% and 49.3% ± 8.8% of the initial NH4+ dose at 60 min and 120 min, respectively (n = 6 different LMO preparations in independent runs)[15], results that support the importance of architecture.
According to the results, the devices designed in our laboratory could be successfully incorporated into BAL systems depending on the chosen biological component. In Table 7, we compare both ammonia detoxification capacities between LMOs in the “flat bottom” and cylindrical BAL prototypes as well as with hepatocyte suspensions in our previous cylindrical BAL (n = 6 different LMO/hepatocyte preparations in independent runs). For the purpose of comparison between the different biological components (LMOs vs hepatocytes), we performed calculations using the following equivalence: 128 × 106 hepatocytes = 1 g of liver[27-29]. After 60 min of perfusion, LMOs in the “flat bottom” BAL showed a significantly decreased level of ammonia detoxification compared to hepatocytes in the cylindrical model (Table 7). However, after 120 min both models had similar metabolizing capacities. Rat hepatocyte isolation and incubation in the cylindrical BAL were performed as already reported by our group[4] (routine protocol for hepatocyte isolation is shown in Figure S1; and cylindrical BAL operation in Figure S2).
The first step before using LMOs in our BAL device was the adaptation of the prototype designed for hepatocytes, and testing it without any biological components. The results obtained (Table 3) demonstrated a proper exchange of fluids between the blood and the BC since the relationship between osmolality and the hematocrit values remained stable during the 120 min of perfusion. Also, it can be appreciated that solute transport functioned properly in both directions: ammonia diffused from blood to the extra-fiber fluid, while glucose flowed the opposite way, almost matching their concentrations during the initial hour (Table 3). We did not detect unspecific loss of ammonia or interaction with any part of the device (QNH4+ remained constant) and the small percentage of hemolysis measured can be attributed to the action of the peristaltic pump. Taken together, the results showed that the cylindrical shaped device made in our laboratory, with its simple design, could be easily adapted and scaled up, which is a very important issue in the construction of a BAL intended for clinical application.
After corroborating the proper functioning of the device, we performed several experiments with LMOs as its biological component. The results of these tests were unexpected: LMOs showed satisfactory viability levels (assayed by LDH release), oxygen consumption capacity did not change during the perfusion, and conserved levels of transcripts and adequate enzymatic activities of CPSI and OTC, but they were not able to metabolize ammonia in a significant amount. Even more astonishing was the fact that all the assayed parameters showed similar values to the ones determined in the NRS[5], where LMOs could detoxify 35.1% ± 7.0% of the initial 1 mmol/L NH4+ dose after 120 min.
When adapting the cylindrical BAL model to house LMOs as a biological component, we found that, in spite of the shaking applied, LMOs piled up over each other at the bottom of the cartridge in an array that could prevent the correct exchange of nutrients, oxygen, and metabolites. Furthermore, the experiments in the NRS were accomplished in flat wells in 6-well multi dishes. Then, we came up with the idea of mimicking the NRS configuration, and developed a BAL prototype with a flat surface BC. We reasoned that this geometrical disposition would allow LMOs to adopt a looser, not stacked distribution that would benefit the exchange mechanisms between LMOs and the bathing media. In this way, provided that the volume of the BC is equivalent to that of the previous prototype (50 cm3), this flat bottomed device offers a surface that allows an enhanced bathing and shaking of plane LMOs due to their better distribution.
When we tested fresh LMO ammonia detoxification efficiency in this new “flat bottom” BAL, the results obtained supported our hypothesis. They were able to detoxify 49.3% ± 8.8% of the initial ammonia overload after 120 min of perfusion[15], contrary to the LMOs applied to the cylindrical BAL (Table 7). This observation demonstrates the importance of adjusting the architecture and design of the artificial compartment to a given biological component in order to obtain an optimal BAL performance. Although we cannot rule out other factors influencing LMO detoxification activities that remained unnoticed, the only change between the cylindrical and flat bottom BALs was the shape of the BC. The BC volume, shaking speed, blood flow velocity, oxygen tension, and the brand of fibers used in their construction were identical.
Both systems published, i.e., the cylindrical one operating with isolated hepatocytes[4] and the “flat bottom” one operating with LMOs[15], function satisfactorily in relation to ammonia detoxification (Table 7) and, according to the needs of the patient, could be optionally selected in the future. The device could also be used as a suitable mini-bioreactor to analyze drug toxicity or other parameters of interest. We are performing further studies to establish the synthetic capacity of the biological components in our prototypes.
At present no BAL is in use to clinically treat liver failure. Some clinical trials have been conducted (for an updated review consult the work of Sakiyama et al[30] and references therein) with some success. All of these devices use isolated liver-derived cells. Four of them use porcine primary hepatocytes either fresh (named LSS, Excorp Medical BLSS and AMC-BAL) or cryopreserved and microcarrier attached (HepatAssit), one uses human primary hepatocytes (MELS), and one uses HepG2/C3A cell line (Vitagen ELAD). These devices have not become mainstream in clinical settings yet because of the complexity of isolating hepatocytes and the difficulties for maintaining the viability of these cells viable for prolonged periods of time. This limits the assembly and transport of the devices and confines its use to the centers where they were developed. An exception to this could be the ELAD system that uses a cell line, but the costs of producing the required amount of cellular material makes it expensive. It is also limited to places with the facilities and expertise to conduct cell culture at a large scale. In this sense our BAL prototype, although it needs further testing, requires hand-cut LMOs, which are obtained by a low-cost technique that is easily learned. Also, the required biological material could be obtained from donor livers not acceptable for transplantation but meeting the criteria to obtain adequate pieces to feed this kind of BAL and possibly used in multiple treatments. Therefore, pre-assembled BALs could be filled with LMOs obtained in the same center where they will be immediately applied. The prototype we are presenting in this work is constructed with standard laboratory and medical supplies, and we envisage that they could be constructed at reasonable costs and, consequently, commercialized at reasonable prices.
After publishing our previous results[15], we became aware that it is difficult to predict the conditions needed for good performance of the devices based on rational design and, instead, much of the experience we have accumulated over the years mostly originated by trial and error. Especially in this field of BAL research, every laboratory seems to apply different strategies to achieve the objective of extracorporeal treatment of liver failure. Although some basic principles and features are followed by all of us, there seems to really exist one different BAL for each group of work[31,32]. As far as we know, this is the first time that a comparison of the same biological component applied to different configurations of BALs is reported. In the literature, we have always found comparisons of different BALs only in review articles that use data from different research groups, which renders the analysis incomplete and inadequate. However, we have made comparisons in the same set of experiments using cells and tissues from the same brood of experimental animals, same batches of solutions, same laboratory instruments, etc. These conditions render our results coherent and valid, and strongly demonstrate that architecture of BALs can determine the success of this kind of device.
Further investigations must be done in order to bring these types of devices to the clinic. Very few clinical trials have been performed, and they are typically carried out on patients with advanced deterioration[32-35]. In conclusion, we demonstrate the importance of adapting the artificial component architecture to the biological component characteristics to obtain an adequate BAL performance regarding blood ammonia detoxification.
Liver failure is a condition that usually requires liver transplantation, but in some cases acute liver failure resolves spontaneously due to the viable hepatic mass remaining after the cause of the damage has disappeared. If the amount of this functional tissue has the sufficient capacity to handle the detoxification of harmful metabolites produced by the insult and to provide the needed essential hepatic molecules and factors, then the regeneration capacity of the organ allows the recovery. This is why many attempts have been pursued to help the patient´s liver to pass through this acute failure and either recover or extend the time frame for a liver transplantation. Artificial livers, either dialysis based or incorporating hepatic cells and tissues (these later referred to as bio-artificial livers or BALs), are extracorporeal devices intended to aid the failing livers to overcome failure or at least to permit the patient to improve to undergo transplantation. In this sense, BALs are considered the choice to accomplish this job but until now they have been applied only by medical care teams that are able to obtain the biological component and to assemble the device at the same location making the practice limited to very few centers in the world.
The BAL research field is several decades old but still no successful device has been developed. Several prototypes have been submitted to clinical trials but none are routinely used in clinical settings or commercially available. These prototypes use isolated cells of hepatic origin (isolated primary human or pig hepatocytes and HepG2/C3A cell line), which is a biological material that requires expertise and money to be obtained and/or maintained. Additionally, cryopreservation of primary hepatocytes is not a very successful technique and recovery after thawing is poor. Among other researchers in the field, we propose and are testing the use of liver microorgans (LMOs) as the biological component for BAL devices, which are promising in terms of bearing all hepatic cellular types and microarchitecture and involve a simple method to obtain. These characteristics are appealing because they bring the possibility of using procured organs not suitable for transplantation to get the material necessary to feed pre-assembled cartridges at the same centers were the BAL would be needed. Our experience in the field has taught us that changes in the artificial part of these devices can have an impact on the biological component function. The finding that these changes in design, that can be minor or significant, have an influence on performance with effects ranging from subtle to massive, address the importance of finely tuning the interplay between the components of the device to optimize BAL operation.
LMOs failed to detoxify ammonia in a scaled-up BAL configuration that was previously successful. We set out to solve this problem and analyze the possible reasons of the phenomenon we were observing.
The methodology used is the standard in our laboratory and has been previously published. The novelty is to perform and report the comparison of different BAL designs using the same biological component. This is the first report making such a comparison in the same set of experiments using cells and tissues from the same brood of experimental animals, same batches of solutions, same laboratory instruments, same materials to construct the devices, and so forth.
The main result we achieved in this work is that LMOs were totally incompetent to detoxify ammonia when placed in a cylindrical shaped BAL while they were fully able to detoxify ammonia inside a flat bottomed BAL.
The accumulation of high levels of ammonia in the blood is an important issue in patients presenting liver failure, and is of the highest interest when studying this kind of device. In the literature, we have always found comparisons of different BALs only in review articles that use data from different research groups, which renders the comparative analysis incomplete. Our experimental design makes our comparison coherent and valid, and strongly demonstrates that the architecture of BALs can determine the success of this kind of device.
We consider that this is an especially important finding, particularly in the light of the results presented, compelling future research to put an effort to finely tune the interplay between the artificial and biological components of BALs in order to achieve optimal performance and finally reach the clinical setting.
We would like to thank GAMBRO, especially to Mr. Jorge Auerbuch, for kindly providing the dialysis cartridge from which we obtained PolyamixTM fibers.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kao JT, Xiao J S- Editor: Ji FF L- Editor: Filipodia E- Editor: Song H
| 1. | Rahman TM, Hodgson HJ. Review article: liver support systems in acute hepatic failure. Aliment Pharmacol Ther. 1999;13:1255-1272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Selden C. Bioartificial liver. In: Nedović V, Willaert R, editors. Applications of Cell Immobilisation Biotechnology. 1st ed. 2005;69-83. [DOI] [Cited in This Article: ] |
| 3. | Bernal W, Hall C, Karvellas CJ, Auzinger G, Sizer E, Wendon J. Arterial ammonia and clinical risk factors for encephalopathy and intracranial hypertension in acute liver failure. Hepatology. 2007;46:1844-1852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 271] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 4. | Rodriguez JV, Pizarro MD, Scandizzi AL, Guibert EE, Almada LL, Mamprin ME. Construction and performance of a minibioreactor suitable as experimental bioartificial liver. Artif Organs. 2008;32:323-328. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Pizarro MD, Mediavilla MG, Berardi F, Tiribelli C, Rodríguez JV, Mamprin ME. Cold storage of liver microorgans in ViaSpan and BG35 solutions: study of ammonia metabolism during normothermic reoxygenation. Ann Hepatol. 2014;13:256-264. [PubMed] [Cited in This Article: ] |
| 6. | Matoori S, Leroux JC. Recent advances in the treatment of hyperammonemia. Adv Drug Deliv Rev. 2015;90:55-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | McEwan P, Simpson D, Kirk JM, Barr DG, McKenzie KJ. Short report: Hyperammonaemia in critically ill septic infants. Arch Dis Child. 2001;84:512-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Bharat A, Cunningham SA, Scott Budinger GR, Kreisel D, DeWet CJ, Gelman AE, Waites K, Crabb D, Xiao L, Bhorade S. Disseminated Ureaplasma infection as a cause of fatal hyperammonemia in humans. Sci Transl Med. 2015;7:284re3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Häberle J, Boddaert N, Burlina A, Chakrapani A, Dixon M, Huemer M, Karall D, Martinelli D, Crespo PS, Santer R. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J Rare Dis. 2012;7:32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 421] [Cited by in F6Publishing: 357] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 10. | Mouat S, Bishop J, Glamuzina E, Chin S, Best EJ, Evans HM. Fatal hyperammonemia associated with disseminated Serratia marcescens infection in a pediatric liver transplant recipient. Pediatr Transplant. 2018;22:e13180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1158] [Cited by in F6Publishing: 1225] [Article Influence: 122.5] [Reference Citation Analysis (1)] |
| 12. | Olde Damink SW, Jalan R, Deutz NE, Redhead DN, Dejong CH, Hynd P, Jalan RA, Hayes PC, Soeters PB. The kidney plays a major role in the hyperammonemia seen after simulated or actual GI bleeding in patients with cirrhosis. Hepatology. 2003;37:1277-1285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 98] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | De Jonghe B, Janier V, Abderrahim N, Hillion D, Lacherade JC, Outin H. Urinary tract infection and coma. Lancet. 2002;360:996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Mandolino C, Pizarro MD, Quintana AB, Rodríguez JV, Mamprin ME. Hypothermic preservation of rat liver microorgans (LMOs) in bes-gluconate solution. Protective effects of polyethyleneglycol (PEG) on total water content and functional viability. Ann Hepatol. 2011;10:196-206. [PubMed] [Cited in This Article: ] |
| 15. | Pizarro MD, Mediavilla MG, Quintana AB, Scandizzi ÁL, Rodriguez JV, Mamprin ME. Performance of cold-preserved rat liver Microorgans as the biological component of a simplified prototype model of bioartificial liver. World J Hepatol. 2016;8:1442-1451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Calligaris SD, Almada LL, Guibert EE, Tiribelli C, Rodriguez JV. Ammonium detoxifying activity is maintained after 72 hours of cold preservation of rat hepatocytes in University of Wisconsin (UW) solution. Cryo Letters. 2002;23:245-254. [PubMed] [Cited in This Article: ] |
| 17. | Proelss HF, Wright BW. Rapid determination of ammonia in a perchloric acid supernate from blood, by use of an ammonia-specific electrode. Clin Chem. 1973;19:1162-1169. [PubMed] [Cited in This Article: ] |
| 18. | Blei AT. Diagnosis and treatment of hepatic encephalopathy. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:959-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Kalinov A. El Laboratorio y Su Interpretación Semiológica. 1st ed. Buenos Aires: Lopez Libreros Editores 1975; . [Cited in This Article: ] |
| 20. | Arnaud FG, Khirabadi BS, Fahy GM. Normothermic blood perfusion of isolated rabbit kidneys. III. In vitro physiology of kidneys after perfusion with Euro-Collins solution or 7.5 M cryoprotectant (VS4). Transpl Int. 2002;15:278-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 21. | Olinga P, Merema MT, Hof IH, De Jager MH, De Jong KP, Slooff MJ, Meijer DK, Groothuis GM. Effect of cold and warm ischaemia on drug metabolism in isolated hepatocytes and slices from human and monkey liver. Xenobiotica. 1998;28:349-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | van Anken HC, Schiphorst ME. A kinetic determination of ammonia in plasma. Clin Chim Acta. 1974;56:151-157. [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 98] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Miszczuk G, Mediavilla MG, Pizarro MD, Tiribelli C, Rodríguez J, Mamprin ME. Expression and distribution of aquaporin 8 in rat hepatocytes cold stored 72 hours in modified University of Wisconsin and bes-gluconate-sucrose solutions. Study of their correlation with water content. Cryo Letters. 2012;33:75-85. [PubMed] [Cited in This Article: ] |
| 24. | Pierson DL. A rapid colorimetric assay for carbamyl phosphate synthetase I. J Biochem Biophys Methods. 1980;3:31-37. [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Ceriotti G. Ornithine Carbamoyltransferase. Methods Enzym Anal vol III. 1983;2:319-325. [Cited in This Article: ] |
| 26. | Bilzer M, Gerbes AL. Preservation injury of the liver: mechanisms and novel therapeutic strategies. J Hepatol. 2000;32:508-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 140] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Bayliss MK, Bell JA, Jenner WN, Park GR, Wilson K. Utility of hepatocytes to model species differences in the metabolism of loxtidine and to predict pharmacokinetic parameters in rat, dog and man. Xenobiotica. 1999;29:253-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Pang KS, Kong P, Terrell JA, Billings RE. Metabolism of acetaminophen and phenacetin by isolated rat hepatocytes. A system in which the spatial organization inherent in the liver is disrupted. Drug Metab Dispos. 1985;13:42-50. [PubMed] [Cited in This Article: ] |
| 29. | Sohlenius-Sternbeck AK. Determination of the hepatocellularity number for human, dog, rabbit, rat and mouse livers from protein concentration measurements. Toxicol In Vitro. 2006;20:1582-1586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 172] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 30. | Sakiyama R, Blau BJ, Miki T. Clinical translation of bioartificial liver support systems with human pluripotent stem cell-derived hepatic cells. World J Gastroenterol. 2017;23:1974-1979. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 34] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (3)] |
| 31. | Bader A, Knop E, Frühauf N, Crome O, Böker K, Christians U, Oldhafer K, Ringe B, Pichlmayr R, Sewing KF. Reconstruction of liver tissue in vitro: geometry of characteristic flat bed, hollow fiber, and spouted bed bioreactors with reference to the in vivo liver. Artif Organs. 1995;19:941-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Carpentier B, Gautier A, Legallais C. Artificial and bioartificial liver devices: present and future. Gut. 2009;58:1690-1702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Krisper P, Stadlbauer V, Stauber RE. Clearing of toxic substances: are there differences between the available liver support devices? Liver Int. 2011;31 Suppl 3:5-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Sussman NL, McGuire BM, Kelly JH. Hepatic assist devices: will they ever be successful? Curr Gastroenterol Rep. 2009;11:64-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 35. | Sussman NL, Kelly JH. Artificial liver. Clin Gastroenterol Hepatol. 2014;12:1439-1442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |